Maternal and Early Postnatal Diet Supplemented with Conjugated Linoleic Acid Isomers Affect Lipid Profile in Hearts of Offspring Rats with Mammary Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
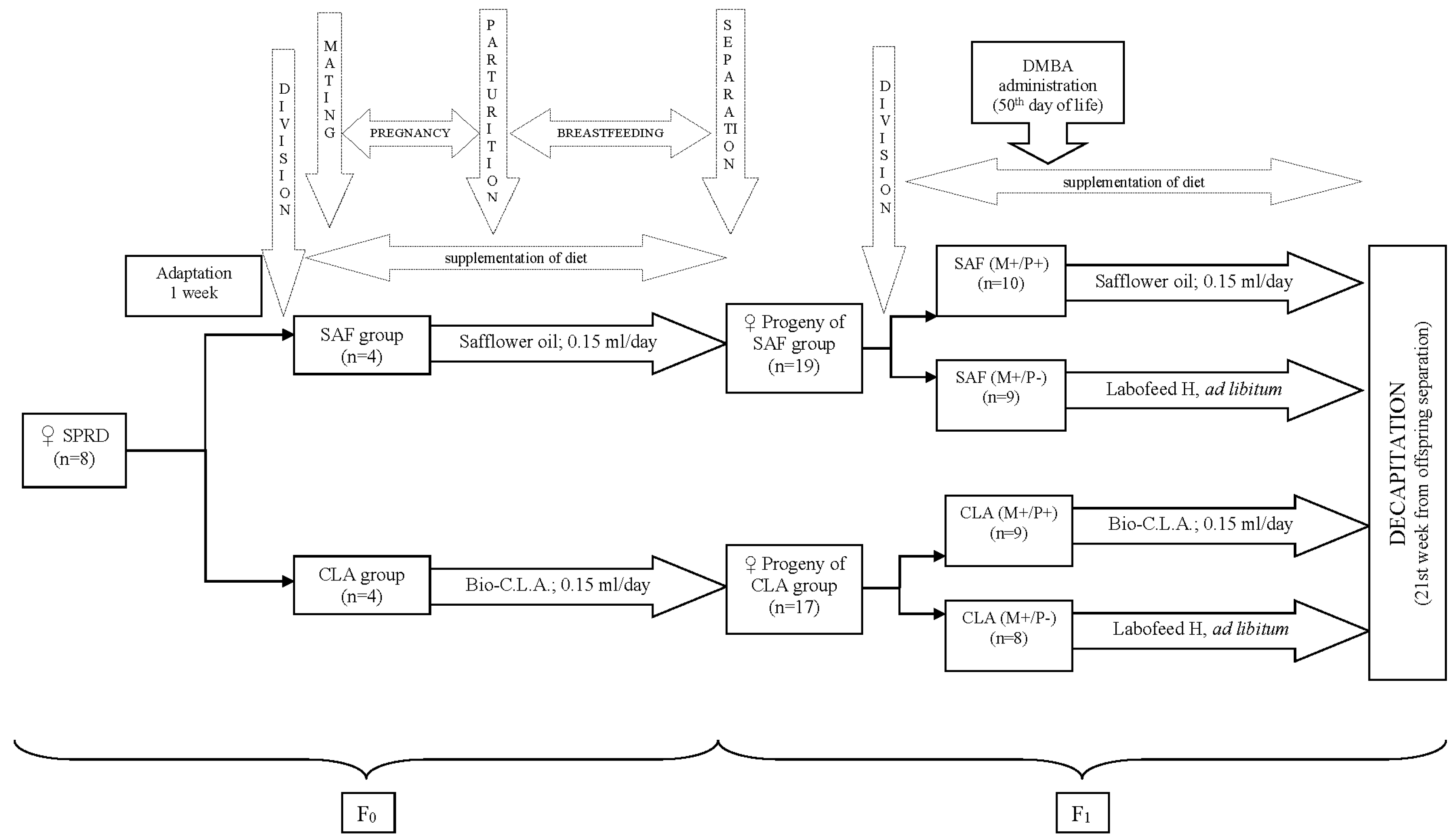
2.1.1. Dietary Ingredients
2.1.2. Animal Experiment
2.2. Methods
2.2.1. CFA and FA Profile
2.2.2. Total Cholesterol and Oxysterol Content
2.3. Malondialdehyde (MDA) Content
2.3.1. Tocopherol Content
2.3.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McAloon, C.J.; Osman, F.; Glennon, P.; Lim, P.B.; Hayat, S.A. Global epidemiology and incidence of cardiovascular disease. In Cardiovascular Diseases: Genetic Susceptibility, Environmental Factors and their Interaction; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 57–96. ISBN 9780128033135. [Google Scholar]
- World Health Organization—WHO. Available online: http://www.who.int/cardiovascular_diseases/en/ (accessed on 20 November 2018).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef] [PubMed]
- Blaes, A.; Prizment, A.; Koene, R.J.; Konety, S. Cardio-oncology Related to Heart Failure: Common Risk Factors Between Cancer and Cardiovascular Disease. Heart Fail. Clin. 2017, 13, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.M.; Anzevino, S.; Rao, R. Cardiovascular disease in cancer survivors. Postgrad. Med. J. 2017, 93, 82–90. [Google Scholar] [CrossRef]
- Bradshaw, P.T.; Stevens, J.; Khankari, N.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Cardiovascular Disease Mortality Among Breast Cancer Survivors. Epidemiology 2016, 27, 6–13. [Google Scholar] [CrossRef]
- Clark, R.A.; Marin, T.S.; Berry, N.M.; Atherton, J.J.; Foote, J.W.; Koczwara, B. Cardiotoxicity and cardiovascular disease risk assessment for patients receiving breast cancer treatment. Cardio-Oncology 2017, 3, 6. [Google Scholar] [CrossRef]
- Vo, J.B.; Nolan, T.S.; Vance, D.E. Cardiovascular disease risk among breast cancer survivors: An evolutionary concept analysis. Nurs. Res. Rev. 2017, 7, 9–16. [Google Scholar] [CrossRef]
- Venneri, L.; Caliccio, F.; Manivarmane, R.; Pareek, N.; Baksi, J.; Rosen, S.; Senior, R.; Lyon, A.; Khattar, R.S. Subclinical myocardial dysfunction in cancer patients: Is there a direct effect of tumour growth? In Proceedings of the European Heart Journal Cardiovasc Imaging Abstracts Supplement (2015) 16 (Supplement 2), Euro Echo 2015 Conference, Seville, Spain, 3 December 2015; p. ii127. [Google Scholar]
- Singleterry, J. The Costs of Cancer Therapy. Am. Cancer Soc. Cancer Action Netw. 2017, 1–28. [Google Scholar]
- Thornburg, K.L. The programming of cardiovascular disease. J. Dev. Orig. Health Dis. 2015, 6, 366–376. [Google Scholar] [CrossRef]
- Heindel, J.J.; Vandenberg, L.N. Developmental origins of health and disease. Curr. Opin. Pediatr. 2015, 27, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Rached, M.T. Developmental establishment of epigenotype: A role for dietary fatty acids? Scand. J. Food Nutr. 2006, 50, 21–26. [Google Scholar] [CrossRef]
- Agosti, M.; Tandoi, F.; Morlacchi, L.; Bossi, A. Nutritional and metabolic programming during the first thousand days of life. La Pediatr. Med. Chir. 2017, 39, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S. Nutritional programming of disease: Unravelling the mechanism. J. Anat. 2009, 215, 36–51. [Google Scholar] [CrossRef]
- Mennitti, L.V.; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; Oller do Nascimento, C.M.; Pisani, L.P. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J. Nutr. Biochem. 2015, 26, 99–111. [Google Scholar] [CrossRef]
