Relationship of Milk Odd- and Branched-Chain Fatty Acids with Urine Parameters and Ruminal Microbial Protein Synthesis in Dairy Cows Fed Different Proportions of Maize Silage and Red Clover Silage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sampling and Chemical Analyses
2.3. Calculations and Statistical Analysis
3. Results
3.1. Estimated Microbial Crude Protein Synthesis
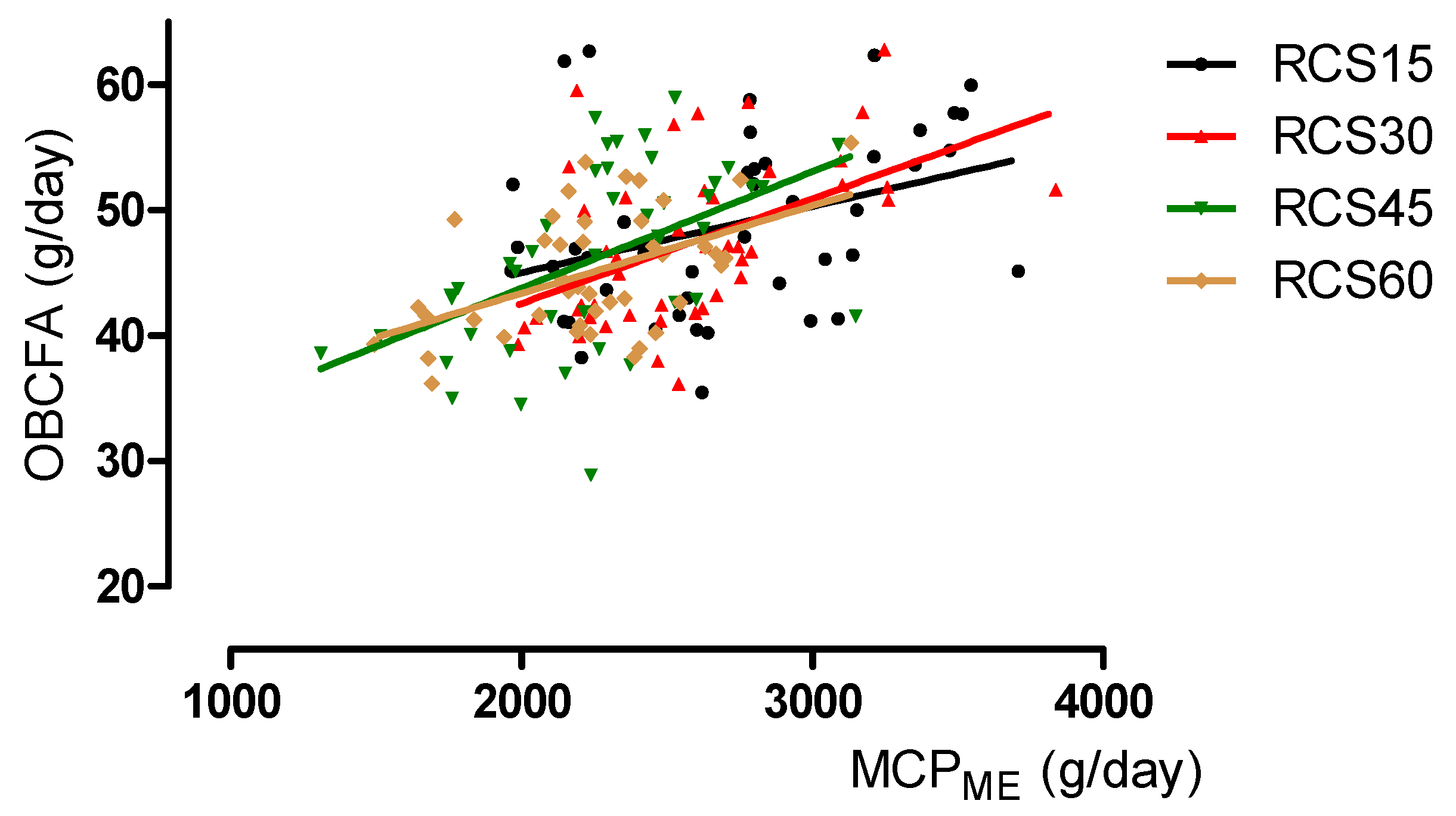
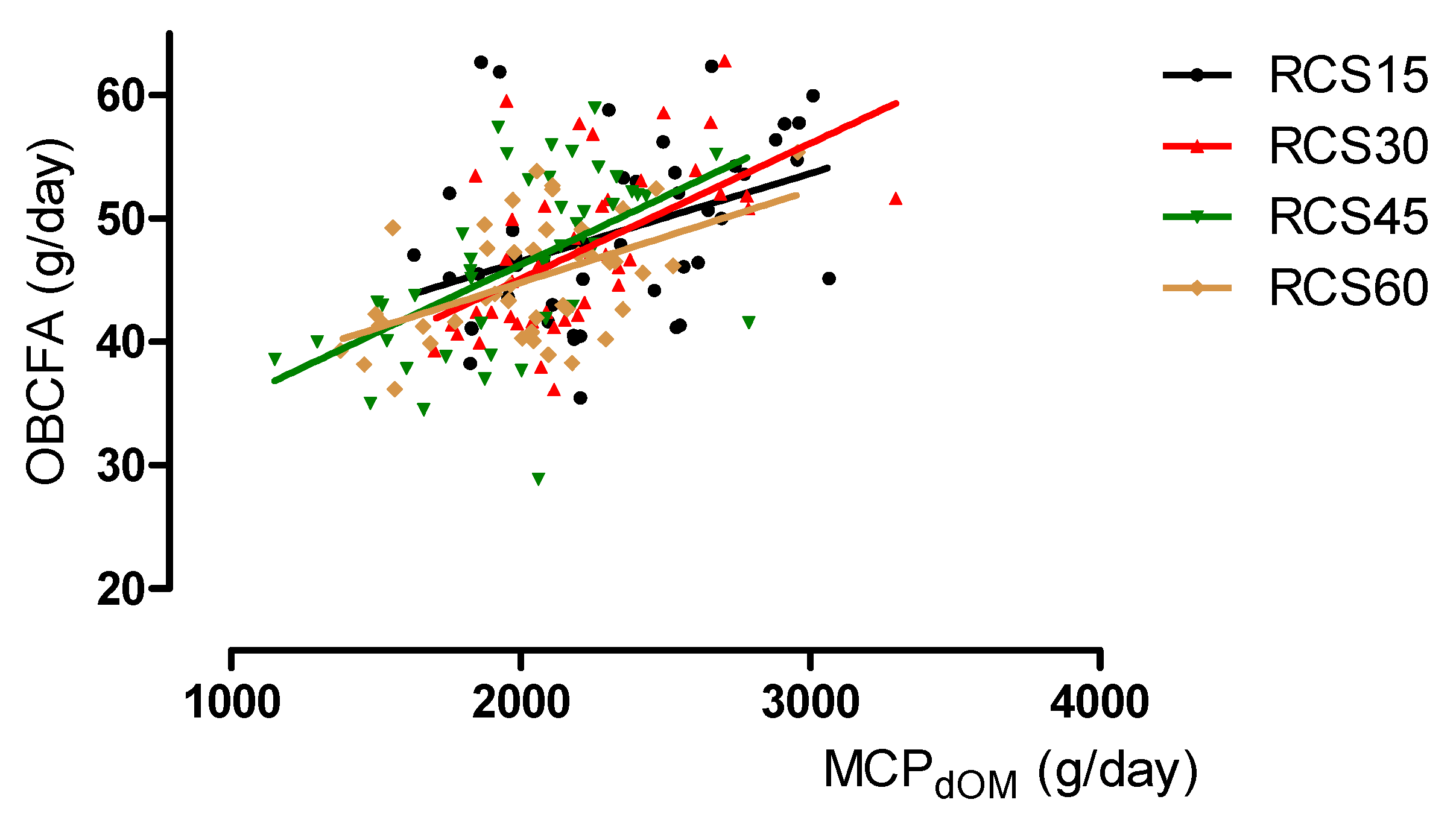
3.2. Relationship Between OBCFA and Estimated Microbial Crude Protein Synthesis
4. Discussion
4.1. Estimated Microbial Crude Protein Synthesis
4.2. Relationship of OBCFA with Urinary Purine Derivates and Estimated Microbial Crude Protein Synthesis
4.3. Limitations of the Present Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Storm, E.; Ørskov, E.R.; Smart, R. The nutritive value of rumen micro-organism in ruminants. 2. The apparent digestibility and net utilization of microbial N for growing lambs. Br. J. Nutr. 1983, 50, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Südekum, K.-H.; Brüsemeister, F.; Schröder, A.; Stangassinger, M. Effects of amount of intake and stage of forage maturity on urinary allantoin excretion and estimated microbial crude protein synthesis in the rumen of steers. J. Anim. Physiol. Anim. Nutr. 2006, 90, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Tas, B.M.; Susenbeth, A. Urinary purine derivates excretion as an indicator of in vivo microbial N flow in cattle: A review. Livest. Sci. 2007, 111, 181–192. [Google Scholar] [CrossRef]
