Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gray Scale Examination
2.2. Doppler US Examination
2.3. CEUS Examination
2.4. Real-Time Elastography (RTE)
2.5. Statistical Analysis
3. Results
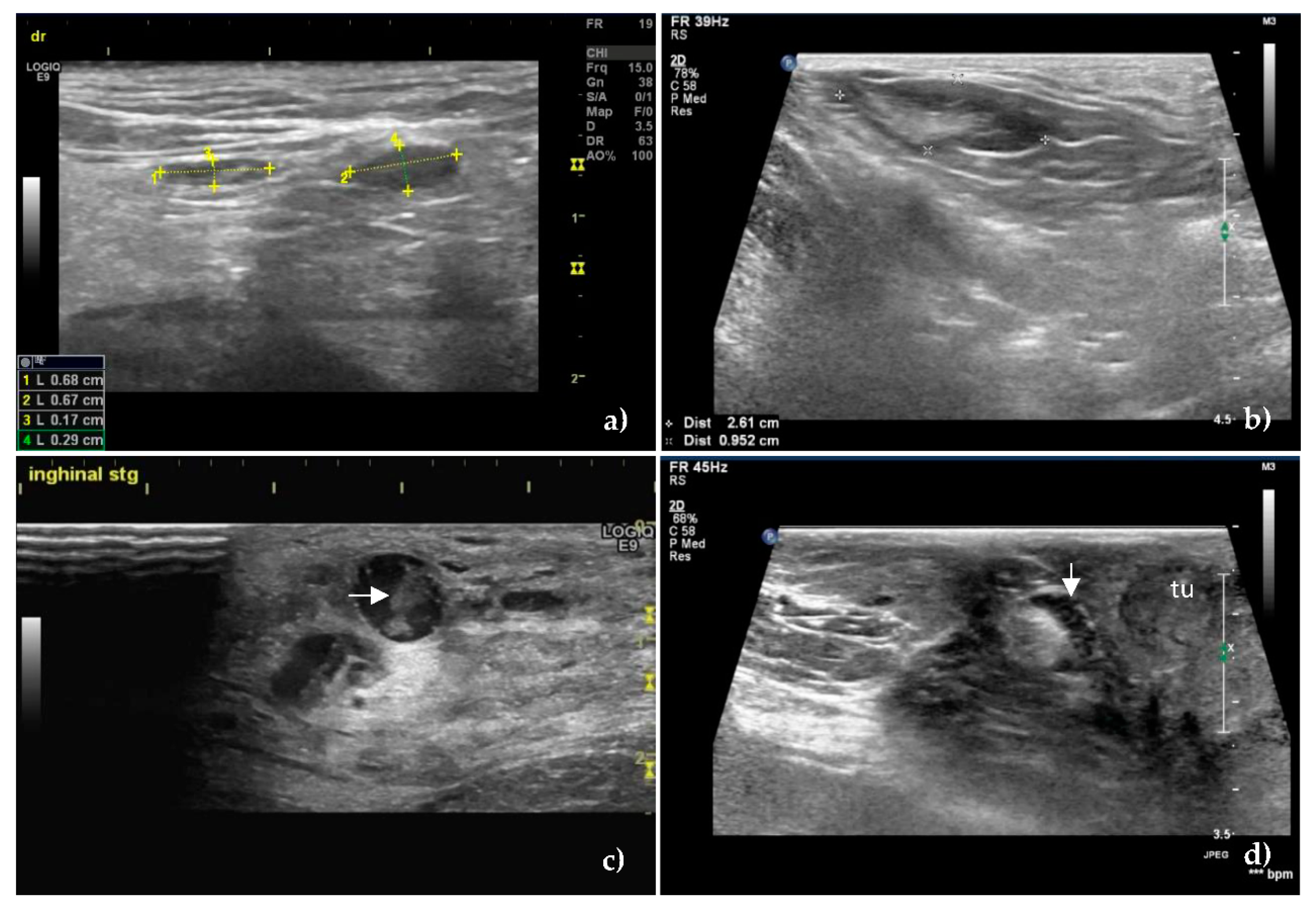
3.1. B-Mode Ultrasonography
3.2. Color Doppler Ultrasonography
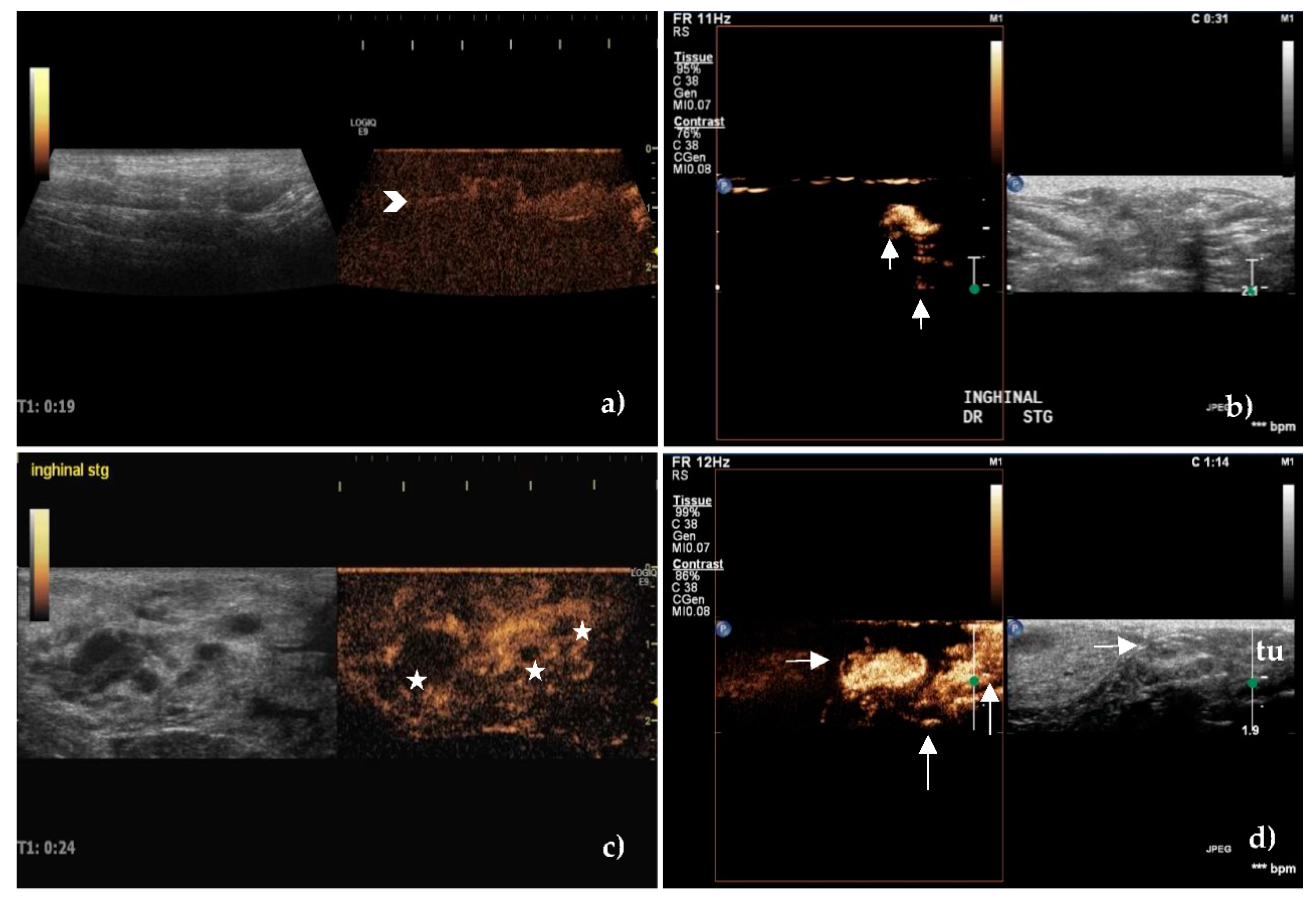
3.3. Contrast-Enhanced Ultrasonography
3.4. Real-Time Elastography
3.5. Lymphatic Mapping
4. Discussion
4.1. B-Mode Ultrasonography
4.2. Color Doppler Ultrasonography
4.3. Contrast-Enhanced Ultrasonography
4.4. Real Time Elastography
4.5. Lymphatic Mapping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L.; Ibisch, C.; et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: Immunophenotypes and prognostic significance. Breast Cancer Res. Treat. 2018, 167, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, F.; Peña, L.; Ibisch, C.; Loussouarn, D.; Gama, A.; Rieder, N.; Belousov, A.; Campone, M.; Abadie, J. Canine invasive mammary carcinomas as models of human breast cancer. Part 1: Natural history and prognostic factors. Breast Cancer Res. Treat. 2018, 167, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleto, A.F.; Wilson, T.M.; Soares, N.P.; Gundim, L.F.; Castro, I.P.; Guimarães, E.C.; Bandarra, M.B.; Medeiros-Ronchi, A.A. Prognostic value of occult isolated tumour cells within regional lymph nodes of dogs with malignant mammary tumours. J. Comp. Pathol. 2018, 158, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chocteau, F.; Abadie, J.; Loussouarn, D.; Nguyen, F. Proposal for a histological staging system of mammary carcinomas in dogs and cats. part 1: Canine mammary carcinomas. Front. Vet. Sci. 2019, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, T.W.; Spitz, F.R.; Callans, L.S.; Alavi, A.; Mick, R.; Weinstein, S.P.; Bedrosian, I.; Fraker, D.L.; Bauer, T.L.; Czerniecki, B.J. Subareolar and peritumoral injection identify similar sentinel nodes for breast cancer. Ann. Surg. Oncol. 2002, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Edwina Doting, M.H.; Annemiek Stiekema, H.M.; de Vries, J.; Lemstra, C.; Hoekstra, H.J.; Vrieling, M.; Rietman, L.; Jager, P.L. Immediate dynamic lymphoscintigraphy delivers no additional value to lymphoscintigraphy 3 hr after tracer injection in sentinel lymph node biopsy in breast cancer patients. J. Surg. Oncol. 2007, 95, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, T.M.; Colbert, M.; Christensen, R.; Ose, K.J.; Jones, T.; Wetherille, R.; Friedman, J.; Swenson, K.; McMasters, K.M. Subareolar injection of 99mTc facilitates sentinel iymph node identification. Ann. Surg. Oncol. 2002, 9, 77–81. [Google Scholar] [CrossRef]
- McGregor, A.; Pavri, S.N.; Tsay, C.; Kim, S.; Narayan, D. Use of indocyanine green for sentinel lymph node biopsy: Case series and methods comparison. Plast. Reconstr. Surg. Glob. Open 2017, 5, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Vermersch, C.; Raia-Barjat, T.; Chapelle, C.; Lima, S.; Chauleur, C. Randomized comparison between indocyanine green fluorescence plus 99m technetium and 99m technetium alone methods for sentinel lymph node biopsy in breast cancer. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Favril, S.; Stock, E.; Hernot, S.; Hesta, M.; Polis, I.; Vanderperren, K.; de Rooster, H. Sentinel lymph node mapping by near-infrared fluorescence imaging and contrast-enhanced ultrasound in healthy dogs. Vet. Comp. Oncol. 2019, 17, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Kawase, K.; Gayed, I.W.; Hunt, K.K.; Kuerer, H.M.; Akins, J.; Yi, M.; Grimes, L.; Babiera, G.V.; Ross, M.I.; Feig, B.W.; et al. Use of lymphoscintigraphy defines lymphatic drainage patterns before sentinel lymph node biopsy for breast cancer. J. Am. Coll. Surg. 2006, 203, 64–72. [Google Scholar] [CrossRef]
- Rossi, F.; Körner, M.; Suárez, J.; Carozzi, G.; Meier, V.S.; Roos, M.; Rohrer Bley, C. Computed tomographic-lymphography as a complementary technique for lymph node staging in dogs with malignant tumors of various sites. Vet. Radiol. Ultrasound 2018, 59, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Soultani, C.; Patsikas, M.N.; Karayannopoulou, M.; Jakovljevic, S.; Chryssogonidis, I.; Papazoglou, L.; Papaioannou, N.; Papadopoulou, P.; Pavlidou, K.; Ilia, G.M.; et al. Assessment of sentinel lymph node metastasis in canine mammary gland tumors using computed tomographic indirect lymphography. Vet. Radiol. Ultrasound 2017, 58, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.M.F.; Baumgartner, C.; Hirschberger, J.; Beer, A.J.; Brühschwein, A.; Kreutzmann, N.; Laberke, S.; Wergin, M.C.; Meyer-Lindenberg, A.; Brandl, J.; et al. Comparative oncology: Evaluation of 2-deoxy-2-[18F]. Fluoro-d-glucose (FDG) Positron emission tomography/computed tomography (PET/CT) for the staging of dogs with malignant tumors. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.; Romero, L.; López, S.; Campuzano, M.; Ortega, R.; Morales, A.; Guadarrama, M.; Cesarman-Maus, G.; García-Pérez, O.; Lizano, M. 18F-FDG—PET/CT in canine mammary gland tumors. Front. Vet. Sci. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Suga, K.; Yuan, Y.; Ogasawara, N.; Okada, M.; Matsunaga, N. Localization of breast sentinel lymph nodes by MR lymphography with a conventional gadolinium contrast agent. Acta Radiol. 2003, 44, 35–42. [Google Scholar] [CrossRef]