- Schulz, L.C. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16757–16758. [Google Scholar] [CrossRef]
- Prentice, A. The Influence of Maternal, Fetal and Child Nutrition on the Development of Chronic Disease in Later Life; Scientific Advisory Committee on Nutrition; The Stationery Office Limited: London, UK, 2011. [Google Scholar]
- Park, J.H.; Kim, S.H.; Lee, M.S.; Kim, M.S. Epigenetic modification by dietary factors: Implications in metabolic syndrome. Mol. Asp. Med. 2017, 54, 58–70. [Google Scholar] [CrossRef]
- Chaplin, A.; Palou, A.; Serra, F. Methylation analysis in fatty-acid-related genes reveals their plasticity associated with conjugated linoleic acid and calcium supplementation in adult mice. Eur. J. Nutr. 2017, 56, 879–891. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Sun, C.; Velazquez, M.A.; Smyth, N.R.; Eckert, J.J. Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo’s potential, affecting adult health. Reprod. Fertil. Dev. 2015, 27, 684–692. [Google Scholar] [CrossRef]
- Andrade, F.D.O.; De Assis, S.; Jin, L.; Fontelles, C.C.; Barbisan, L.F.; Purgatto, E.; Hilakivi-Clarke, L.; Ong, T.P. Lipidomic fatty acid profile and global gene expression pattern in mammary gland of rats that were exposed to lard-based high fat diet during fetal and lactation periods associated to breast cancer risk in adulthood. Chem. Biol. Interact. 2015, 239, 118–128. [Google Scholar] [CrossRef]
- Chmurzynska, A.; Mlodzik, M.A.; Radziejewska, A.; Szwengiel, A.; Malinowska, A.M.; Nowacka-Woszuk, J. Caloric restriction can affect one-carbon metabolism during pregnancy in the rat: A transgenerational model. Biochimie 2018, 152, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Delgado-Olguin, P. Embryonic programming of heart disease in response to obesity during pregnancy. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1886, 165402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yang, S.; Jia, Y.; Sun, B.; He, B.; Zhao, R. Maternal betaine supplementation attenuates glucocorticoid-induced hepatic lipid accumulation through epigenetic modification in adult offspring rats. J. Nutr. Biochem. 2018, 54, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Menikdiwela, K.R.; Clevenger, S.; Eboh, T.; Allen, L.; Koboziev, I.; Scoggin, S.; Rashid, A.M.; Moussa, H.; Moustaid-Moussaa, N. Maternal and Postnatal Supplementation of Fish Oil Improves Metabolic Health of Mouse Male Offspring. Obesity 2018, 26, 1740–1748. [Google Scholar] [CrossRef]
- Capobianco, E.; Fornes, D.; Roberti, S.L.; Powell, T.L.; Jansson, T.; Jawerbaum, A. Supplementation with polyunsaturated fatty acids in pregnant rats with mild diabetes normalizes placental PPARγ and mTOR signaling in female offspring developing gestational diabetes. J. Nutr. Biochem. 2018, 53, 39–47. [Google Scholar] [CrossRef]
- Herrera, E.; Ortega-Senovilla, H. Maternal lipid metabolism during normal pregnancy and its implications to fetal development. Clin. Lipidol. 2010, 5, 899–911. [Google Scholar] [CrossRef]
- Bobinski, R.; Mikulska, M. The ins and outs of maternal-fetal fatty acid metabolism. Acta Biochim Pol. 2015, 62, 499–507. [Google Scholar] [CrossRef]
- Vidakovic, A.J.; Jaddoe, V.W.V.; Voortman, T.; Demmelmair, H.; Koletzko, B.; Gaillard, R. Maternal plasma polyunsaturated fatty acid levels during pregnancy and childhood lipid and insulin levels. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 78–85. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Lupu, D.S.; Craciunescu, C.N. Perinatal manipulation of alpha-linolenic acid intake induces epigenetic changes in maternal and offspring livers. FASEB J. 2013, 27, 350–358. [Google Scholar] [CrossRef]