- Ahnert, S.; Dickhoefer, U.; Schulz, F.; Susenbeth, A. Influence of ruminal quebracho tannin extract infusion on apparent nutrient digestibility, nitrogen balance, and urinary purine derivatives excretion in heifers. Livest. Sci. 2015, 177, 63–70. [Google Scholar] [CrossRef]
- Henke, A.; Dickhoefer, U.; Westreicher-Kristen, E.; Knappstein, K.; Molkentin, J.; Hasler, M.; Susenbeth, A. Effects of quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in lactating dairy cows. Arch. Anim. Nutr. 2017, 71, 37–53. [Google Scholar] [CrossRef]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function-An update. Anim. Feed Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Davis, D.R.; Merry, R.J. Microbial protein supply from the rumen. Anim. Feed Sci. Technol. 2000, 85, 1–21. [Google Scholar] [CrossRef]
- Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J.; Gomes, E. Nitrogen supplementation of corn silages. 2. Assessing rumen function using fatty acid profiles of bovine milk. J. Dairy Sci. 2003, 86, 4020–4032. [Google Scholar] [CrossRef] [Green Version]
- Vlaeminck, B.; Dufour, C.; Van Vuuren, A.M.; Cabrita, A.R.J.; Dewhurst, R.J.; Demeyer, D.; Fievez, V. Use of odd and branched-chain fatty acids in rumen contents and milk as a potential microbial marker. J. Dairy Sci. 2005, 88, 1031–1042. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Li, Y.; Xin, H.; Zhang, Y.; Li, G. Relations of ruminal fermentation parameters and microbial matters to odd- and branched-chain fatty acids in rumen fluid of dairy cows at different milk stages. Animals. 2019, 9, 1019. [Google Scholar] [CrossRef] [Green Version]