- De Swarte, M.; Alexander, K.; Rannou, B.; D’Anjou, M.A.; Blond, L.; Beauchamp, G. Comparison of sonographic features of benign and neoplastic deep lymph nodes in dogs. Vet. Radiol. Ultrasound 2011, 52, 451–456. [Google Scholar] [CrossRef]
- Llabrés-Díaz, F.J. Ultrasonography of the medial iliac lymph nodes in the dog. Vet. Radiol. Ultrasound 2004, 45, 156–165. [Google Scholar] [CrossRef]
- Nyman, H.T.; O’Brien, R.T. The sonographic evaluation of lymph nodes. Clin. Tech. Small Anim. Pract. 2007, 22, 128–137. [Google Scholar] [CrossRef]
- Stan, F.; Gudea, A.; Baba, A.I.; Feier, D.; Badea, R. Correlation between ultrasonographic features and morphological pattern after blue dye injection of normal superficial lymph nodes in carnivores. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca Vet. Med. 2012, 69, 211–219. [Google Scholar] [CrossRef]
- Cox, K.; Taylor-Phillips, S.; Sharma, N.; Weeks, J.; Mills, H.; Sever, A.; Lim, A.; Haigh, I.; Hashem, M.; De Silva, T.; et al. Enhanced pre-operative axillary staging using intradermal microbubbles and contrast-enhanced ultrasound to detect and biopsy sentinel lymph nodes in breast cancer: A potential replacement for axillary surgery. Br. J. Radiol. 2018, 91, 1082. [Google Scholar] [CrossRef]
- Mei, M.; Ye, L.; Quan, J.; Huang, P. Contrast-enhanced ultrasound for the differential diagnosis between benign and metastatic superficial lymph nodes: A meta-analysis. Cancer Manag. Res. 2018, 10, 4987–4997. [Google Scholar] [CrossRef] [Green Version]
- Nielsen Moody, A.; Bull, J.; Culpan, A.M.; Munyombwe, T.; Sharma, N.; Whitaker, M.; Wolstenhulme, S. Preoperative sentinel lymph node identification, biopsy and localisation using contrast enhanced ultrasound (CEUS) in patients with breast cancer: A systematic review and meta-analysis. Clin. Radiol. 2017, 72, 959–971. [Google Scholar] [CrossRef]
- Sharma, N.; Cox, K. Axillary nodal staging with contrast-enhanced ultrasound. Curr. Breast Cancer Rep. 2017, 9, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Liptak, J.M.; Boston, S.E. Nonselective lymph node dissection and sentinel lymph node mapping and biopsy. Vet. Clin. North Am. Small Anim. Pract. 2019, 49, 793–807. [Google Scholar] [CrossRef]
- Stan, F.G. Non-invasive assessment of sentinel lymph nodes that drain the tumoral mammary glands in female dog. Bull. UASVM Vet. Med. 2016, 73, 382–388. [Google Scholar] [CrossRef]
- Gelb, H.R.; Freeman, L.J.; Rohleder, J.J.; Snyder, P.W. Feasibility of contrast-enhanced ultrasound-guided biopsy of sentinel lymph nodes in dogs. Vet. Radiol. Ultrasound 2010, 51, 628–633. [Google Scholar] [CrossRef]
- Belotta, A.F.; Gomes, M.C.; Rocha, N.S.; Melchert, A.; Giuffrida, R.; Silva, J.P.; Mamprim, M.J. Sonography and sonoelastography in the detection of malignancy in superficial lymph nodes of dogs. J. Vet. Intern. Med. 2019, 33, 1403–1413. [Google Scholar] [CrossRef] [Green Version]
- Seiler, G.S.; Griffith, E. Comparisons between elastographic stiffness scores for benign versus malignant lymph nodes in dogs and cats. Vet. Radiol. Ultrasound 2018, 59, 79–88. [Google Scholar] [CrossRef]
- Silva, P.; Uscategui, R.A.R.; Maronezi, M.C.; Gasser, B.; Pavan, L.; Gatto, I.R.H.; de Almeida, V.T.; Vicente, W.R.R.; Feliciano, M.A.R. Ultrasonography for lymph nodes metastasis identification in bitches with mammary neoplasms. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.H.; Baek, J.H. Ultrasound elastography for evaluation of cervical lymph nodes. Ultrasonography 2015, 34, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, S.M.; Kim, B.; La Yun, B.; Jang, M.; Ko, Y.; Lee, S.H.; Jeong, H.; Chang, J.M.; Cho, N. Comparison of strain and shear wave elastography for qualitative and quantitative assessment of breast masses in the same population. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Pereira, C.T.; Rahal, S.C.; De Carvalho Balieiro, J.C.; Ribeiro, A.A.C.M. Lymphatic drainage on healthy and neoplasic mammary glands in female dogs: Can it really be altered? J. Vet. Med. Ser. C Anat. Histol. Embryol. 2003, 32, 282–290. [Google Scholar] [CrossRef]