- Lupu, D.S.; Cheatham, C.L.; Corbin, K.D.; Niculescu, M.D. Genetic and epigenetic transgenerational implications related to omega-3 fatty acids. Part I: Maternal FADS2 genotype and DNA methylation correlate with polyunsaturated fatty acid status in toddlers: An exploratory analysis. Nutr. Res. 2015, 35, 939–947. [Google Scholar] [CrossRef]
- Pariza, M.W.; Park, Y.; Cook, M.E. The biologically active isomers of conjugated linoleic acid. Prog. Lipid Res. 2001, 40, 283–298. [Google Scholar] [CrossRef]
- Fuke, G.; Nornberg, J.L. Systematic evaluation on the effectiveness of conjugated linoleic acid in human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1–7. [Google Scholar] [CrossRef]
- Koba, K.; Yanagita, T. Health benefits of conjugated linoleic acid (CLA). Obes. Res. Clin. Pract. 2014, 8, e525–e532. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, M.H.; Aliasghari, F.; Babaei Beigi, M.A.; Hasanzadeh, J. Effect of conjugated linoleic acid and omega-3 fatty acid supplementation on inflammatory and oxidative stress markers in atherosclerotic patients. ARYA Atheroscler. 2013, 9, 311–318. [Google Scholar]
- Białek, A.; Tokarz, A. Conjugated linoleic acid as a potential protective factor in prevention of breast cancer. Postȩpy Hig. Med. Doświadczalnej 2013, 67, 6–14. [Google Scholar] [CrossRef]
- Bruen, R.; Fitzsimons, S.; Belton, O. Atheroprotective Effects of Conjugated Linoleic Acid. Br. J. Clin. Pharmacol. 2017, 83, 46–53. [Google Scholar] [CrossRef]
- Białek, A.; Tokarz, A.; Zagrodzki, P. Conjugated linoleic acids in diet of female rats inhibit the breast cancer formation in their offspring. J. Food Nutr. Res. 2014, 53, 39–50. [Google Scholar]
- Czauderna, M.; Kowalczyk, J.; Korniluk, K.; Wasowska, I. Improved saponification then mild base and acid-catalyzed methylation is a useful method for quantifying fatty acids, with special emphasis on conjugated dienes. Acta Chromatogr. 2007, 18, 59–71. [Google Scholar]
- Białek, M.; Czauderna, M.; Białek, A. Partial replacement of rapeseed oil with fish oil, and dietary antioxidants supplementation affects concentrations of biohydrogenation products and conjugated fatty acids in rumen and selected lamb tissues. Anim. Feed Sci. Technol. 2018, 241, 63–74. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Krajewska, K.; Rozbicka, A.; Michalski, J. Dietary selenite and conjugated linoleic acid isomers influence fatty acid concentrations in the liver and femoral muscles of rats. J. Anim. Feed Sci. 2009, 18, 564–581. [Google Scholar] [CrossRef]
- Lopes, L.D.; Böger, B.R.; Cavalli, K.F.; Silveira-Júnior, J.F.D.S.; Osório, D.V.C.L.; de Oliveira, D.F.; Luchetta, L.; Tonial, I.B. Fatty acid profile, quality lipid index and bioactive compounds of flour from grape residues. Cienc. Investig. Agrar. 2014, 41, 225–234. [Google Scholar]
- Ghaeni, M.; Ghahfarokhi, K.N. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices in Leiognathusbindus and Upeneussulphureus. J. Mar. Sci. Res. Dev. 2013, 3, 3–5. [Google Scholar] [CrossRef]
- Bialek, A.; Bialek, M.; Jelinska, M.; Tokarz, A. Fatty acid composition and oxidative characteristics of novel edible oils in Poland. CyTA J. Food 2017, 15, 1–18. [Google Scholar] [CrossRef]