- Dewhurst, R.J.; Moorby, J.M.; Vlaeminck, B.; Fievez, V. Apparent recovery of duodenal odd- and branched faty acids in milk dairy cows. J. Dairy Sci. 2007, 90, 1775–1780. [Google Scholar] [CrossRef] [Green Version]
- Castro-Montoya, J.; Henke, A.; Molkentin, J.; Knappstein, K.; Susenbeth, A.; Dickhoefer, U. Relationship between milk odd and branched-chain fatty acids and urinary purine derivatives in dairy cows supplemented with quebracho tannins—A study to test milk fatty acids as predictors of rumen microbial protein synthesis. Anim. Feed Sci. Technol. 2016, 214, 22–33. [Google Scholar] [CrossRef]
- Broderick, G.A.; Walgenbach, R.P.; Maignan, S. Production of lactating dairy cows fed alfalfa or red clover silage at equal dry matter or crude protein contents in the diet. J. Dairy Sci. 2001, 84, 1728–1737. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Davies, L.J.; Kim, E.J. Effects of mixtures of red clover and maize silages on the partitioning of dietary nitrogen between milk and urine by dairy cows. Animal 2010, 4, 732–738. [Google Scholar] [CrossRef] [Green Version]
- Moorby, J.M.; Ellis, N.M.; Davies, D.R. Assessment of dietary ratios of red clover and corn silages on milk production and milk quality in dairy cows. J. Dairy Sci. 2016, 99, 7982–7992. [Google Scholar] [CrossRef] [Green Version]
- Schulz, F.; Westreicher-Kristen, E.; Knappstein, K.; Molkentin, J.; Susenbeth, A. Replacing maize silage plus soybean meal with red clover silage plus wheat in diets for lactating dairy cows. J. Dairy Sci. 2018, 101, 1216–1226. [Google Scholar] [CrossRef]
- Federal Republic of Germany. Tierschutzgesetz. 2014. Available online: https://www.gesetze-im-internet.de/tierschg/BJNR012770972.html (accessed on 9 May 2019).