- Patsikas, M.N.; Karayannopoulou, M.; Kaldrymidoy, E.; Papazoglou, L.G.; Papadopoulou, P.L.; Tzegas, S.I.; Tziris, N.E.; Kaitzis, D.G.; Dimitriadis, A.S.; Dessiris, A.K. The lymph drainage of the neoplastic mammary glands in the bitch: A lymphographic study. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2006, 35, 228–234. [Google Scholar] [CrossRef]
- Stan, F. Correlation between subareolar and peritumoral blue dye injection to identify sentinel lymph nodes in canine mammary neoplazia. Lucr. Ştiinţifice Ser. Med. Vet. Iasi 2012, 55, 125–132. [Google Scholar]
- Patsikas, M.N.; Dessiris, A. The lymph drainage of the mammary glands in the bitch: A lymphographic study. Part I: The 1st, 2nd, 4th and 5th mammary glands. Anat. Histol. Embryol. 1996, 25, 131–138. [Google Scholar] [CrossRef]
- Patsikas, M.N.; Dessiris, A. The lymph drainage of the mammary glands in the bitch: a lymphographic study. Part II: The 3rd mammary gland. Anat. Histol. Embryol. 1996, 25, 139–143. [Google Scholar] [CrossRef]
- Rossi, F.; Fina, C.; Stock, E.; Vanderperren, K.; Duchateau, L.; Saunders, J.H. Effect of sedation on contrast-enhanced ultrasonography of the spleen in healthy dogs. Vet. Radiol. Ultrasound 2016, 57, 276–281. [Google Scholar] [CrossRef]
- Stock, E.; Vanderperren, K.; Van der Vekens, E.; Haers, H.; Duchateau, L.; Polis, I.; Hesta, M.; Saunders, J.H. The effect of anesthesia with propofol and sedation with butorphanol on quantitative contrast-enhanced ultrasonography of the healthy feline kidney. Vet. J. 2014, 202, 637–639. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Dudea, S.M.; Lenghel, M.; Botar-Jid, C.; Vasilescu, D.; Duma, M. Ultrasonography of superficial lymph nodes: benign vs. malignant. Med. Ultrason. 2012, 14, 294–306. [Google Scholar]
- Nyman, H.T.; Kristensen, A.T.; Skovgaard, I.M.; McEvoy, F.J. Characterization of normal and abnormal canine superficial lymph nodes using gray-scale b-mode, color flow mapping, power, and spectral doppler ultrasonography: A multivariate study. Vet. Radiol. Ultrasound 2005, 46, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.; Dominguez, E.; Lucas, X.; Novellas, R.; Gomes-Coelho, K.V.; Espada, Y.; Agut, A. Comparison between ultrasonographic findings of benign and malignant canine mammary gland tumours using b-mode, colour doppler, power doppler and spectral doppler. Res. Vet. Sci. 2016, 107, 141–146. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Uscategui, R.A.R.; Maronezi, M.C.; Simões, A.P.R.; Silva, P.; Gasser, B.; Pavan, L.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Ultrasonography methods for predicting malignancy in canine mammary tumors. PLoS ONE 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Choi, M.Y.; Lee, J.W.; Jang, K.J. Distinction between benign and malignant causes of cervical, axillary, and inguinal lymphadenopathy: Value of doppler spectral waveform analysis. Am. J. Roentgenol. 1995, 165, 981–984. [Google Scholar] [CrossRef]
- Sever, A.R.; Mills, P.; Jones, S.E.; Mali, W.; Jones, P.A. Sentinel node identification using microbubbles and contrast-enhanced ultrasonography. Clin. Radiol. 2012, 67, 687–694. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, D.; Cheng, L.; Yu, L.; Yang, L.; Tong, F.; Liu, H.; Wang, S.; Wang, S. Intradermal microbubbles and contrast-enhanced ultrasound (CEUS) is a feasible approach for sentinel lymph node identification in early-stage breast cancer. World J. Surg. Oncol. 2015, 13, 1–8. [Google Scholar] [CrossRef]
- Poanta, L.; Serban, O.; Pascu, I.; Pop, S.; Cosgarea, M.; Fodor, D. The place of CEUS in distinguishing benign from malignant cervical lymph nodes: A prospective study. Med. Ultrason. 2014, 16, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Alam, F.; Naito, K.; Horiguchi, J.; Fukuda, H.; Tachikake, T.; Ito, K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: Comparison with conventional b-mode sonography. Am. J. Roentgenol. 2008, 191, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, M.H.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef]
- Vassallo, P.; Edel, G.; Roos, N.; Naguib, A.; Peters, P.E. In-vitro high-resolution ultrasonography of benign and malignant lymph nodes. Invest. Radiol. 1993, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Cassali, G.D.; Lavalle, G.E.; de Nardi, A.B.; Ferreira, E.; Bertagnolli, A.C.; Estrela-Lima, A.; Alessi, A.C.; Daleck, C.R.; Salgado, B.S.; Fernandes, C.G.; et al. Consensus for the diagnosis, prognosis and treatment of canine mammary tumors. Braz. J. Vet. Pathol. 2011, 4, 153–180. [Google Scholar]
- Karin, U.; Sorenmo, D.R.; Worley, M.H.G. Tumors of the mammary gland. In Small Animal Clinical Oncology; Stephen, J., Withrow, D.M., Vail, R.L.P., Eds.; Saunders Company: Philadelphia, PA, USA, 2013; pp. 538–556. [Google Scholar]
