Low Expression of Sirtuin 1 in the Dairy Cows with Mild Fatty Liver Alters Hepatic Lipid Metabolism
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Average Milk Production Collection and Milk Components Analysis
2.4. Liver chemistry measurement
2.5. ELISA Assay for the Contents of SIRT1 and Downstream Lipid- metabolism-related Proteins and Key Enzymes
2.6. Liver Histology
2.7. Total RNA Isolation and qRT-PCR
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Milk Production, Milk Component and SCC
3.2. Chol and TG Concentrations in the Control and Fatty Liver Dairy Cows
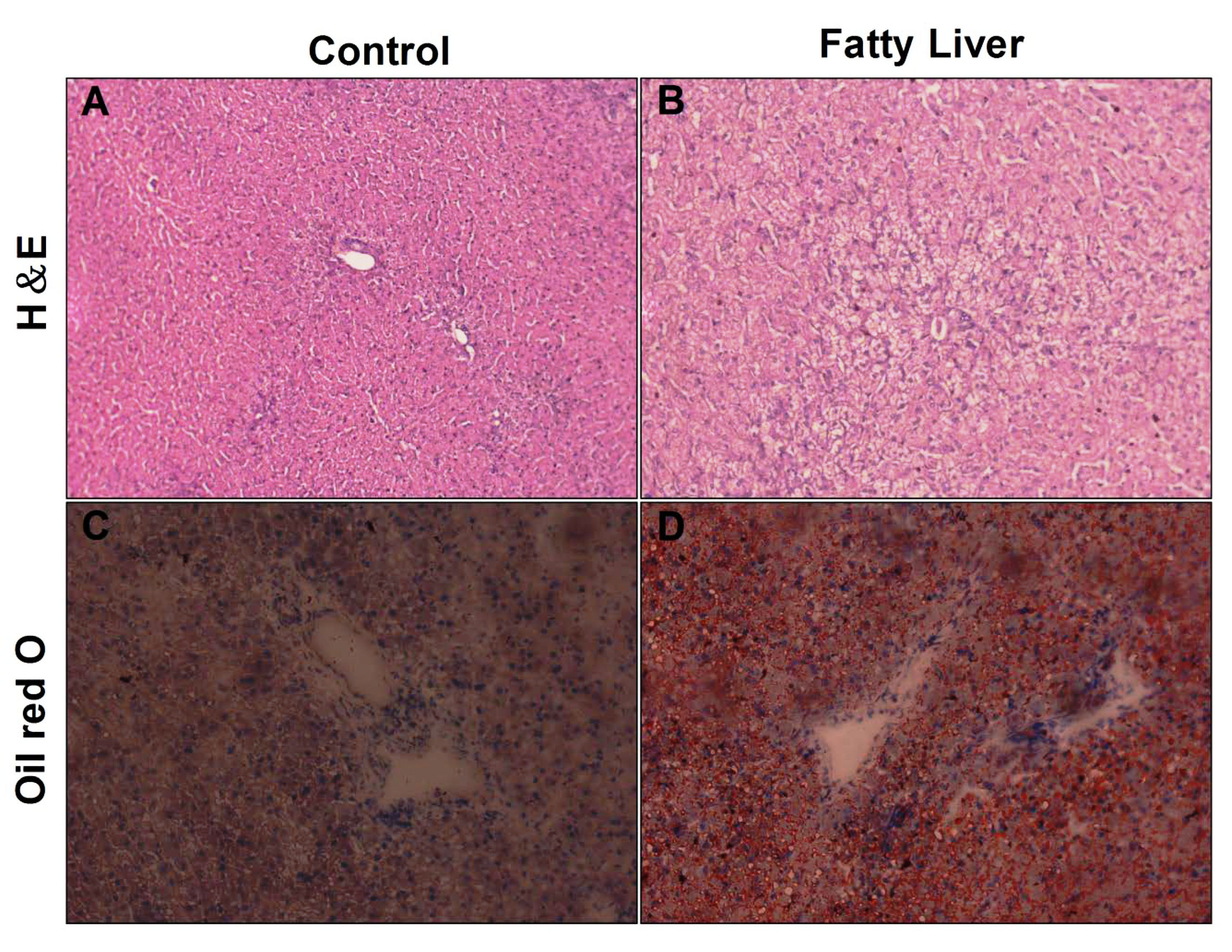
3.3. Histological Analysis of Liver
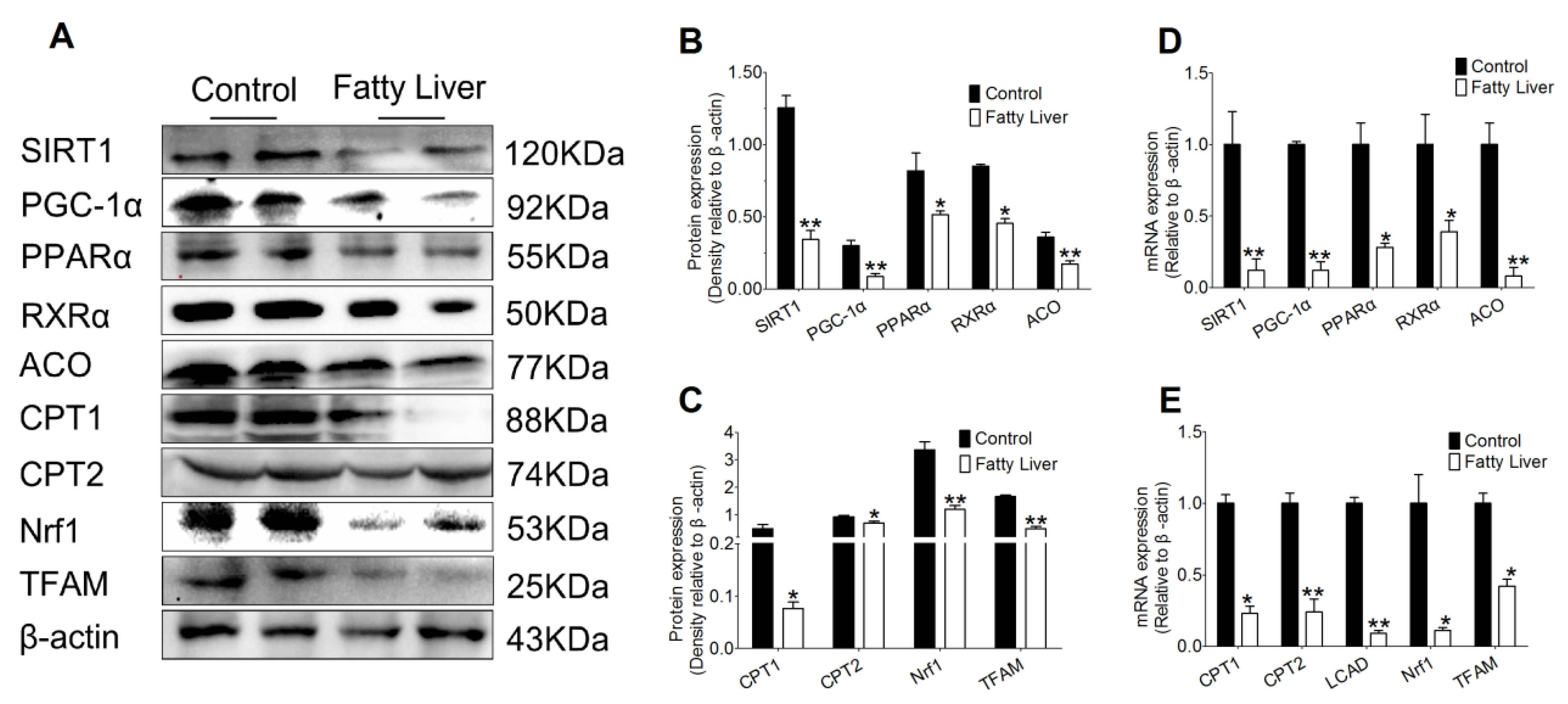
3.4. Effects of Hepatic Steatosis on Enzyme Activities of SIRT1, SREBP-1c, PGC-1α and Redox Index
3.5. Effect of Hepatic Steatosis on the Protein and mRNA Abundance of Fatty Acid Oxidation Proteins
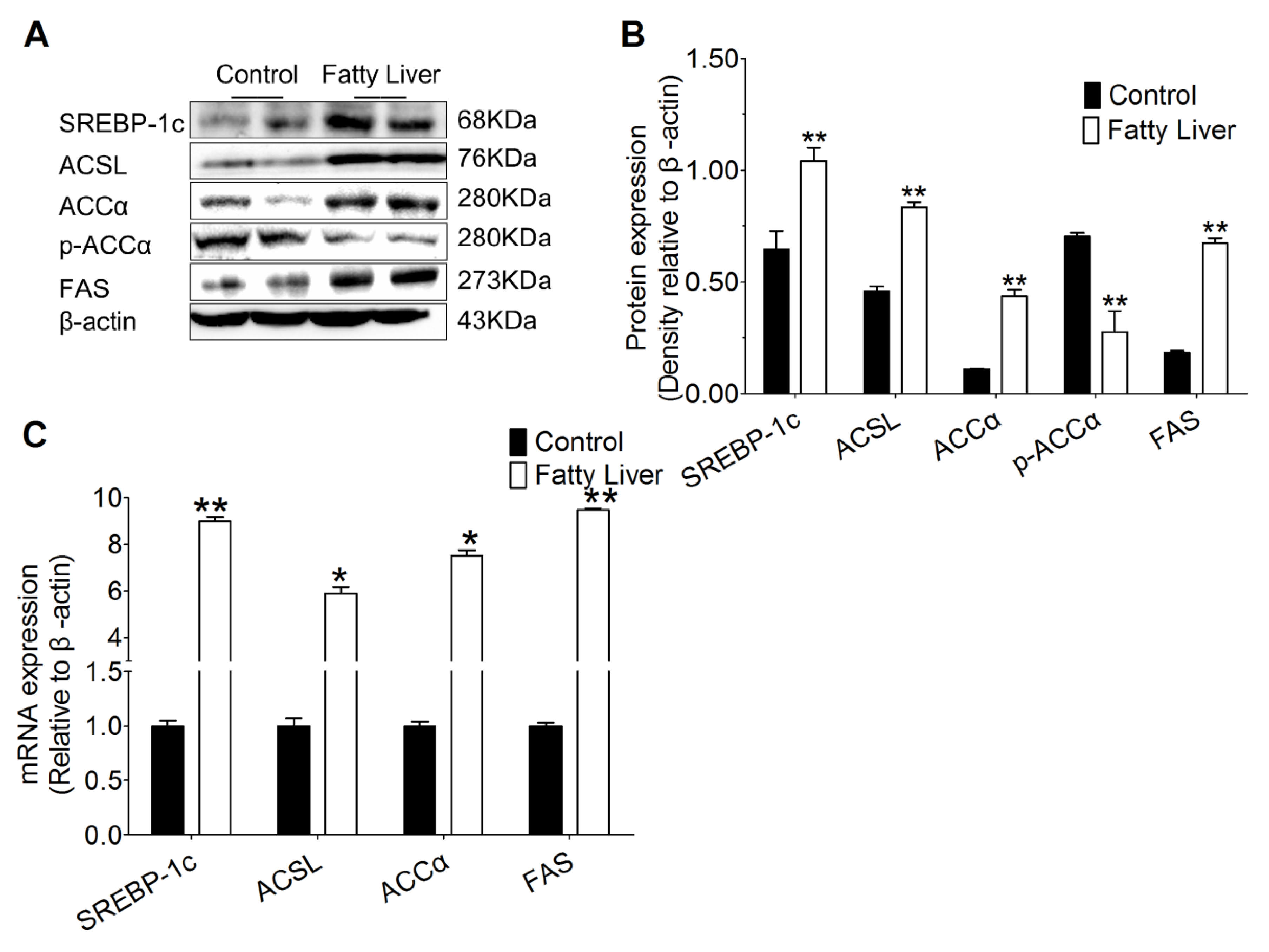
3.6. Hepatic Steatosis Enhances Protein and mRNA Abundance of Hepatic Lipogenesis
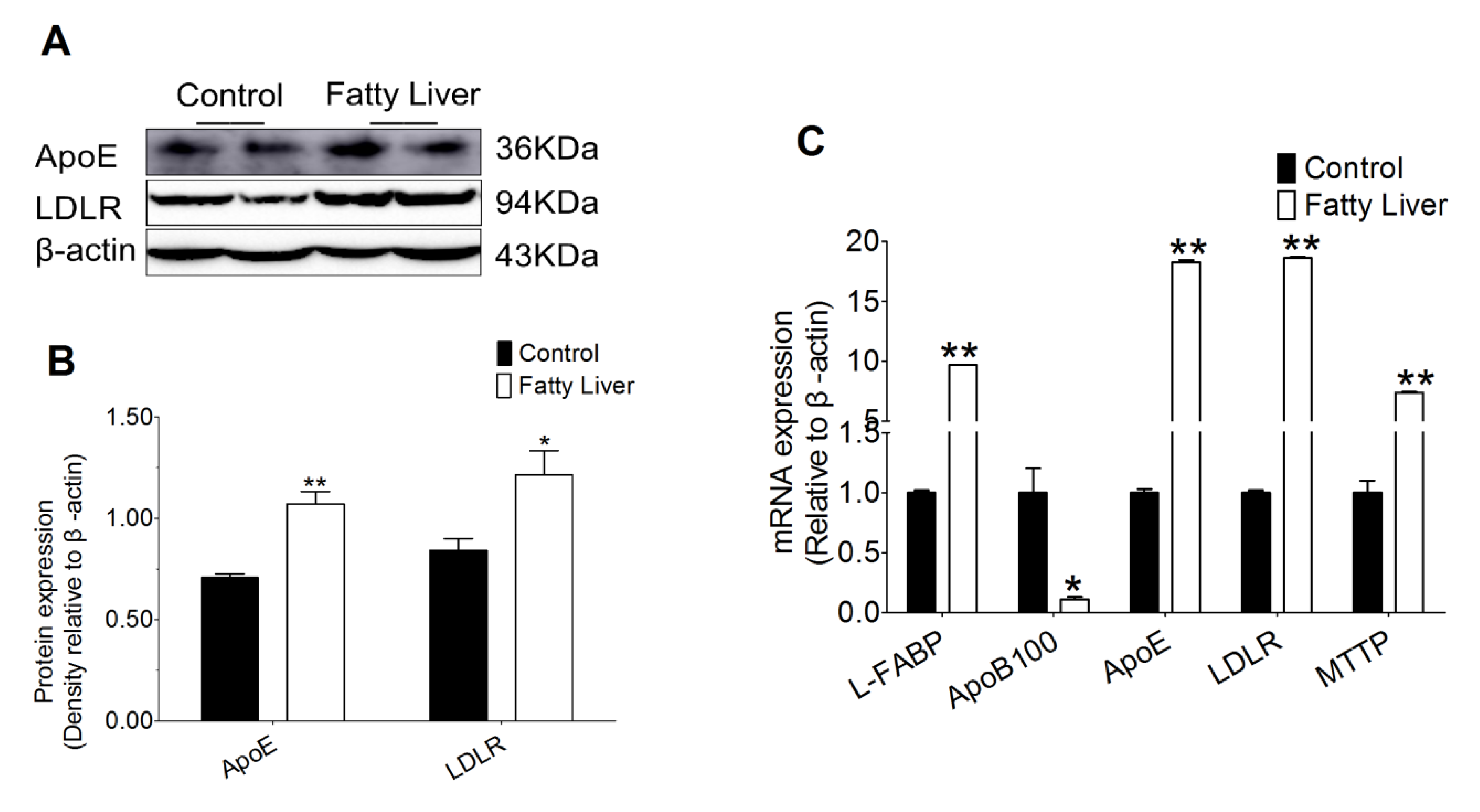
3.7. Effect of Hepatic Steatosis on Hepatic Lipid Transport Related Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Havlin, J.M.; Robinson, P.H.; Garrett, J.E. Niacin feeding to fresh dairy cows: Immediate effects on health and milk production. Anim. Prod. Sci. 2017, 57, 1069–1078. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.; Wang, X.; Liu, L.; Huang, D.; Zhang, R.; Guo, L.; Wang, Z.; Li, X.; Liu, G. High levels of acetoacetate and glucose increase expression of cytokines in bovine hepatocytes, through activation of the NF-κB signalling pathway. J. Dairy Res. 2016, 83, 51–57. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R.; Overton, T.R.; Ospina, P.A. Elevated non-esterified fatty acids and β-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 2013, 198, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, A.L.; Shulman, G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014, 59, 713–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Yuan, X.; Chen, L.; Wang, T.; Wang, Z.; Sun, G.; Li, X.; Li, X.; Liu, G. Histamine induces bovine rumen epithelial cell inflammatory response via NF-κB pathway. Cell. Physiol. Biochem. 2017, 42, 1109–1119. [Google Scholar] [CrossRef]