- Schulz, F.; Westreicher-Kristen, E.; Molkentin, J.; Knappstein, K.; Susenbeth, A. Effect of replacing maize silage with red clover silage in the diet on milk fatty acid composition in cows. J. Dairy Sci. 2018, 101, 7156–7167. [Google Scholar] [CrossRef] [Green Version]
- IDF (International Dairy Federation). Milk—Determination of Fat Content—Gravimetric Method (Reference Method). Standard 1 (ISO 1211), 3rd ed.; International Dairy Federation: Brussels, Belgium, 2010. [Google Scholar]
- IDF (International Dairy Federation). Milk Fat—Preparation of Fatty Acid Methyl Esters. Standard 182 (ISO 15884), 1st ed.; International Dairy Federation: Brussels, Belgium, 2002. [Google Scholar]
- IDF (International Dairy Federation). Milk Fat—Determination of the Fatty Acid Composition by Gas-Liquid Chromatography. Standard 184 (ISO 15885), 1st ed.; International Dairy Federation: Brussels, Belgium, 2002. [Google Scholar]
- Molkentin, J.; Giesemann, A. Differentiation of originally and conventionally produced milk by stable isotope and fatty acid analysis. Anal. Bioanal. Chem. 2007, 388, 297–305. [Google Scholar] [CrossRef]
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten). Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA Methodenhandbuch), Bd. III. Die chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2007. [Google Scholar]
- Chen, X.B.; Ørskov, E.R. Research on Urinary Excretion of Purine Derivates in Ruminants: Past, present and Future; International Feed Resources Unit, Macaulay Land Use Research Institute: Craigiebuckler, Aberdeen, UK, 2003. [Google Scholar]
- Westreicher-Kristen, E.; Blank, R.; Schulz, F.; Susenbeth, A. Replacing maize silage with red clover silage in total mixed rations for dairy cows: In vitro ruminal fermentation characteristics and associative effects. Anim. Feed Sci. Technol. 2017, 227, 131–141. [Google Scholar] [CrossRef]
- GfE (Gesellschaft für Ernährungsphysiologie). Empfehlungen zur Energie- und Nährstoffversorgung der Milchkühe und Aufzuchtrinder; DLG-Verlag: Frankfurt am Main, Germany, 2001. [Google Scholar]
- AFRC (Agricultural and Food Research Council). Energy and protein requirements of ruminants. In An Advisory Manual Prepared by the AFRC Technical Committee on Response to Nutrients; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Castro-Montoya, J.; Witzig, M.; Rahman, M.; Westreicher-Kristen, E.; Dickhoefer, U. Replacing maize silage with red clover silage in total mixed rations of dairy cows: Ruminal fermentation, microbial protein synthesis and composition of microbial community in vitro. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1450–1463. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Hao, X.; Xin, H. The relationships between odd- and branched-chain fatty acids to ruminal fermentation parameters and bacterial populations with different dietary ratios of forage and concentrate. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1103–1114. [Google Scholar] [CrossRef]