- De Araújo, M.R.; Campos, L.C.; Ferreira, E.; Cassali, G.D. Quantitation of the regional lymph node metastatic burden and prognosis in malignant mammary tumors of dogs. J. Vet. Intern. Med. 2015, 29, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Beserra, H.E.O.; Grandi, F.; Dufloth, R.M.; Pinheiro, L.G.P.; Miot, H.A.; Vexenat, S.C.O.R.; Rocha, N.S. Metastasis of mammary carcinoma in bitches: Evaluation of the sentinel lymph node technique. Adv. Breast Cancer Res. 2016, 5, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Lorek, A.; Stojčev, Z.; Zarębski, W.; Kowalczyk, M.; Szyluk, K. Analysis of postoperative complications after 303 sentinel lymph node identification procedures using the sentimag® method in breast cancer patients. Med. Sci. Monit. 2019, 25, 3154–3160. [Google Scholar] [CrossRef]
- Luigi Solbiati, V.; Cioffi, E.B. Ultrasonography of the neck. Radiol. Clin. N. Am. 1992, 30, 941–954. [Google Scholar]
- Nyman, H.T.; Nielsen, O.L.; McEvoy, F.J.; Lee, M.H.; Martinussen, T.; Hellmén, E.; Kristensen, A.T. Comparison of b-mode and doppler ultrasonographic findings with histologic features of benign and malignant mammary tumors in dogs. Am. J. Vet. Res. 2006, 67, 985–991. [Google Scholar] [CrossRef]
- Silver, T.I.; Lawson, J.A.; Mayer, M.N. Sonographic characteristics of presumptively normal main axillary and superficial cervical lymph nodes in dogs. Am. J. Vet. Res. 2012, 73, 1200–1206. [Google Scholar] [CrossRef]
- Salwei, R.M.; O’Brien, R.T.; Matheson, J.S. Characterization of lymphomatous lymph nodes in dogs using contrast harmonic and power doppler ultrasound. Vet. Radiol. Ultrasound 2005, 46, 411–416. [Google Scholar] [CrossRef]
- Zandvliet, M. Canine Lymphoma: A Review. Vet. Q. 2016, 36, 76–104. [Google Scholar] [CrossRef]
- Alvarez, S.; Añorbe, E.; Alcorta, P.; López, F.; Alonso, I.; Cortés, J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: A systematic review. Am. J. Roentgenol. 2006, 186, 1342–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, X.; Fan, Z.; Li, J.; Xie, Y.; Wang, T.; Ouyang, T. Ultrasound as a replacement for physical examination in clinical staging of axillary lymph nodes in breast cancer patients. Thorac. Cancer 2020, 11, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, M.N.; Lawson, J.A.; Silver, T.I. Sonographic characteristics of presumptively normal canine medial iliac and superficial inguinal lymph nodes. Vet. Radiol. Ultrasound 2010, 51, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, M.J.; Pollard, R.E.; Willcox, J.L. Ultrasonographic characterization of cervical lymph nodes in healthy dogs. Vet. Radiol. Ultrasound 2019, 60, 560–566. [Google Scholar] [CrossRef]
- Burns, G.O.; Scrivani, P.V.; Thompson, M.S.; Erb, H.N. Relation between age, body weight, and medial retropharyngeal lymph node size in apparently healthy dogs. Vet. Radiol. Ultrasound 2008, 49, 277–281. [Google Scholar] [CrossRef]
- Krol, L.; O’Brien, R. Ultrasonographic assessment of abdominal lymph nodes in puppies. Vet. Radiol. Ultrasound 2012, 53, 455–458. [Google Scholar] [CrossRef]
- Ahmadi, O.; Mccall, J.L.; Stringer, M.D. Does senescence affect lymph node number and morphology? a systematic review. ANZ J. Surg. 2013, 83, 612–618. [Google Scholar] [CrossRef]
- Xin, L.; Yan, Z.; Zhang, X.; Zang, Y.; Ding, Z.; Xue, H.; Zhao, C. Parameters for contrast-enhanced ultrasound (CEUS) of enlarged superficial lymph nodes for the evaluation of therapeutic response in lymphoma: a preliminary study. Med. Sci. Monit. 2017, 23, 5430–5438. [Google Scholar] [CrossRef] [Green Version]
- Dialani, V.; James, D.F.; Slanetz, P.J. A Practical approach to imaging the axilla. Insights Imaging 2015, 6, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, A.T.; Ying, M.; Ho, S.Y.; Antonio, G.; Lee, Y.P.; King, A.D.; Wong, K.T. Ultrasound of malignant cervical lymph nodes. Cancer Imaging 2008, 8, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Nieciecki, M.; Dobruch-Sobczak, K.; Wareluk, P.; Gumińska, A.; Białek, E.; Cacko, M.; Królicki, L. Rola badania ultrasonograficznego oraz limfoscyntygrafii w diagnostyce węzłów chłonnych pachowych u pacjentek z rakiem piersi. J. Ultrason. 2016, 16, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Kinns, J.; Mai, W. Association between malignancy and sonographic heterogeneity in canine and feline abdominal lymph nodes. Vet. Radiol. Ultrasound 2007, 48, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Agthe, P.; Caine, A.R.; Posch, B.; Herrtage, M.E. Ultrasonographic appearance of jejunal lymph nodes in dogs without clinical signs of gastrointestinal disease. Vet. Radiol. Ultrasound 2009, 50, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, F.; De Margerie Mellon, C.; Bricout, M.; Cauderlier, E.; Chapelier, M.; Albiter, M.; Bourrier, P.; Espié, M.; De Kerviler, E.; De Bazelaire, C. Diagnostic strategy for the assessment of axillary lymph node status in breast cancer. Diagn. Interv. Imaging 2015, 96, 1089–1101. [Google Scholar] [CrossRef]