- Chung, S.W.; Yao, H.W.; Caito, S.; Hwang, J.-W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arc. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Takeuchi, H.; Sonobe, Y.; Jin, S.; Wang, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Suzumura, A. Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes. J. Neuroimmunol. 2014, 269, 38–43. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Washida, N.; Tokuyama, H.; Hayashi, K.; Itoh, H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem. Bioph. Res. Co. 2008, 372, 51–56. [Google Scholar] [CrossRef]
- Chan, S.-H.; Hung, C.-H.; Shih, J.-Y.; Chu, P.-M.; Cheng, Y.-H.; Lin, H.-C.; Tsai, K.-L. SIRT1 inhibition causes oxidative stress and inflammation in patients with coronary artery disease. Redox Bio. 2017, 13, 301–309. [Google Scholar] [CrossRef]
- De, D.; Halder, D.; Shin, I.; Kim, K.K. Small molecule-induced cellular conversion. Chem. Soc. Rev. 2017, 46, 6241–6254. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Liu, X.; Pan, L.; Xiong, Q.; Zhu, Y.Z. Hydrogen sulfide protects against apoptosis under oxidative stress through SIRT1 pathway in H9c2 cardiomyocytes. Nitric Oxide 2015, 46, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shi, Z.; Peng, Z.; Zhao, C.; Zhang, Y.; Wang, Z.; Li, X.; Liu, G.; Li, X. Acetoacetate induces hepatocytes apoptosis by the ROS-mediated MAPKs pathway in ketotic cows. J. Cell. Physiol. 2017, 232, 3296–3308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, H.Y.; Wang, X.C.; Feng, S.B.; Li, X.B.; Wang, Z.; Liu, G.W.; Li, X.W. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. An. N. 2017, 100, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Thakran, S.; Sharma, P.; Attia, R.R.; Hori, R.T.; Deng, X.; Elam, M.B.; Park, E.A. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. J. Biol. Chem. 2013, 288, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xu, M.; Lee, J.; He, C.; Xie, Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endoc. M. 2012, 303, E1234–E1244. [Google Scholar] [CrossRef] [Green Version]
- Jeon, B.T.; Kim, K.E.; Shin, H.J.; Yi, C.-o.; Hah, Y.-S.; Kim, W.-H.; Lee, S.-I.; Roh, G.S. Myeloid-specific deletion of SIRT1 increases hepatic steatosis and hypothalamic inflammation in mice fed a high-fat diet. Metab. Brain Dis. 2014, 29, 635–643. [Google Scholar] [CrossRef]