- Shingfield, K.J. Estimationof microbial protein supply in ruminant animals based on renal and mammary purine metabolite excretion. A review. J. Anim. Feed Sci. 2000, 9, 169–212. [Google Scholar] [CrossRef] [Green Version]
- Firkins, J.L.; Hristov, A.N.; Hall, M.B.; Varga, G.A.; St-Pierre, N.R. Integration of ruminal metabolism in dairy cattle. J. Dairy Sci. 2006, 89, E31–E51. [Google Scholar] [CrossRef]
- Clark, J.H.; Klusmeyer, T.H.; Cameron, M.R. Microbial protein synthesis and flows of nitrogen fractions and amino acid nutrition in dairy cattle. J. Dairy Sci. 1992, 75, 2304–2323. [Google Scholar] [CrossRef]
- Dickhoefer, U.; Ahnert, S.; Susenbeth, A. Effects of quebracho tannin extract on rumen fermentation and yield and composition of microbial mass in heifers. J. Anim. Sci. 2016, 94, 1561–1575. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Hao, X.; Li, Y.; Luo, G.; Zhang, Y.; Xin, H. The relantionship between odd- and branched-chain fatty acids and microbial nucleic acid bases in rumen. Asian-Australas J. Anim. Sci. 2017, 30, 1590–1597. [Google Scholar] [CrossRef] [Green Version]
- Croom, W.J.; Bauman, D.E.; Davis, C.L. Methylmalonic acid in low-fat milk syndrome. J. Dairy Sci. 1981, 64, 649–654. [Google Scholar] [CrossRef]
- Massart-Leën, A.M.; Roets, E.; Peeters, G.; Verbeke, R. Propionate for fatty acid synthesis by the mammary gland of the lactating goat. J. Dairy Sci. 1983, 66, 1445–1454. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006, 172, 51–65. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Gervais, R.; Rahman, M.M.; Gadeyne, F.; Gorniak, M.; Doreau, M.; Fievez, V. Postruminal synthesis modifies the odd- and branched-chain fatty acid profile from the duodenum to milk. J. Dairy Sci. 2015, 98, 4829–4840. [Google Scholar] [CrossRef]
- Westreicher-Kristen, E.; Südekum, K.-H. Replacing maize silage plus soybean meal with red clover silage plus wheat grain in diets of dairy cows: Modelling the utilizable crude protein at the duodenum, a precursor to metabolizable protein. Anim. Feed Sci. Technol. 2018, 246, 29–35. [Google Scholar] [CrossRef]
| Item | TMR 1 | |||
|---|---|---|---|---|
| RCS15 | RCS30 | RCS45 | RCS60 | |
| Ingredients (g/kg DM) | ||||
| Maize silage | 610 | 466 | 316 | 162 |
| Red clover silage | 136 | 275 | 421 | 571 |
| Soybean meal | 159 | 108 | 55.0 | - |
| Wheat | - | 54.9 | 110 | 168 |
| Lupine seed | 85.9 | 87.0 | 88.8 | 89.6 |
| Premix 2 | 9.1 | 9.1 | 9.2 | 9.4 |
| Chemical composition (g/kg DM) | ||||
| Crude ash | 60.5 | 70.7 | 84.2 | 93.2 |
| Organic matter 3 | 940 | 929 | 916 | 907 |
| Crude protein | 172 | 174 | 173 | 175 |
| Ether extract | 21.4 | 21.3 | 20.5 | 20.2 |
| Sugar | 56.2 | 29.3 | 25.1 | 28.4 |
| Starch | 232 | 205 | 180 | 170 |
| Neutral detergent fibre (aNDFom) 4 | 340 | 332 | 342 | 341 |
| Acid detergent fibre (ADFom) 5 | 212 | 219 | 239 | 244 |