- Czauderna, M.; Marounek, M.; Duskova, D.; Kowalczyk, J. The sensitive and simple measurement of underivatized cholesterol and its oxygen derivatives in biological materials by capillary gas chromatography coupled to a mass-selective detector. Acta Chromatogr. 2013, 25, 655–667. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Marounek, M. The simple and sensitive measurement of malondialdehyde in selected specimens of biological origin and some feed by reversed phase high performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2251–2258. [Google Scholar] [CrossRef]
- Czauderna, M.; Kowalczyk, J.; Niedźwiedzka, K.M. Simple HPLC Analysis of Tocopherols and Cholesterol from Specimens of Animal Origin. Chem. Anal. 2009, 203, 203–214. [Google Scholar]
- StaSoft Inc. StatSoft. Stat. Data Anal. Softw. Syst. 2016, 13. [Google Scholar]
- Kabaran, S.; Besler, T.T. Do fatty acids affect fetal programming? J. Health Popul. Nutr. 2015, 33, 1–9. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T.; Schellong, K.; Schulz, S.; Stupin, J.H. Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 641–653. [Google Scholar] [CrossRef]
- Białek, A.; Jelińska, M.; Tokarz, A. Influence of maternal diet enrichment with conjugated linoleic acids on lipoxygenase metabolites of polyunsaturated fatty acids in serum of their offspring with 7,12-dimethylbenz[a]anthracene induced mammary tumors. Prostaglandins Other. Lipid Mediat. 2015, 116–117, 10–18. [Google Scholar] [CrossRef]
- Reynolds, C.M.; Segovia, S.A.; Zhang, X.D.; Gray, C.; Vickers, M.H. Conjugated Linoleic Acid Supplementation During Pregnancy and Lactation Reduces Maternal High-Fat-Diet-Induced Programming of Early-Onset Puberty and Hyperlipidemia in Female Rat Offspring. Biol. Reprod. 2015, 9240, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Segovia, S.A.; Vickers, M.H.; Zhang, X.D.; Gray, C.; Reynolds, C.M. Maternal supplementation with conjugated linoleic acid in the setting of diet-induced obesity normalises the inflammatory phenotype in mothers and reverses metabolic dysfunction and impaired insulin sensitivity in offspring. J. Nutr. Biochem. 2015, 26, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, C.A.; Segovia, S.A.; Markworth, J.F.; Gray, C.; Zhang, X.D.; Milan, A.M.; Mitchell, C.J.; Barnett, M.P.; Roy, N.C.; Vickers, M.H.; et al. Maternal conjugated linoleic acid supplementation reverses high-fat diet-induced skeletal muscle atrophy and inflammation in adult male rat offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R432–R439. [Google Scholar] [CrossRef] [PubMed]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Zhang, X.D.; Reynolds, C.M. Conjugated Linoleic Acid Supplementation Improves Maternal High Fat Diet-Induced Programming of Metabolic Dysfunction in Adult Male Rat Offspring. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, B.G.; Wu, D.K.; Kelley, M.W.; Tam, P.L.; Nichols, J.; Smith, A. Signaling and Transcriptional Networks in Heart Development and Regeneration. Cold Spring Harb. Lab. Perspect. Biol. 2013, 5, a008292. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient Regenerative Potential of the Neonatal Mouse Heart. Science 2011, 331, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, M.; Kowalczyk, J.; Wąsowska, I.; Niedźwiedzka, K.; Pastuszewska, B. The effects of selenium and conjugated linoleic acid (CLA) isomers on fatty acid composition, CLA isomer content in tissues, and growth of rats. J. Anim. Feed Sci. 2003, 12, 865–881. [Google Scholar] [CrossRef]