- Rao, Y.; Yu, H.; Gao, L.; Lu, Y.; Xu, Z.; Liu, H.; Gu, L.; Ye, J.; Huang, Z. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Brit. J. Pharmacol. 2017, 174, 2457–2470. [Google Scholar] [CrossRef]
- Deng, X.; Chen, L.; Li, N. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007, 27, 708–715. [Google Scholar] [CrossRef]
- Xu, F.; Gao, Z.; Zhang, J.; Rivera, C.A.; Yin, J.; Weng, J.; Ye, J. Lack of SIRT1 (Mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/- mice: A role of lipid mobilization and inflammation. Endocrinology 2010, 151, 2504–2514. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, S.; Blander, G.; Jeanette, G.T.; Krieger, M.; Guarente, L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 2007, 28, 91–106. [Google Scholar] [CrossRef]
- Pfluger, P.T.; Herranz, D.; Velasco-Miguel, S.; Serrano, M.; Tschöp, M.H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl Acad. Sci. USA 2008, 105, 9793–9798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.W.; Guan, Y.; Loor, J.J.; Sha, X.Y.; Coleman, D.N.; Zhang, C.; Du, X.L.; Shi, Z.; Li, X.B.; Wang, Z.; et al. Fatty acid-induced endoplasmic reticulum stress promoted lipid accumulation in calf hepatocytes, and endoplasmic reticulum stress existed in the liver of severe fatty liver cows. J. Dairy Sci. 2019, 102, 7359–7370. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.H.; Loor, J.J.; Zuo, R.K.; Chen, X.Y.; Liang, Y.S.; Wang, Y.Z.; Shu, X.; Sun, X.D.; Jia, H.D.; Liu, G.W.; et al. Low abundance of mitofusin 2 in dairy cows with moderate fatty liver is associated with alterations in hepatic lipid metabolism. J. Dairy Sci. 2019, 102, 7536–7547. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.B.; Bu, D.P.; Wang, J.Q.; Sun, X.Z.; Pan, L.; Zhou, L.Y.; Liu, W. Effects of rumen-protected γ-aminobutyric acid on performance and nutrient digestibility in heat-stressed dairy cows. J. Dairy Sci. 2014, 97, 5599–5607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Liu, C.; Hu, K.; Greenberg, A.; Wu, D.; Ausman, L.M.; McBurney, M.W.; Wang, X.-D. Ablation of systemic SIRT1 activity promotes nonalcoholic fatty liver disease by affecting liver-mesenteric adipose tissue fatty acid mobilization. BBA-Mol. Basis Dis. 2017, 1863, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Ospina, P.A.; McArt, J.A.; Overton, T.R.; Stokol, T.; Nydam, D.V. Using nonesterified fatty acids and β-hydroxybutyrate concentrations during the transition period for herd-level monitoring of increased risk of disease and decreased reproductive and milking performance. Vet. Clin. Food A. 2013, 29, 387–412. [Google Scholar] [CrossRef]