- Choi, H.Y.; Park, M.; Seo, M.; Song, E.; Shin, S.Y.; Sohn, Y.M. Preoperative Axillary Lymph Node Evaluation in Breast Cancer: Current Issues and Literature Review. Ultrasound Q. 2017, 33, 6–14. [Google Scholar] [CrossRef]
- Prativadi, R.; Dahiya, N.; Kamaya, A.; Bhatt, S. Chapter 5 ultrasound characteristics of benign vs malignant cervical lymph nodes. Semin. Ultrasound CT MRI 2017, 38, 506–515. [Google Scholar] [CrossRef]
- Ying, M.; Bhatia, K.S.S.; Lee, Y.P.; Yuen, H.Y.; Ahuja, A.T. Review of ultrasonography of malignant neck nodes: Greyscale, doppler, contrast enhancement and elastography. Cancer Imaging 2014, 13, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Almerey, T.; Villacreses, D.; Li, Z.; Patel, B.; McDonough, M.; Gibson, T.; Maimone, S.; Gray, R.; McLaughlin, S.A. Value of axillary ultrasound after negative axillary mri for evaluating nodal status in high-risk breast cancer. J. Am. Coll. Surg. 2019, 228, 792–797. [Google Scholar] [CrossRef] [Green Version]
- Misra, D.; Panjwani, S.; Rai, S.; Misra, A.; Prabhat, M.; Gupta, P.; Talukder, S. Diagnostic efficacy of color doppler ultrasound in evaluation of cervical lymphadenopathy. Dent. Res. J. (Isfahan) 2016, 13, 217–224. [Google Scholar] [CrossRef]
- Stan, F.G. Power Dopler Ultrasonography vs color dopler of the sentinel lymph mammary glands at female dog. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca Vet. Med. 2010, 67, 298–304. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, Y.M.; Cho, S.H.; Jung, H.K.; Kim, S.J.; Ryu, H.S. imaging findings of variable axillary mass and axillary lymphadenopathy. Ultrasound Med. Biol. 2014, 40, 1934–1948. [Google Scholar] [CrossRef]
- Ahuja, A.T.; Ying, M.; Ho, S.S.Y.; Metreweli, C. Distribution of intranodal vessels in differentiating benign from metastatic neck nodes. Clin. Radiol. 2001, 56, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Rotim, T.; Kristek, B.; Turk, T.; Kretić, D.; Perić, M.; Pušeljić, I.; Pandurović, T.; Štefanić, M. Measurable and unmeasurable features of ultrasound lymph node images in detection of malignant infiltration. Acta Clin. Croat. 2017, 56, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Stan, F. Qualitative Morphological Assessement of Tumor Associated Lymphatic Vasculature in Mammary Gland Neoplasia of Female Dog in Relation With Sentinel Lymph Nodes Metastatic Infiltration. Sci. Work. Ser. C. Vet. Med. 2015, 61, 16–23. [Google Scholar]
- Apple, S.K. Sentinel lymph node in breast cancer: review article from a pathologist’s point of view. J. Pathol. Transl. Med. 2016, 50, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, S.; Gomez-Ochoa, P.; De Blas, I.; Gascón, M.; Aceña, C.; Corda, A.; Sosa, I.; Gregori, T.; Couto, G. Pathologic correlation of resistive and pulsatility indices in canine abdominal lymph nodes. Vet. Radiol. Ultrasound 2009, 50, 525–529. [Google Scholar] [CrossRef]
- El-Gohary, Y.M.; Metwally, G.; Saad, R.S.; Robinson, M.J.; Mesko, T.; Poppiti, R.J. Prognostic significance of intratumoral and peritumoral lymphatic density and blood vessel density in invasive breast carcinomas. Am. J. Clin. Pathol. 2008, 129, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, B.B.; Merton, D.A.; Liu, J. Contrast-enhanced sonographic imaging of lymphatic channels and sentinel lymph nodes. J. Ultrasound Med. 2005, 24, 953–965. [Google Scholar] [CrossRef]
- Goldberg, B.B.; Merton, D.A.; Liu, J.; Thakur, M.; Murphy, G.F.; Needleman, L.; Tornes, A. Sentinel lymph nodes in a swine model with melanoma: contrast enhanced lymphatic US. Radiology 2004, 230, 727–734. [Google Scholar] [CrossRef]
- Platt, A.M.; Randolph, G.J. Dendritic Cell Migration Through the Lymphatic Vasculature to Lymph Nodes, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 120. [Google Scholar] [CrossRef]