- Białek, M.; Białek, A.; Czauderna, M. Conjugated linoleic acid isomers affect profile of lipid compounds and intensity of their oxidation in heart of rats with chemically induced mammary tumors—preliminary study. Nutrients 2019, 11, 2032. [Google Scholar] [CrossRef]
- Chaplin, A.; Parra, P.; Serra, F.; Palou, A. Conjugated Linoleic Acid Supplementation under a High-Fat Diet Modulates Stomach Protein Expression and Intestinal Microbiota in Adult Mice. PLoS ONE 2015, 10, e0125091. [Google Scholar] [CrossRef]
- Alasnier, C.; Berdeaux, O.; Chardigny, J.M.; Sébédio, J.L. Fatty acid composition and conjugated linoleic acid content of different tissues in rats fed individual conjugated linoleic acid isomers given as triacylglycerols. J. Nutr. Biochem. 2002, 13, 337–345. [Google Scholar] [CrossRef]
- Kelley, D.S.; Bartolini, G.L.; Newman, J.W.; Vemuri, M.; Mackey, B.E. Fatty acid composition of liver, adipose tissue, spleen, and heart of mice fed diets containing t10, c12-, and c9, t11-conjugated linoleic acid. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Stawarska, A.; Tokarz, A.; Czuba, K.; Konarska, A.; Mazurkiewicz, M.; Stanimirova-Daszykowska, I. Enrichment of maternal diet with conjugated linoleic acids influences desaturases activity and fatty acids profile in livers and hepatic microsomes of the offspring with 7,12-dimethylbenz[A]anthracene-induced mammary tumors. Acta Pol. Pharm. Drug Res. 2014, 71, 747–761. [Google Scholar]
- Kulig, W.; Cwiklik, L.; Jurkiewicz, P.; Rog, T.; Vattulainen, I. Cholesterol oxidation products and their biological importance. Chem. Phys. Lipids 2016, 199, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Leonarduzzi, G.; Testa, G.; Gargiulo, S.; Poli, G.; Biasi, F. Lipid Oxidation Derived Aldehydes and Oxysterols Between Health and Disease. Eur. J. Lipid Sci. Technol. 2018, 1700047, 1–16. [Google Scholar] [CrossRef]
- Gargiulo, S.; Testa, G.; Gamba, P.; Staurenghi, E.; Poli, G.; Leonarduzzi, G. Oxysterols and 4-hydroxy-2-nonenal contribute to atherosclerotic plaque destabilization. Free Radic. Biol. Med. 2017, 111, 140–150. [Google Scholar] [CrossRef]
- Nakamura, Y.K.; Flintoff-Dye, N.; Omaye, S.T. Conjugated linoleic acid modulation of risk factors associated with atherosclerosis. Nutr. Metab. 2008, 5, 22. [Google Scholar] [CrossRef]
- Poli, G.; Biasi, F.; Leonarduzzi, G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013, 1, 125–130. [Google Scholar] [CrossRef]
- Adachi, J.; Kudo, R.; Ueno, Y.; Hunter, R.; Rajendram, R.; Want, E.; Preedy, V.R. Heart 7-Hydroperoxycholesterol and Oxysterols Are Elevated in Chronically Ethanol-Fed Rats. J. Nutr. 2001, 131, 2916–2920. [Google Scholar] [CrossRef][Green Version]
- Jusakul, A.; Yongvanit, P.; Loilome, W.; Namwat, N.; Kuver, R. Mechanisms of oxysterol-induced carcinogenesis. Lipids Health Dis. 2011, 10, 44. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Aruelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Grotto, D.; Santa Maria, L.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Dialdehyd malonowy (MDA) jako wskaźnik procesów peroksydacji lipidów w organizmie. Wiadomości Lek. 2004, 57, 453–455. [Google Scholar]
- Zeitz, J.O.; Most, E.; Eder, K. Conjugated linoleic acid influences the metabolism of tocopherol in lactating rats but has little effect on tissue tocopherol concentrations in pups. Lipids Health Dis. 2016, 15, 102. [Google Scholar] [CrossRef][Green Version]

| Fatty Acid | Labofeed H | Safflower Oil (SAF Oil) | Bio-C.L.A. |