- Ntambi, J.M. Hepatic de Novo Lipogenesis and Regulation of Metabolism; Springer: New York, NY, USA, 2015. [Google Scholar]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Han, C.; Wan, H.; Ma, S.; Liu, D.; He, F.; Wang, J.; Pan, Z.; Liu, H.; Li, L.; He, H. RETRACTED: Role of mammalian sirtuin 1 (SIRT1) in lipids metabolism and cell proliferation of goose primary hepatocytes. Mol. Cell. Endocrin. 2014, 382, 282–291. [Google Scholar] [CrossRef]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Auwerx, J. Protein deacetylation by SIRT1: An emerging key post-translational modification in metabolic regulation. Pharmacol. Res. 2010, 62, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.-H.; Kim, H.-S.; Xiao, C.; Xu, X.; Gavrilova, O.; Deng, C.-X. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J. Clin. Invest. 2011, 121, 4477–4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajmo, J.M.; Liang, X.; Rogers, C.Q.; Pennock, B.; You, M. Resveratrol alleviates alcoholic fatty liver in mice. Am. J. Physiol. Gastr. L. 2008, 295, G833–G842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Chen, L.-l.; Xiao, F.-x.; Sun, H.; Ding, H.-c.; Xiao, H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol. Sin. 2008, 29, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, J.T.; Puigserver, P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. USA 2007, 104, 12861–12866. [Google Scholar] [CrossRef] [Green Version]
- Satapati, S.; Kucejova, B.; Duarte, J.A.G.; Fletcher, J.A.; Reynolds, L.; Sunny, N.E.; He, T.; Nair, L.A.; Livingston, K.; Fu, X. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J. Clin. Invest. 2015, 125, 4447–4462. [Google Scholar] [CrossRef] [Green Version]
- Du, X.L.; Chen, L.; Huang, D.; Peng, Z.C.; Zhao, C.X.; Zhang, Y.M.; Zhu, Y.W.; Wang, Z.; Li, X.W.; Liu, G.W. Elevated apoptosis in the liver of dairy cows with ketosis. Cell. Physiol. Biochem. 2017, 43, 568–578. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pr. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Oliveira, C.P.M.S.; da Costa Gayotto, L.C.; Tatai, C.; Della Bina, B.I.; Janiszewski, M.; Lima, E.S.; Abdalla, D.S.P.; Lopasso, F.P.; Laurindo, F.R.M.; Laudanna, A.A. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J. Cell. Mol. Med. 2002, 6, 399–406. [Google Scholar] [CrossRef]