- Skobe, M.; Detmar, M. Structure, function, and molecular control of the skin lymphatic system. J. Investig. Dermatol. Symp. Proc. 2000, 5, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Sever, A.; Broillet, A.; Schneider, M.; Cox, K.; Jones, S.; Weeks, J.; Mills, P.; Fish, D.; Jones, P. Dynamic visualization of lymphatic channels and sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasound in a swine model and patients with breast cancer. J. Ultrasound Med. 2010, 29, 1699–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimazu, K.; Ito, T.; Uji, K.; Miyake, T.; Aono, T.; Motomura, K.; Naoi, Y.; Shimomura, A.; Shimoda, M.; Kagara, N.; et al. Identification of sentinel lymph nodes by contrast-enhanced ultrasonography with sonazoid in patients with breast cancer: a feasibility study in three hospitals. Cancer Med. 2017, 6, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; He, J.; Gou, B.; Luo, Y.; Deng, S.; Wen, H.; Zhou, L. Percutaneous contrast-enhanced ultrasound for localization and diagnosis of sentinel lymph node in early breast cancer. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, J.; Zhu, Q.L.; Jiang, Y.X.; Sun, Q.; Zhou, Y.D.; Wang, M.Q.; Meng, Z.L.; Mao, X.X. The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: a prospective study. Eur. Radiol. 2018, 28, 1654–1661. [Google Scholar] [CrossRef]
- Mori, N.; Mugikura, S.; Miyashita, M.; Kudo, Y.; Suzuki, M.; Li, L.; Mori, Y.; Takahashi, S.; Takase, K. Perfusion contrast-enhanced ultrasound to predict early lymph-node metastasis in breast cancer. Jpn. J. Radiol. 2019, 37, 145–153. [Google Scholar] [CrossRef]
- Ouyang, Q.; Chen, L.; Zhao, H.; Xu, R.; Lin, Q. Detecting metastasis of lymph nodes and predicting aggressiveness in patients with breast carcinomas. J. Ultrasound Med. 2010, 29, 343–352. [Google Scholar] [CrossRef]
- Zenk, J.; Bozzato, A.; Hornung, J.; Gottwald, F.; Rabe, C.; Gill, S.; Iro, H. Neck lymph nodes: prediction by computer-assisted contrast medium analysis? Ultrasound Med. Biol. 2007, 33, 246–253. [Google Scholar] [CrossRef]
- Yang, H.K.; Burns, P.N.; Jang, H.J.; Kono, Y.; Khalili, K.; Wilson, S.R.; Kim, T.K. Contrast-enhanced ultrasound approach to the diagnosis of focal liver lesions: the importance of washout. Ultrasonography 2019, 38, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Piscaglia, F.; Nolsøe, C.; Dietrich, C.F.; Cosgrove, D.O.; Gilja, O.H.; Bachmann Nielsen, M.; Albrecht, T.; Barozzi, L.; Bertolotto, M.; Catalano, O.; et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall der Medizin 2012, 33, 33–59. [Google Scholar] [CrossRef] [Green Version]
- Badea, R.; Ciobanu, L. Contrast enhanced and doppler ultrasonography in the characterization of the microcirculation. expectancies and performances. Med. Ultrason. 2012, 14, 307–317. [Google Scholar]
- Choi, M.; Yoon, J.; Choi, M. Semi-quantitative strain elastography may facilitate pre-surgical prediction of mandibular lymph nodes malignancy in dogs. J. Vet. Sci. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.S.; Cho, C.C.; Yuen, Y.H.; Rasalkar, D.D.; King, A.D.; Ahuja, A.T. Real-time qualitative ultrasound elastography of cervical lymph nodes in routine clinical practice: Interobserver agreement and correlation with malignancy. Ultrasound Med. Biol. 2010, 36, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.S.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.C.; Liao, L.J. Comparison of two elasticity scoring systems in the assessment of the cervical lymph nodes. J. Med. Ultrasound 2014, 22, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.L.; Xia, X.N.; Zhang, Y.; He, J.J.; Sheng, W.; Ruan, L.T.; Yin, Y.M.; Hou, H.L. Elastosonography and two-dimensional ultrasonography in diagnosis of axillary lymph node metastasis in breast cancer. Clin. Radiol. 2018, 73, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojcinski, S.; Dupont, J.; Schmidt, W.; Cassel, M.; Hillemanns, P. Real-time ultrasound elastography in 180 axillary lymph nodes: Elasticity distribution in healthy lymph nodes and prediction of breast cancer metastases. BMC Med. Imaging 2012, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Lenghel, L.M.; Bolboacǎ, S.D.; Botar-Jid, C.; Bǎciut, G.; Dudea, S.M. The value of a new score for sonoelastographic differentiation between benign and malignant cervical lymph nodes. Med. Ultrason. 2012, 14, 271–277. [Google Scholar]
- Bhatia, K.S.S.; Lee, Y.Y.P.; Yuen, E.H.Y.; Ahuja, A.T. Ultrasound elastography in the head and neck. part ii. accuracy for malignancy. Cancer Imaging 2013, 13, 260–276. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, K.S.S.; Lee, Y.Y.P.; Yuen, E.H.Y.; Ahuja, A.T. Ultrasound elastography in the head and neck. part, i. basic principles and practical aspects. Cancer Imaging 2013, 13, 253–259. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Barr, R.G.; Farrokh, A.; Dighe, M.; Hocke, M.; Jenssen, C.; Dong, Y.; Saftoiu, A.; Havre, R.F. 1 introduction to elastography. Elastography 2016. [Google Scholar] [CrossRef]