|---|---|---|---|
| C6:0 (μg/g) | 10.4 | nd | nd |
| C8:0 (mg/g) | nd | nd | 1.23 |
| C10:0 (μg/g) | nd | nd | 917 |
| C12:0 (μg/g) | 4.8 | nd | 29.2 |
| C14:0 (μg/g) | 18.6 | 209 | 209 |
| C15:0 (μg/g) | 10.0 | 53.8 | nd |
| C16:0 (mg/g) | 1.05 | 13.9 | 16.2 |
| c7 C16:1 (μg/g) | 11.9 | 167 | 31.8 |
| c9 C16:1 (μg/g) | 16.4 | 266 | 235 |
| C17:0 (μg/g) | 10.0 | 61.4 | 77.5 |
| c6C17:1 (μg/g) | 6.2 | nd | nd |
| c9C17:1 (μg/g) | nd | 69.0 | 54.2 |
| C18:0 (mg/g) | 0.44 | 0.01 | 6.48 |
| t11C18:1 (μg/g) | nd | nd | 53.4 |
| c9 C18:1 (mg/g) | 1.12 | 130 | 37.0 |
| c11 C18:1 (mg/g) | 0.04 | 2.12 | 2.66 |
| t9c12 C18:2 (μg/g) | nd | nd | 202 |
| LA (mg/g) | 4.12 | 75.3 | 42.7 |
| ALA (mg/g) | 2.21 | 0.95 | 0.00 |
| C20:0 (μg/g) | 10.5 | 957 | 820 |
| c9t11C18:2 (mg/g) | nd | nd | 99.6 |
| t7c9C18:2 (μg/g) | nd | nd | 944 |
| t10c12C18:2 (mg/g) | nd | nd | 97.6 |
| c11c13C18:2 (mg/g) | nd | nd | 4.13 |
| c9c11C18:2 (μg/g) | nd | nd | 699 |
| c11C20:1 (mg/g) | 10.4 | 619 | nd |
| c8c11c14c17C20:4n-3 (μg/g) | nd | 672 | nd |
| C22:0 (μg/g) | 5.4 | nd | 359 |
| C24:0 (μg/g) | nd | 202 | 64.9 |
| c15 C24:1 (μg/g) | nd | 240 | 187 |
| Conjugated fatty acids: (mg/g) | |||
| ƩCFA: | nd | 0.49 | 192 |
| ƩCD: | nd | 0.23 | 189 |
| tt CD | nd | 0.17 | 5.18 |
| ct/tc CD | nd | 0.05 | 178 |
| cc CD | nd | nd | 6.23 |
| ƩCT: | 0.00 | 0.26 | 3.00 |
| ttt CT | nd | 0.22 | 2.78 |
| ttc CT | nd | 0.02 | 0.22 |
| cct CT | 0.00 | 0.02 | 0.00 |
| Cholesterol (μg/g) | 155 | nd | nd |
| Tocopherols (μg/g) | |||
| δ (delta) tocopherol | 27.2 | 46.7 | 64.2 |
| γ (gamma) tocopherol | 4.43 | 18.5 | 21.0 |
| α (alpha) tocopherol | 14.7 | 22.3 | 186 |
| α (alpha) tocopherol acetate | 82.4 | 129 | 48.6 |
| Mothers’ Diet | SAF Oil | Bio-C.L.A. | p Values for Two-Way Anova | |||||
|---|---|---|---|---|---|---|---|---|
| Group | SAF (M+/P+) (n = 10) | SAF (M+/P−) (n = 9) | CLA (M+/P+) (n = 8) | CLA (M+/P−) (n = 9) | Mothers’ Diet (MD) | Offspring Supplementation (OS) | Interaction (MD × OS) | |
| Variables | ||||||||
| Fatty acids: | ||||||||
| ƩFAs (mg/g) | 6.20 ± 0.71 | 7.03 ± 0.77 | 6.43 ± 0.63 | 6.14 ± 1.24 | 0.26 | 0.37 | 0.06 | |
| C12:0 (μg/g) | 5.84 ± 2.73 | 5.77 ± 2.59 | 5.58 ± 1.89 | 2.86 ± 0.75 | 0.04 | 0.07 | 0.09 | |
| C14:0 (μg/g) | 13.6 ± 2.92 a | 17.5 ± 3.43 ab | 20.5 ± 4.75 b | 16.9 ± 5.21 ab | 0.03 | 0.92 | 0.01 | |
| C15:0 (μg/g) | 7.84 ± 2.47 | 9.37 ± 1.26 | 10.9 ± 3.52 | 9.75 ± 1.93 | 0.04 | 0.82 | 0.10 | |
| C16:0 (μg/g) | 914 ± 53.8 | 941 ± 120 | 868 ± 63.9 | 821 ± 104 | 0.01 | 0.74 | 0.23 | |
| C17:0 (μg/g) | 37.7 ± 7.27 | 40.6 ± 5.71 | 40.1 ± 7.79 | 46.0 ± 13.2 | 0.22 | 0.17 | 0.64 | |
| C18:0 (mg/g) | 1.72 ± 0.10 ab | 1.71 ± 0.23 ab | 1.77 ± 0.13 b | 1.52 ± 0.20 a | 0.24 | 0.04 | 0.04 | |
| C20:0 (μg/g) | 7.34 ± 2.76 | 7.55 ± 2.87 | 9.10 ± 2.75 | 6.39 ± 2.03 | 0.74 | 0.16 | 0.11 | |
| C21:0 (μg/g) | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.31 ± 1.07 | 4.08 ± 1.03 | <0.01 | 0.65 | 0.65 | |
| C22:0 (μg/g) | 5.77 ± 2.96 | 4.33 ± 1.22 | 6.93 ± 1.23 | 3.67 ± 0.97 | 0.89 | 0.20 | 0.60 | |
| C24:0 (μg/g) | 15.7 ± 3.97 | 22.5 ± 4.27 | 15.4 ± 2.58 | 18.4 ± 3.95 | 0.09 | <0.01 | 0.14 | |
| ƩSFA (mg/g) | 2.71 ± 0.15 | 2.66 ± 0.24 | 2.63 ± 0.29 | 2.44 ± 0.312 | 0.10 | 0.19 | 0.40 | |
| A-SFA (μg/g) | 932 ± 53.8 | 964 ± 123 | 786 ± 311 | 840 ± 108 | 0.02 | 0.45 | 0.85 | |
| T-SFA (mg/g) | 2.47 ± 0.54 | 2.67 ± 0.34 | 2.55 ± 0.29 | 2.35 ± 0.31 | 0.37 | 0.97 | 0.14 | |
| c7C16:1 (μg/g) | 9.84 ± 4.38 | 13.4 ± 4.99 | 14.4 ± 9.11 | 10.2 ± 3.75 | 0.75 | 0.88 | 0.06 | |