| Lignin (sa) | 28.0 | 32.4 | 36.9 | 41.0 |
| Item | Min. | Max. | Mean | SD 1 | CV 2 (%) |
|---|---|---|---|---|---|
| OBCFA 3 proportions (g/100g fatty acid) | |||||
| Iso-C14:0 | 0.050 | 0.130 | 0.084 | 0.016 | 19.4 |
| Iso-C15:0 | 0.140 | 0.270 | 0.221 | 0.022 | 10.0 |
| Anteiso-C15:0 | 0.260 | 0.540 | 0.437 | 0.041 | 9.46 |
| C15:0 | 0.730 | 1.330 | 1.033 | 0.115 | 11.1 |
| Iso-C16:0 | 0.140 | 0.320 | 0.226 | 0.036 | 16.1 |
| Iso-C17:0 | 0.220 | 0.370 | 0.295 | 0.031 | 10.4 |
| Anteiso-C17:0 | 0.340 | 0.560 | 0.437 | 0.046 | 10.6 |
| C17:0 | 0.440 | 0.750 | 0.564 | 0.066 | 11.7 |
| cis-9 C17:1 | 0.140 | 0.410 | 0.216 | 0.049 | 22.7 |
| Iso-C18:0 | 0.010 | 0.110 | 0.058 | 0.016 | 28.3 |
| OBCFA yields (g/d) | |||||
| Iso-C14:0 | 0.604 | 1.783 | 1.098 | 0.254 | 23.1 |
| Iso-C15:0 | 1.358 | 4.412 | 2.895 | 0.526 | 18.2 |
| Anteiso-C15:0 | 2.414 | 9.017 | 5.736 | 1.027 | 17.9 |
| C15:0 | 5.884 | 19.09 | 13.51 | 2.252 | 16.7 |
| Iso-C16:0 | 1.559 | 5.091 | 2.966 | 0.659 | 22.2 |
| Iso-C17:0 | 1.710 | 5.770 | 3.862 | 0.685 | 17.7 |
| Anteiso-C17:0 | 2.213 | 9.711 | 5.739 | 1.081 | 18.8 |
| C17:0 | 3.169 | 10.63 | 7.364 | 1.185 | 16.1 |
| cis-9 C17:1 | 1.157 | 6.050 | 2.815 | 0.686 | 24.4 |
| Iso-C18:0 | 0.119 | 1.650 | 0.755 | 0.217 | 28.8 |
| Urine parameters | |||||
| Nitrogen (g/L) | 5.093 | 13.43 | 8.978 | 1.593 | 17.7 |
| Uric acid (mmol/L) | 0.313 | 0.804 | 0.458 | 0.80 | 17.4 |
| Allantoin (mmol/L) | 7.450 | 19.30 | 13.75 | 2.389 | 17.4 |
| Total purine derivates (mmol/L) | 8.010 | 19.70 | 14.21 | 2.380 | 16.7 |
| Nitrogen (g/d) | 61.70 | 424.7 | 220.3 | 64.00 | 29.1 |
| Uric acid (mmol/d) | 3.039 | 24.31 | 11.45 | 3.967 | 34.7 |
| Allantoin (mmol/d) | 102.3 | 677.7 | 337.8 | 99.22 | 29.4 |
| Total purine derivates (mmol/d) | 105.4 | 699.3 | 349.2 | 101.8 | 29.2 |
| MCP 4 synthesis (g/d) | |||||
| MCPPD | 1205 | 3448 | 1812 | 459 | 25.3 |
| MCPME | 1309 | 3837 | 2450 | 456 | 18.6 |
| MCPdOM | 1151 | 3296 | 2137 | 372 | 17.4 |
| MCP 1 Synthesis (g/d) | TMR 2 | p-Value 3 | ||||
|---|---|---|---|---|---|---|
| RCS15 | RCS30 | RCS45 | RCS60 | Linear | Quadratic | |
| MCPPD | 1998 a ± 437 | 1840 b ± 544 | 1541 c ± 319 | 1707 b,c ± 381 | <0.001 | <0.001 |
| MCPME | 2729 a ± 486 | 2580 b ± 393 | 2266 c ± 392 | 2224 c ± 342 | <0.001 | 0.01 |
| MCPdOM | 2328 a ± 397 | 2208 b ± 324 | 1999 c ± 345 | 2015 c ± 324 | <0.001 | 0.007 |
| OBCFA 2 Proportions | MCP Synthesis 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitrogen (g/L) | Uric Acid (mmol/L) | Allantoin (mmol/L) | Total PD 3 (mmol/L) | Nitrogen (g/d) | Uric Acid (mmol/d) | Allantoin (mmol/d) | Total PD (mmol/d) | MCPPD | MCPME | MCPdOM | |
| Iso-C14:0 | −0.18 | −0.21 | −0.21 | ||||||||
| Iso-C15:0 | 0.23 | −0.26 | 0.22 | 0.21 | 0.19 | ||||||
| Anteiso-C15:0 | 0.40 | −0.37 | 0.36 | 0.35 | −0.30 | ||||||
| C15:0 | −0.18 | 0.26 | 0.25 | 0.18 | |||||||
| Iso-C16:0 | |||||||||||
| Iso-C17:0 | 0.28 | −0.29 | 0.36 | 0.35 | −0.23 | ||||||