- Jung, J.W.; Je, H.; Lee, S.K.; Jang, Y.; Choi, J. Two-Dimensional shear wave elastography of normal soft tissue organs in adult beagle dogs; interobserver agreement and sources of variability. Front. Bioeng. Biotechnol. 2020, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, W.; Zhao, Y.; Xia, T.; Zha, X.; Ding, Q.; Liu, X.; Zhao, Y.; Ling, L.; Chen, L.; et al. A novel finding of sentinel lymphatic channels in early stage breast cancer patients: which may influence detection rate and false-negative rate of sentinel lymph node biopsy. PLoS ONE 2012, 7, e51226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhou, W.; Li, C.; Gong, H.; Li, C.; Yang, N.; Zha, X.; Chen, L.; Xia, T.; Liu, X.; et al. variation of sentinel lymphatic channels (SLCs) and sentinel lymph nodes (SLNs) assessed by contrast-enhanced ultrasound (CEUS) in breast cancer patients. World J. Surg. Oncol. 2017, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, H.; Wang, S.; Cao, Y.; Liu, M.; Xie, F.; Liu, P.; Zhou, B.; Tong, F.; Cheng, L.; et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: A prospective cohort study. World J. Surg. Oncol. 2017, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Joseph, F.J.; Van Oepen, A.; Friebe, M. Breast sentinel lymph node biopsy with imaging towards minimally invasive surgery. Biomed. Tech. 2017, 62, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, N.; Koyanagi, A.; Yamamoto, H.; Shimura, K.; Fujii, R. Comparison of the indocyanine green dye method versus the combined method of indigo carmine blue dye with indocyanine green fluorescence imaging for sentinel lymph node biopsy in patients with stage i or ii breast cancer. Ann. Oncol. 2014, 25, i3. [Google Scholar] [CrossRef] [Green Version]
- Siddique, M.; Nawaz, M.K.; Bashir, H. The usefulness of SPECT/CT in sentinel node mapping of early stage breast cancer patients showing negative or equivocal findings on planar scintigraphy. Asia Ocean. J. Nucl. Med. Biol. 2018, 6, 80–89. [Google Scholar] [CrossRef]
- Taumberger, N.; Pernthaler, B.; Schwarz, T.; Bjelic-Radisic, V.; Pristauz, G.; Aigner, R.M.; Tamussino, K. lymphoscintigraphy for sentinel lymph node biopsy in breast cancer: do we need a delayed image? Breast Care 2020, 15, 55–59. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Lymphatic imaging: Focus on imaging probes. Theranostics 2015, 5, 686–697. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Purushotham, A.D.; Horgan, K.; Klaase, J.M.; Douek, M. Meta-Analysis of superficial versus deep injection of radioactive tracer and blue dye for lymphatic mapping and detection of sentinel lymph nodes in breast cancer. Br. J. Surg. 2015, 102. [Google Scholar] [CrossRef]
- Zada, A.; Peek, M.C.L.; Ahmed, M.; Anninga, B.; Baker, R.; Kusakabe, M.; Sekino, M.; Klaase, J.M.; Haken, B.; Douek, M. Meta-analysis of sentinel lymph node biopsy in breast cancer using the magnetic technique. Br. J. Surg. 2016. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, A.; Michael, P.; Lone, C. The nordic sentimag trial: A comparison of super paramagnetic iron oxide (spio) nanoparticles versus tc 99 and patent blue in the detection of sentinel node (sn) in patients with breast cancer and a meta-analysis of earlier studies. Breast cancer Res. Treat. 2016, 281–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martonos, C.; Gudea, A.; Dezdrobitu, C.; Damian, A.; Crisan, M.; Bud, I.; Pentea, M. Morphological description of sentinel lymphatic channels in canine mammary tumours. In Proceedings of the Multidisciplinary Conference on Sustainable Development, Timisoara, Romania, 23–24 May 2019; Filodiritto Editore: Bologna, Italy, 2019; pp. 637–643, ISBN 978-88-85813-60-1. [Google Scholar]
- Zhao, Y.C.; Ni, X.J.; Li, Y.; Dai, M.; Yuan, Z.X.; Zhu, Y.Y.; Luo, C.Y. Peritumoral lymphangiogenesis induced by vascular endothelial growth factor c and d promotes lymph node metastasis in breast cancer patients. World, J. Surg. Oncol. 2012, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widodo, I.; Dwianingsih, E.K.; Utoro, T.; Anwar, S.L.; Aryandono, T. Prognostic value of lymphangiogenesis determinants in luminal and non-luminal breast carcinomas. Asian Pacific J. Cancer Prev. 2018, 19, 2461–2467. [Google Scholar] [CrossRef]




| Elasticity Score | Description | Interpretation |
|---|---|---|
| 1 | Total green or yellow, absent blue areas or very small blue area/s | Soft |
| 2 | Small scattered blue areas or total blue area <45% | Moderately soft |
| 3 | Large blue area/s, total blue area ≥45% | Moderately stiff |
| 4 | Peripheral large hard area and central small green areas suggesting central necrosis | Predominantly stiff |
| 5 | Hard area occupying the entire lymph node, with or without green rim | Stiff |
| Type of Neoplasms | Number of Neoplasms |
|---|---|
| Carcinoma in a mixed tumor | 25 |
| Carcinoma—simple tubular | 19 |
| Carcinoma—simple tubulopapillary | 17 |
| Carcinoma—solid | 5 |
| Carcinoma—complex type | 3 |
| Carcinoma—anaplastic | 2 |
| Total subjects | 71 |
| Ultrasound Parameter n (%) | Unaffected Nodes (N = 42) | Metastatic/Affected Nodes (N = 54) | p Value |