| c9C16:1 (μg/g) | 12.9 ± 4.50 | 14.7 ± 3.52 | 12.5 ± 4.71 | 11.9 ± 3.19 | 0.24 | 0.68 | 0.37 | |
| c9C18:1 (μg/g) | 208 ± 39.8 | 228 ± 38.8 | 189 ± 47.1 | 199 ± 14.1 | 0.05 | 0.19 | 0.81 | |
| c11C18:1 (μg/g) | 171 ± 13.1 | 189 ± 17.0 | 162 ± 17.5 | 179 ± 18.9 | 0.16 | 0.01 | 0.91 | |
| c11C20:1 (μg/g) | 5.43 ± 2.20 ab | 13.3 ± 5.32 b | 8.41 ± 3.11 ab | 0.00 ± 0.00 a | 0.85 | <0.01 | <0.01 | |
| c15C24:1 (μg/g) | 8.25 ± 0.47 | 8.31 ± 3.17 | 0.00 ± 0.00 | 0.00 ± 0.00 | <0.01 | 0.97 | 0.97 | |
| ƩMUFA (μg/g) | 403 ± 47.4 | 433 ± 92.3 | 381 ± 74.4 | 384 ± 95.4 | 0.20 | 0.54 | 0.63 | |
| LA (mg/g) | 1.71 ± 0.21 | 1.99 ± 0.21 | 1.77 ± 0.31 | 1.80 ± 0.16 | 0.42 | 0.50 | 0.13 | |
| ALA (μg/g) | 13.8 ± 2.10 | 17.0 ± 2.26 | 13.3 ± 1.76 | 13.4 ± 3.71 | 0.03 | 0.06 | 0.09 | |
| c11c14C20:2 (μg/g) | 10.2 ± 4.36 | 9.53 ± 3.00 | 7.30 ± 3.72 | 8.91 ± 1.98 | 0.11 | 0.67 | 0.29 | |
| DGLA (μg/g) | 12.7 ± 3.17 | 15.4 ± 2.98 | 15.7 ± 3.60 | 14.4 ± 2.36 | 0.34 | 0.55 | 0.07 | |
| AA (mg/g) | 0.96 ± 0.08 | 1.12 ± 0.11 | 0.99 ± 0.11 | 1.04 ± 0.18 | 0.54 | 0.02 | 0.23 | |
| EPA (μg/g) | 0.00 ± 0.00 a | 7.70 ± 1.46 b | 7.29 ± 0.67 b | 5.69 ± 2.22 b | <0.01 | <0.01 | <0.01 | |
| DPA (μg/g) | 96.4 ± 19.7 | 126 ± 25.6 | 92.3 ± 16.7 | 121 ± 22.1 | 0.53 | <0.01 | 0.96 | |
| DHA (μg/g) | 482 ± 85.4 | 575 ± 92.8 | 526 ± 79.1 | 503 ± 96.9 | 0.64 | 0.24 | 0.06 | |
| ƩPUFA (mg/g) | 3.28 ± 0.29 | 3.85 ± 0.39 | 3.42 ± 0.47 | 3.31 ± 0.86 | 0.28 | 0.21 | 0.07 | |
| n-3PUFA (μg/g) | 590 ± 87.0 | 720 ± 108 | 636 ± 187 | 642 ± 116 | 0.64 | 0.05 | 0.08 | |
| n-6PUFA (mg/g) | 2.69 ± 0.26 | 3.13 ± 0.29 | 2.77 ± 0.31 | 2.66 ± 0.77 | 0.25 | 0.30 | 0.09 | |
| n-6/n-3 | 4.62 ± 0.33 | 4.38 ± 0.38 | 4.40 ± 0.13 | 4.09 ± 1.04 | 0.30 | 0.27 | 0.90 | |
| Indices: | ||||||||
| D4D | 0.83 ± 0.04 | 0.82 ± 0.02 | 0.85 ± 0.02 | 0.81 ± 0.02 | 0.84 | <0.01 | 0.06 | |
| D5D | 0.99 ± 0.01 | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.99 ± 0.00 | 0.14 | 0.74 | 0.11 | |
| D9D_C16 | 0.01 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.57 | 0.27 | 0.23 | |
| D9D_C18 | 0.10 ± 0.01 | 0.12 ± 0.03 | 0.09 ± 0.02 | 0.10 ± 0.04 | 0.38 | 0.19 | 0.44 | |
| D9D_total | 0.07 ± 0.03 | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.07 ± 0.03 | 0.59 | 0.32 | 0.29 | |
| PI | 121 ± 11.0 | 125 ± 4.30 | 121 ± 9.19 | 128 ± 5.21 | 0.57 | 0.05 | 0.77 | |
| AI | 0.27 ± 0.03 | 0.24 ± 0.01 | 0.23 ± 0.09 | 0.26 ± 0.11 | 0.77 | 0.94 | 0.15 | |
| TI | 0.75 ± 0.16 | 0.68 ± 0.04 | 0.74 ± 0.13 | 0.70 ± 0.12 | 0.86 | 0.18 | 0.69 | |
| HH | 3.72 ± 0.35 a | 4.24 ± 0.21 ab | 23.09 ± 4.96 b | 4.09 ± 0.94 ab | 0.03 | 0.03 | 0.03 | |
| iso_LA | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.14 ± 0.32 | 0.21 | 0.40 | 0.38 | |
| iso_ALA | 0.61 ± 0.18 c | 0.29 ± 0.16 a | 0.45 ± 0.23 abc | 0.38 ± 0.12 ab | 0.52 | <0.01 | 0.04 | |
| Mothers’ Diet | SAF Oil | Bio-C.L.A. | p Values for Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Group | SAF (M+/P+) (n = 10) | SAF (M+/P−) (n = 9) | CLA (M+/P+) (n = 8) | CLA (M+/P−) (n = 9) | Mothers’ Diet (MD) | Offspring Supplementation (OS) | Interaction (MD × OS) | |
| Variables | ||||||||
| Conjugated fatty acids: | ||||||||
| Ʃ CFAs (μg/g) | 60.4 ± 18.6 | 52.7 ± 14.5 | 100 ± 15.7 | 84.0 ± 28.8 | <0.01 | 0.08 | 0.50 | |
| Ʃ CD (μg/g) | 40.1 ± 14.6 | 44.6 ± 14.9 | 81.2 ± 10.5 | 75.1 ± 29.7 | <0.01 | 0.90 | 0.40 | |
| tt CD (μg/g) | 35.1 ± 9.81 | 41.0 ± 14.1 | 46.5 ± 9.43 | 65.5 ± 26.8 | 0.03 | 0.03 | 0.24 | |