- Lee, J.W.; Davis, J.M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011, 23, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef] [Green Version]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.X.; Wu, X.C.; Chau, J.F.L.; Szeto, I.Y.Y.; Tam, W.Y.; Guo, Z.S.; Chung, S.K.; Oates, P.J.; Chung, S.S.M.; Yang, J.Y. Aldose reductase regulates hepatic peroxisome proliferator-activated receptor α phosphorylation and activity to impact lipid homeostasis. J. Biol. Chem. 2008, 283, 17175–17183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugden, M.C.; Bulmer, K.; Gibbons, G.F.; Knight, B.L.; Holness, M.J. Peroxisome-proliferator-activated receptor-α (PPARα) deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochem. J. 2002, 364, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Eaton, S. Control of mitochondrial β-oxidation flux. Prog. Lipid Res. 2002, 41, 197–239. [Google Scholar] [CrossRef]
- Li, X.W.; Li, X.B.; Chen, H.; Lei, L.; Liu, J.X.; Guan, Y.; Liu, Z.X.; Zhang, L.; Yang, W.T.; Zhao, C.X.; et al. Non-esterified fatty acids activate the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes. Cell Biochem. Biophy. 2013, 67, 1157–1169. [Google Scholar] [CrossRef]
- Noh, H.; Lee, H.; Kim, E.; Mu, L.; Rhee, Y.K.; Cho, C.-W.; Chung, J. Inhibitory effect of a Cirsium setidens extract on hepatic fat accumulation in mice fed a high-fat diet via the induction of fatty acid β-oxidation. Biosci. Biotech. Bioch. 2013, 77, 1424–1429. [Google Scholar] [CrossRef] [Green Version]
- Laubenthal, L.; Hoelker, M.; Frahm, J.; Dänicke, S.; Gerlach, K.; Südekum, K.H.; Sauerwein, H.; Häussler, S. Mitochondrial DNA copy number and biogenesis in different tissues of early-and late-lactating dairy cows. J. Dairy Sci. 2016, 99, 1571–1583. [Google Scholar] [CrossRef] [Green Version]
- Laubenthal, L.; Ruda, L.; Sultana, N.; Winkler, J.; Rehage, J.; Meyer, U.; Dänicke, S.; Sauerwein, H.; Häussler, S. Effect of increasing body condition on oxidative stress and mitochondrial biogenesis in subcutaneous adipose tissue depot of nonlactating dairy cows. J. Dairy Sci. 2017, 100, 4976–4986. [Google Scholar] [CrossRef]