|---|---|---|---|
| B-MODE ULTRASONOGRAPHY OF SENTINEL LYMPH NODES | |||
| SA/LA ratio < 0.55 | 33 (78.57) | 9(16.66) | <0.001 |
| Short axis (mean ± SD) cm | 0.67 ± 0.26 | 1.17 ± 0.53 | |
| Long axis (mean ± SD) cm | 1.33 ± 0.46 | 1.83 ± 0.79 | |
| Internal structure (Echostructure) | |||
| Homogeneous | 36(85.71) | 10(18.51) | <0.001 |
| Inhomogeneous | 6(14.28) | 44(81.48) | |
| Echogenicity | |||
| Hypoechoic | 28(66.66) | 37(68.51) | =0.81 |
| Isoechoic | 10(23.80) | 14(25.92) | |
| Hyperechoic | 4(9.52) | 3(5.55) | |
| Hillum tissue definition | |||
| Present | 30(71.48) | 29(53.70) | =0.07 |
| Absent/invisible | 12(28.57) | 25(46.29) | |
| Capsule (Borders) | |||
| Well-defined | 37(88.09) | 39(72.22) | 0.057 |
| Ill-defined | 5 (11.90) | 15(27.77) | |
| COLOR DOPPLER ULTRASONOGRAPHY—VASCULAR PATTERN | |||
| Localization | |||
| Absence of vascular signal | 2(4.76) | 1(1.85) | <0.001 |
| Hilar pattern | 36(85.71) | 12(22.22) | |
| Peripheral pattern | 1(2.38) | 30(55.55) | |
| Mixed | 3(7.14) | 11(20.37) | |
| Type and distribution | |||
| Ordered | 36(85.71) | 5(9.25) | <0.001 |
| Chaotic | 4(9.52) | 48(88.88) | |
| NA | 2(4.76) | 1(1.85) | |
| Intranodal vascular resistance (expressed by mean ± SD) | |||
| RI | 0.5060 ± 0.147 | 0.7011 ± 0.138 | <0.001 |
| PI | 0.9828 ± 0.223 | 1.2426 ± 0.259 | |
| CONTRAST-ENHANCED ULTRASONOGRAPHY (CEUS) | |||
| Enhancement patterns | |||
| Intense homogeneouss | 32(76.19) | 6(11.11) | <0.001 |
| Inhomogeneous | 8(19.04) | 44(81.48) | |
| No enhancement | 2(4.76) | 4(7.40) | |
| Perfusion times (expressed by mean ± SD) | |||
| Wash in time/s | 18.12 ± 7.15 | 15.34 ± 7.23 | =0.07 |
| Wash out time/s | 151.48 ± 34.62 | 104.26 ± 39.67 | <0.001 |
| REAL-TIME ELASTOGRAPHY—LYMPH NODE STIFFNESS ASSESSED BY ELASTICITY STIFFNESS SCORES | |||
| Soft (scores 1 and 2) | 38(90.47) | 6(11.11) | <0.001 |
| Hard (scores 3, 4 and 5) | 6(14.28) | 48(88.88) | |
| Lymph Nodes | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Unaffected (N = 42) | 25 (59.5) | 13 (31.0) | 3 (7.1) | - | 1 (2.4) |
| Metastatic (N = 54) | 2 (3.7) | 4 (7.4) | 14 (25.9) | 19 (35.2) | 15 (27.8) |
| p value | <0.001 | ||||
| Criterion | AUC | SE (%) | SP (%) | Statistical Sig. | Cutoff Value | CI 95% | PPV (%) | NPV (%) | Acc (%) |
|---|---|---|---|---|---|---|---|---|---|
| S/L Ratio | 0.812 | 83.3 | 78.6 | Good | 0.550 | 0.724–0.899 | 83.33 | 78.57 | 81.25 |
| L axis | 0.689 | 50.0 | 92.9 | Poor | 1.860 | 0.583–0.794 | 90.00 | 59.09 | 68.75 |
| S axis | 0.798 | 64.8 | 85.7 | Fair | 0.854 | 0.711–0.885 | 85.37 | 65.45 | 73.96 |
| RI | 0.837 | 83.0 | 75.0 | Good | 0.565 | 0.753–0.921 | 81.02 | 77.45 | 80.00 |
| PI | 0.798 | 83.0 | 65.0 | Fair | 1.025 | 0.705–0.891 | 75.31 | 74.86 | 75.14 |
| WOT (s) | 0.818 | 84.0 | 74.4 | Good | 133.0 | 0.727–0.910 | 80.81 | 78.33 | 79.78 |
| ES | 0.928 | 88.9 | 90.5 | Excellent | 2.5 | 0.871–0.986 | 89.69 | 86.36 | 89.71 |
| US Parameter | Pattern | Score | |
|---|---|---|---|
| Gray scale ultrasound | |||
| 1. | S/L ratio | <0.55 | 0 |
| ≥0.55 | 1 | ||
| 2. | Echostructure | Homogeneous | 0 |
| Inhomogeneous | 1 | ||
| Doppler ultrasound | |||
| 3. | Localization | Hilar | 0 |
| Peripheral | 1 | ||
| 4. | Type and distribution | Ordered | 0 |
| Chaotic | 1 | ||
| 5. | RI | <0.54 | 0 |
| ≥0.54 | 1 | ||
| 6. | PI | <0.83 | 0 |
| ≥0.83 | 1 | ||
| Score < 3—unaffected lymph node; Score = 3 CEUS and elastography should be performed; Score ≥ 3 metastatic lymph node should be taken into account | |||
| CEUS | |||
| 1. | Enhancement pattern | Intense, homogeneous | 0 |
| Inhomogeneous ± no enhanced areas | 1 | ||
| 2. | Wash-out time (s) peritumoral ad. | >133 | 0 |
| ≤133 | 1 | ||
| Strain elastography | |||
| 3. | Stiffness | Soft or moderately soft (scores 1 and 2) | 0 |
| Predominantly/moderately stiff or stiff (scores 3,4,5) | 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stan, F.; Gudea, A.; Damian, A.; Gal, A.F.; Papuc, I.; Pop, A.R.; Martonos, C. Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs. Animals 2020, 10, 2366. https://doi.org/10.3390/ani10122366
Stan F, Gudea A, Damian A, Gal AF, Papuc I, Pop AR, Martonos C. Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs. Animals. 2020; 10(12):2366. https://doi.org/10.3390/ani10122366
Chicago/Turabian StyleStan, Florin, Alexandru Gudea, Aurel Damian, Adrian Florin Gal, Ionel Papuc, Alexandru Raul Pop, and Cristian Martonos. 2020. "Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs" Animals 10, no. 12: 2366. https://doi.org/10.3390/ani10122366
APA StyleStan, F., Gudea, A., Damian, A., Gal, A. F., Papuc, I., Pop, A. R., & Martonos, C. (2020). Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs. Animals, 10(12), 2366. https://doi.org/10.3390/ani10122366