| ct/tc CD (μg/g) | 2.05 ± 1.88 a | 3.28 ± 1.86 ab | 32.9 ± 3.76 c | 8.05 ± 2.91 abc | <0.01 | <0.01 | 0.02 | |
| t10c12CLA (μg/g) | 0.97 ± 0.53 a | 1.28 ± 1.08 ab | 14.0 ± 1.83 c | 3.42 ± 1.12 abc | <0.01 | 0.08 | 0.05 | |
| c9t11CLA (μg/g) | 0.67 ± 0.34 a | 0.76 ± 0.45 ab | 14.4 ± 1.99 c | 2.31 ± 0.61 abc | <0.01 | 0.05 | 0.04 | |
| cc CD (μg/g) | 0.00 ± 0.00 a | 1.05 ± 0.64 ab | 1.60 ± 0.35 c | 0.44 ± 0.25 ab | <0.01 | <0.01 | <0.01 | |
| Ʃ CT (μg/g) | 20.2 ± 9.55 | 8.13 ± 5.85 | 15.4 ± 7.25 | 8.93 ± 5.43 | 0.42 | <0.01 | 0.26 | |
| ttt CT (μg/g) | 8.77 ± 3.13 | 5.24 ± 2.79 | 7.03 ± 2.44 | 4.58 ± 1.55 | 0.19 | <0.01 | 0.55 | |
| ttc CT (μg/g) | 3.82 ± 1.48 | 1.02 ± 0.88 | 2.51 ± 1.43 | 1.36 ± 1.02 | 0.39 | <0.01 | 0.15 | |
| cct CT (μg/g) | 6.20 ± 3.56 | 0.66 ± 0.14 | 6.41 ± 4.77 | 2.16 ± 2.03 | 0.83 | <0.01 | 0.97 | |
| Mothers’ Diet | SAF Oil | Bio-C.L.A. | p Values for Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Group | SAF (M+/P+) (n = 10) | SAF (M+/P−) (n = 9) | CLA (M+/P+) (n = 8) | CLA (M+/P−) (n = 9) | Mothers’ Diet (MD) | Offspring Supplementation (OS) | Interaction (MD × OS) | |
| Variables | ||||||||
| MDA (µg/g) | 4.31 ± 0.94 | 3.27 ± 0.76 | 4.11 ± 1.30 | 3.83 ± 0.42 | 0.55 | 0.04 | 0.22 | |
| Tocopherols: | ||||||||
| δ (delta) | 1.86 ± 1.38 a | 2.86 ± 1.15 ab | 5.74 ± 2.04 b | 2.90 ± 0.90 ab | <0.01 | 0.06 | <0.01 | |
| γ (gamma) | 20.7 ± 15.8 | 6.13 ± 2.13 | 9.13 ± 2.85 | 4.69 ± 1.33 | 0.04 | <0.01 | 0.11 | |
| α (alpha) | 2.94 ± 3.28 | 0.54 ± 0.08 | 0.97 ± 0.32 | 0.65 ± 0.12 | 0.14 | 0.03 | 0.10 | |
| α (alpha) acetate | 5.29 ± 2.75 | 2.70 ± 1.09 | 3.52 ± 1.04 | 3.30 ± 0.57 | 0.33 | 0.02 | 0.06 | |
| Cholesterols and oxy-sterols: | ||||||||
| Cholesterol (mg/g) | 2.42 ± 0.80 | 3.07 ± 0.61 | 2.69 ± 1.01 | 2.31 ± 1.20 | 0.34 | 0.53 | 0.76 | |
| 7AOH (μg/g) | 2.04 ± 1.99 | 0.81 ± 0.30 | 0.73 ± 0.38 | 0.47 ± 0.31 | 0.04 | 0.36 | 0.67 | |
| 7BOH (μg/g) | 2.41 ± 2.09 | 2.36 ± 0.66 | 0.35 ± 0.03 | 2.00 ± 0.11 | 0.05 | 0.25 | 0.21 | |
| 5,6AE (μg/g) | 4.79 ± 4.75 b | 0.87 ± 0.01 a | 1.28 ± 0.11 b | 1.33 ± 0.38 ab | 0.12 | 0.10 | 0.04 | |
| 5,6BE (μg/g) | 6.23 ± 2.59 b | 0.69 ± 0.46 a | 1.79 ± 1.38 ab | 1.36 ± 0.57 ab | 0.01 | <0.01 | <0.01 | |
| triol (μg/g) | 1.45 ± 1.43 | 0.00 ± 0.00 | 0.15 ± 0.02 | 0.00 ± 0.00 | 0.10 | 0.05 | 0.10 | |
| 7K (μg/g) | 3.71 ± 1.06 | 1.68 ± 0.18 | 2.01 ± 1.93 | 1.15 ± 0.43 | 0.10 | 0.40 | 0.39 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białek, M.; Białek, A.; Czauderna, M. Maternal and Early Postnatal Diet Supplemented with Conjugated Linoleic Acid Isomers Affect Lipid Profile in Hearts of Offspring Rats with Mammary Tumors. Animals 2020, 10, 464. https://doi.org/10.3390/ani10030464
Białek M, Białek A, Czauderna M. Maternal and Early Postnatal Diet Supplemented with Conjugated Linoleic Acid Isomers Affect Lipid Profile in Hearts of Offspring Rats with Mammary Tumors. Animals. 2020; 10(3):464. https://doi.org/10.3390/ani10030464
Chicago/Turabian StyleBiałek, Małgorzata, Agnieszka Białek, and Marian Czauderna. 2020. "Maternal and Early Postnatal Diet Supplemented with Conjugated Linoleic Acid Isomers Affect Lipid Profile in Hearts of Offspring Rats with Mammary Tumors" Animals 10, no. 3: 464. https://doi.org/10.3390/ani10030464
APA StyleBiałek, M., Białek, A., & Czauderna, M. (2020). Maternal and Early Postnatal Diet Supplemented with Conjugated Linoleic Acid Isomers Affect Lipid Profile in Hearts of Offspring Rats with Mammary Tumors. Animals, 10(3), 464. https://doi.org/10.3390/ani10030464