| Anteiso-C17:0 | 0.44 | −0.26 | 0.39 | 0.39 | −0.31 | ||||||
| C17:0 | −0.21 | ||||||||||
| cis-9 C17:1 | −0.34 | −0.20 | −0.23 | −0.22 | |||||||
| Iso-C18:0 | −0.35 | −0.29 | −0.29 | 0.19 | |||||||
| Iso-C15:0 + iso-C17:0 | 0.31 | −0.33 | 0.35 | 0.34 | −0.22 | ||||||
| C17:0 + cis-9 C17:1 | −0.19 | −0.27 | −0.25 | ||||||||
| Total iso-BCFA 5 | |||||||||||
| Total anteiso-BCFA | 0.41 | −0.31 | 0.42 | 0.41 | −0.31 | ||||||
| Total BCFA | 0.27 | −0.32 | 0.27 | 0.25 | −0.25 | ||||||
| Total OCFA 6 | |||||||||||
| Total OBCFA | −0.29 | 0.22 | 0.21 | −0.23 | |||||||
| OBCFA 2 Yields | MCP 4 Synthesis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitrogen (g/L) | Uric Acid (mmol/L) | Allantoin (mmol/L) | Total PD 3 (mmol/L) | Nitrogen (g/d) | Uric Acid (mmol/d) | Allantoin (mmol/d) | Total PD (mmol/d) | MCPPD | MCPME | MCPdOM | |
| Iso-C14:0 | 0.19 | 0.18 | 0.31 | 0.32 | |||||||
| Iso-C15:0 | 0.21 | 0.18 | 0.18 | 0.20 | 0.20 | 0.19 | 0.53 | 0.55 | |||
| Anteiso-C15:0 | 0.31 | 0.25 | 0.25 | 0.49 | 0.52 | ||||||
| C15:0 | 0.42 | 0.45 | |||||||||
| Iso-C16:0 | 0.19 | 0.29 | 0.29 | ||||||||
| Iso-C17:0 | 0.23 | 0.25 | 0.25 | 0.40 | 0.43 | ||||||
| Anteiso-C17:0 | 0.31 | 0.25 | 0.26 | 0.41 | 0.44 | ||||||
| C17:0 | 0.35 | 0.36 | |||||||||
| cis-9 C17:1 | −0.23 | 0.20 | 0.23 | 0.24 | |||||||
| Iso-C18:0 | −0.28 | 0.25 | −0.24 | −0.23 | 0.26 | 0.23 | 0.21 | ||||
| Iso-C15:0 + iso-C17:0 | 0.23 | 0.23 | 0.24 | 0.48 | 0.51 | ||||||
| C17:0 + cis-9 C17:1 | 0.32 | 0.33 | |||||||||
| Total iso-BCFA 5 | 0.21 | 0.43 | 0.44 | ||||||||
| Total anteiso-BCFA | 0.26 | 0.23 | 0.23 | 0.46 | 0.48 | ||||||
| Total BCFA | 0.47 | 0.49 | |||||||||
| Total OCFA 6 | 0.40 | 0.42 | |||||||||
| Total OBCFA | 0.45 | 0.48 | |||||||||
| Item | Equation | R2 | |
|---|---|---|---|
| Allantoin (mmol/L) | (1) | 6.15 *** + 25.5 × iso-C17:0 *** | 0.67 |
| (2) | 11.1 *** + 1.98 × iso-C17:0 *** − 1.36 × iso-C15:0 ** − 1.45 × iso-C18:0 * | 0.49 | |
| Total PD 2 (mmol/L) | (1) | 6.81 *** + 24.8 × iso-C17:0 *** | 0.64 |
| (2) | 11.5 *** + 1.98 × iso-C17:0 *** − 1.33 × iso-C15:0 ** − 1.53 × iso-C18:0 ** | 0.47 | |
| Allantoin (mmol/d) | (1) | 524 *** − 643 × anteiso-C17:0 *** − 554 × cis-9 C17:1 *** + 695 × iso-C17:0 * | 0.16 |
| (2) | 189 ** + 146 × iso-C15:0 *** − 32.1 × anteiso-C17:0 *** − 33.3 × iso-C16:0 * | 0.22 | |
| Total PD (mmol/d) | (1) | 552 *** − 676 × anteiso-C17:0 *** − 567 × cis-9 C17:1 *** + 693 × iso-C17:0 * | 0.15 |
| (2) | 197 ** + 151 × iso-C15:0 *** − 33.8 × anteiso-C17:0 *** − 33.5 × iso-C16:0 * | 0.21 | |
| MCPPD (g/d) | (1) | 1308 *** − 2525 × iso-C16:0 *** + 4973 × cis-9 C17:1 *** | 0.99 |
| (2) | No delivered equation | − | |
| MCPME (g/d) | (1) | 3890 *** − 3172 × cis-9 C17:1 *** − 1824 × anteiso-C17 *** | 0.24 |
| (2) | 1154 *** + 480 × iso-C15:0 *** − 123 × cis-9 C17:1 * + 310 × iso-C18:0 * | 0.37 | |
| MCPdOM (g/d) | (1) | 3358 *** − 2896 × cis-9 C17:1 *** − 1411 × anteiso-C17:0 ** | 0.21 |