- Schreiber, S.N.; Knutti, D.; Brogli, K.; Uhlmann, T.; Kralli, A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRα). J. Biol. Chem. 2003, 278, 9013–9018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aharoni-Simon, M.; Hann-Obercyger, M.; Pen, S.; Madar, Z.; Tirosh, O. Fatty liver is associated with impaired activity of PPAR γ-coactivator 1 α (PGC1 α) and mitochondrial biogenesis in mice. Lab. Invest. 2011, 91, 1018–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porstmann, T.; Griffiths, B.; Chung, Y.-L.; Delpuech, O.; Griffiths, J.R.; Downward, J.; Schulze, A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 2005, 24, 6465–6481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, R.A.; Lewin, T.M.; Van Horn, C.G.; Gonzalez-Baró, M.R. Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J. Nutr. 2002, 132, 2123–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakil, S.J.; Stoops, J.K.; Joshi, V.C. Fatty acid synthesis and its regulation. Annu. Rev. Biochem. 1983, 52, 537–579. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Invest. 2008, 118, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Greenow, K.; Pearce, N.J.; Ramji, D.P. The key role of apolipoprotein E in atherosclerosis. J. Mol. Med. 2005, 83, 329–342. [Google Scholar] [CrossRef]
- Michaely, P.; Zhao, Z.Z.; Li, W.P.; Garuti, R.; Huang, L.J.; Hobbs, H.H.; Cohen, J.C. Identification of a VLDL-induced, FDNPVY-independent internalization mechanism for the LDLR. EMBO J. 2007, 26, 3273–3282. [Google Scholar] [CrossRef]
- Fabbrini, E.; Magkos, F. Hepatic steatosis as a marker of metabolic dysfunction. Nutrients 2015, 7, 4995–5019. [Google Scholar] [CrossRef] [Green Version]


| Groups | Milk Ketones | Feed Intake | Milk Production | Health Status | Reproductive Performance | Other Disease Conditions |
|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| Mild fatty liver | + | − | − | − | − | 0 |
| Item | Content | |
|---|---|---|
| Prenatal | Postpartum | |
| Silage | 31.4 | 40.0 |
| Guinea grass | 23.4 | |
| Corn | 19.6 | 35.0 |
| Wheat bran | 10.0 | 8.0 |
| Soybean meal | 2.0 | 5.0 |
| Sunflower | 11.5 | 8.0 |
| NaCl | 0.8 | 1.0 |
| Premix1 | 1.3 | 1.8 |
| NaHCO3 | 1.2 | |
| Total | 100.00 | 100.00 |
| Energy (%) 1 | ||
| NEL(MJ/Kg) 2 | 5.7 | 6.7 |
| Crude protein | 11.3 | 15.2 |
| Neutral detergent fiber | 50.2 | 33.45 |
| Acid detergent fiber | 28.5 | 17.2 |
| Ca | 0.3 | 0.7 |
| P | 0.3 | 0.5 |