| (2) | 1008 *** + 424 × iso-C15:0 *** − 110 × cis-9 C17:1 * + 269 × iso-C18:0 * | 0.36 | |
| Item | Equation | R2 | |
|---|---|---|---|
| Total PD (mmol/L) | (1) | 8.76 *** + 3.02 × BCFA * | 0.56 |
| (1) | 10.5 *** + 1.94 × OCFA † | 0.67 | |
| (1) | 6.70 ** + 2.06 × OBCFA ** | 0.65 | |
| (2) | 12.3 *** + 0.07 × BCFA † | 0.55 | |
| (2) | 12.6 *** + 0.06 × OCFA | 0.60 | |
| (2) | 12.3 *** + 0.04 × OBCFA | 0.57 | |
| Total PD (mmol/d) | (1) | 409 *** − 40.7 × BCFA | 0.12 |
| (1) | 276 * + 33.8 × OCFA | 0.11 | |
| (1) | 342 * – 1.23 × OBCFA | 0.11 | |
| (2) | 248 *** + 3.99 × BCFA † | 0.13 | |
| (2) | 244 *** + 3.97 × OCFA * | 0.13 | |
| (2) | 240 *** + 2.13 × OBCFA * | 0.13 | |
| MCPPD (g/d) | (1) | 1620 ** + 83.7 × BCFA | 0.49 |
| (1) | 2027 *** − 141 × OCFA | 0.51 | |
| (1) | 1824 ** – 14.9 × OBCFA | 0.52 | |
| (2) | 1487 *** + 12.4 × BCFA | 0.52 | |
| (2) | 1534 *** + 10.1 × OCFA | 0.56 | |
| (2) | 1496 *** + 5.94 × OBCFA | 0.54 | |
| MCPME (g/d) | (1) | 3265 *** − 493 × BCFA * | 0.15 |
| (1) | 3240 *** − 460 × OCFA † | 0.16 | |
| (1) | 3640 *** − 346 × OBCFA * | 0.16 | |
| (2) | 1140 *** + 56.0 × BCFA *** | 0.32 | |
| (2) | 1423 *** + 41.6 × OCFA *** | 0.22 | |
| (2) | 1199 *** + 26.1 × OBCFA *** | 0.27 | |
| MCPdOM (g/d) | (1) | 2730 *** − 355 × BCFA | 0.12 |
| (1) | 2746 *** − 351 × OCFA | 0.13 | |
| (1) | 3030 *** − 258 × OBCFA † | 0.13 | |
| (2) | 962 *** + 50.5 × BCFA *** | 0.34 | |
| (2) | 1207 *** + 38.4 × OCFA *** | 0.22 | |
| (2) | 1009 *** + 23.8 × OBCFA *** | 0.29 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westreicher-Kristen, E.; Castro-Montoya, J.; Hasler, M.; Susenbeth, A. Relationship of Milk Odd- and Branched-Chain Fatty Acids with Urine Parameters and Ruminal Microbial Protein Synthesis in Dairy Cows Fed Different Proportions of Maize Silage and Red Clover Silage. Animals 2020, 10, 316. https://doi.org/10.3390/ani10020316
Westreicher-Kristen E, Castro-Montoya J, Hasler M, Susenbeth A. Relationship of Milk Odd- and Branched-Chain Fatty Acids with Urine Parameters and Ruminal Microbial Protein Synthesis in Dairy Cows Fed Different Proportions of Maize Silage and Red Clover Silage. Animals. 2020; 10(2):316. https://doi.org/10.3390/ani10020316
Chicago/Turabian StyleWestreicher-Kristen, Edwin, Joaquín Castro-Montoya, Mario Hasler, and Andreas Susenbeth. 2020. "Relationship of Milk Odd- and Branched-Chain Fatty Acids with Urine Parameters and Ruminal Microbial Protein Synthesis in Dairy Cows Fed Different Proportions of Maize Silage and Red Clover Silage" Animals 10, no. 2: 316. https://doi.org/10.3390/ani10020316
APA StyleWestreicher-Kristen, E., Castro-Montoya, J., Hasler, M., & Susenbeth, A. (2020). Relationship of Milk Odd- and Branched-Chain Fatty Acids with Urine Parameters and Ruminal Microbial Protein Synthesis in Dairy Cows Fed Different Proportions of Maize Silage and Red Clover Silage. Animals, 10(2), 316. https://doi.org/10.3390/ani10020316