| NFC Non fiber carbohydrate | 28.0 | 40.4 |
| RDP Neutral detergent fiber | 7.0 | 7.4 |
| NFC/RDP Nonfiber carbohydrate/ Neutral detergent fiber | 4.0 | 5.5 |
| Genes 1 | Primer Sequences (5′-3′) | Gene Bank Accession no. | Amplicon (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| SIRT1 | Forward: TATGGAGTGACATAGAGTGTGCT | XM_015461011.1 | 143 | 57 |
| Reverse: GTCGCTACACCACTTCAATCC | ||||
| SREBP-1c | Forward:CGACACCACCAGCATCAACCACG | NM_001113302.1 | 119 | 62 |
| Reverse: GCAGCCCATTCATCAGCCAGACC | ||||
| ACCα | Forward: TGCTGAATATCCTCACGGAGCT | NM_174224.2 | 212 | 60 |
| Reverse: CGACGTTTCGGACAAGATGAGT | ||||
| FAS | Forward: ACAGCCTCTTCCTGTTTGACG | NM_174662.2 | 226 | 59 |
| Reverse: CTCTGCACGATCAGCTCGAC | ||||
| ACSL | Forward: TCGGAACTGAAGCCATCACC | NM_001076085.1 | 173 | 63 |
| Reverse: GCCTCGTTCCAGCAGATCAC | ||||
| CPT-1 | Forward: ACGCCGTGAAGTATAACCCT | NM_001304989.1 | 119 | 60 |
| Reverse: CCAAAAATCGCTTGTCCCTT | ||||
| CPT-2 | Forward: TGAACATCCTCTCCATCTGG | NM_001045889.2 | 188 | 58 |
| Reverse: GGTCAACAGCAACTACTACG | ||||
| ACO | Forward: TACGGAGGGATGAGGAGTGT | NM_001205495.1 | 143 | 64 |
| Reverse: TCTCAGGAAGCGAGTTTGG | ||||
| LCAD | Forward: GGTCCACAGCACAGACTTGGT | NM_001076936.1 | 151 | 56 |
| Reverse: GGAATTGGCTAGGCTTGTGATC | ||||
| L-FABP | Forward: AAGTACCAAGTCCAGACCCAG | NM_175817.3 | 111 | 61 |
| Reverse: CACGATTTCCGACACCC | ||||
| LDLR | Forward: GCTGTTCTGCCTTTCTCCTT | NM_001166530.1 | 228 | 65 |
| Reverse: ACTTTCTCCCCTGACCCTTG | ||||
| Apo-100 | Reverse: GATACTCAGAACGGAGCAAT | XM_019969506.1 | 222 | 58 |
| Forward: GCACCAATCAGATAACAGGA | ||||
| ApoE | Reverse: TCCTGAATGACCTGGGTGTTG | XM_005219148.3 | 219 | 62 |
| Forward: TCTGTGGGTTGCCGTGGTG | ||||
| MTTP | Reverse: CAGTTTGCAGCCTTGGTTCTG | NM_001101834.1 | 201 | 56 |
| Forward: TTCAAAAGCACCGAGAGCGTT | ||||
| PGC-1α | Reverse: GACCACAAATGATGACCCTC | NM_177945.3 | 123 | 60 |
| Forward: GGTTTGGCTTGTAGATGTT | ||||
| Nrf1 | Reverse: TTTTAGTAACCCTGATGGC | NM_001098002.2 | 198 | 57 |
| Forward: GCTTGCGTTGTCTGGATG | ||||
| TFAM | Reverse: TGGCACATCACAGGTAAA | NM_001034016.2 | 137 | 63 |
| Forward: GTTCCTCCCAAGATTTCA | ||||
| MnSOD | Reverse: TTCAATAAGGAGCAGGGAC | NM_201527.2 | 234 | 64 |
| Forward: CAGTGTAAGGCTGACGGTTT | ||||
| Cu/Zn SOD | Reverse: GAAGAGAGGCATGTTGGAGA | NM_174615.2 | 221 | 60 |
| Forward: CCAATTACACCACGAGCCAA | ||||
| CAT | Reverse: AGATACTCCAAGGCGAAGGTG | NM_001035386.2 | 120 | 61 |
| Forward: AAAGCCACGAGGGTCACGAAC | ||||
| GSH-Px | Reverse: GCGGGAGCAGGACTTCTACGA | NM_001101113.2 | 137 | 65 |
| Forward: CCCGATAGTGCTGGTCTGTGAA | ||||
| PPARα | Reverse: GGGTTTTCTTAGGCTTTT | NM_001034036 | 176 | 60 |
| Forward: AGTCCATCCCTGGGTTTG | ||||
| RXRα | Reverse: GGCAGATGTTGGTGACGGG | NM_001304343 | 163 | 62 |
| Forward: GGCGAGAGCGAGGTGGAGT | ||||
| β-actin | Reverse: GCCCTGAGGCTCTCTTCCA | NM_173979.3 | 101 | 59 |
| Forward:GCGGATGTCGACGTCACA |
| Parameter | Control | Fatty Liver | p-Value |
|---|---|---|---|
| DMI, kg/d | 23.17 ± 3.24 | 18.45 ± 2.84 | <0.01 |
| Milk production, kg/d | 28 ± 7 | 20 ± 6 | <0.01 |
| Milk component | |||
| Fat, % | 3.48 ± 0.35 | 3.33 ± 0.32 | 0.39 |
| Protein, % | 3.25 ± 0.21 | 2.91 ± 0.34 | <0.01 |
| Lactose, % | 4.96 ± 0.11 | 4.88 ± 0.13 | 0.91 |
| MUN 1 (mg/100 mL) | 13.76 ± 1.91 | 11.52 ± 1.43 | 0.01 |
| SCC (×103/mL) | 112.35 ± 20.23 | 365.61 ± 43.25 | <0.01 |
| Genes | Control (n = 6) | Fatty Liver (n = 6) |
|---|---|---|
| Cu/Zn SOD | 1.00 ± 0.12 | 0.31 ± 0.09 |
| CAT | 1.00 ± 0.06 | 0.21 ± 0.15 ** |
| GSH-Px | 1.00 ± 0.20 | 0.09 ± 0.06 ** |
| Mn SOD | 1.00 ± 0.13 | 0.06 ± 0.03 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zou, S.; Ding, H.; Hao, N.; Huang, Y.; Tang, J.; Cheng, J.; Feng, S.; Li, J.; Wang, X.; et al. Low Expression of Sirtuin 1 in the Dairy Cows with Mild Fatty Liver Alters Hepatic Lipid Metabolism. Animals 2020, 10, 560. https://doi.org/10.3390/ani10040560
Li Y, Zou S, Ding H, Hao N, Huang Y, Tang J, Cheng J, Feng S, Li J, Wang X, et al. Low Expression of Sirtuin 1 in the Dairy Cows with Mild Fatty Liver Alters Hepatic Lipid Metabolism. Animals. 2020; 10(4):560. https://doi.org/10.3390/ani10040560
Chicago/Turabian StyleLi, Yu, Suping Zou, Hongyan Ding, Ning Hao, Yingying Huang, Jishun Tang, Jianbo Cheng, Shibin Feng, Jinchun Li, Xichun Wang, and et al. 2020. "Low Expression of Sirtuin 1 in the Dairy Cows with Mild Fatty Liver Alters Hepatic Lipid Metabolism" Animals 10, no. 4: 560. https://doi.org/10.3390/ani10040560
APA StyleLi, Y., Zou, S., Ding, H., Hao, N., Huang, Y., Tang, J., Cheng, J., Feng, S., Li, J., Wang, X., & Wu, J. (2020). Low Expression of Sirtuin 1 in the Dairy Cows with Mild Fatty Liver Alters Hepatic Lipid Metabolism. Animals, 10(4), 560. https://doi.org/10.3390/ani10040560





