Identification of Crucial Genetic Factors, Such as PPARγ, that Regulate the Pathogenesis of Fatty Liver Disease in Dairy Cows Is Imperative for the Sustainable Development of Dairy Industry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Ethics Approval and Consent to Participate
3. Progress of the Pathogenesis Mechanism of Fatty Liver Disease
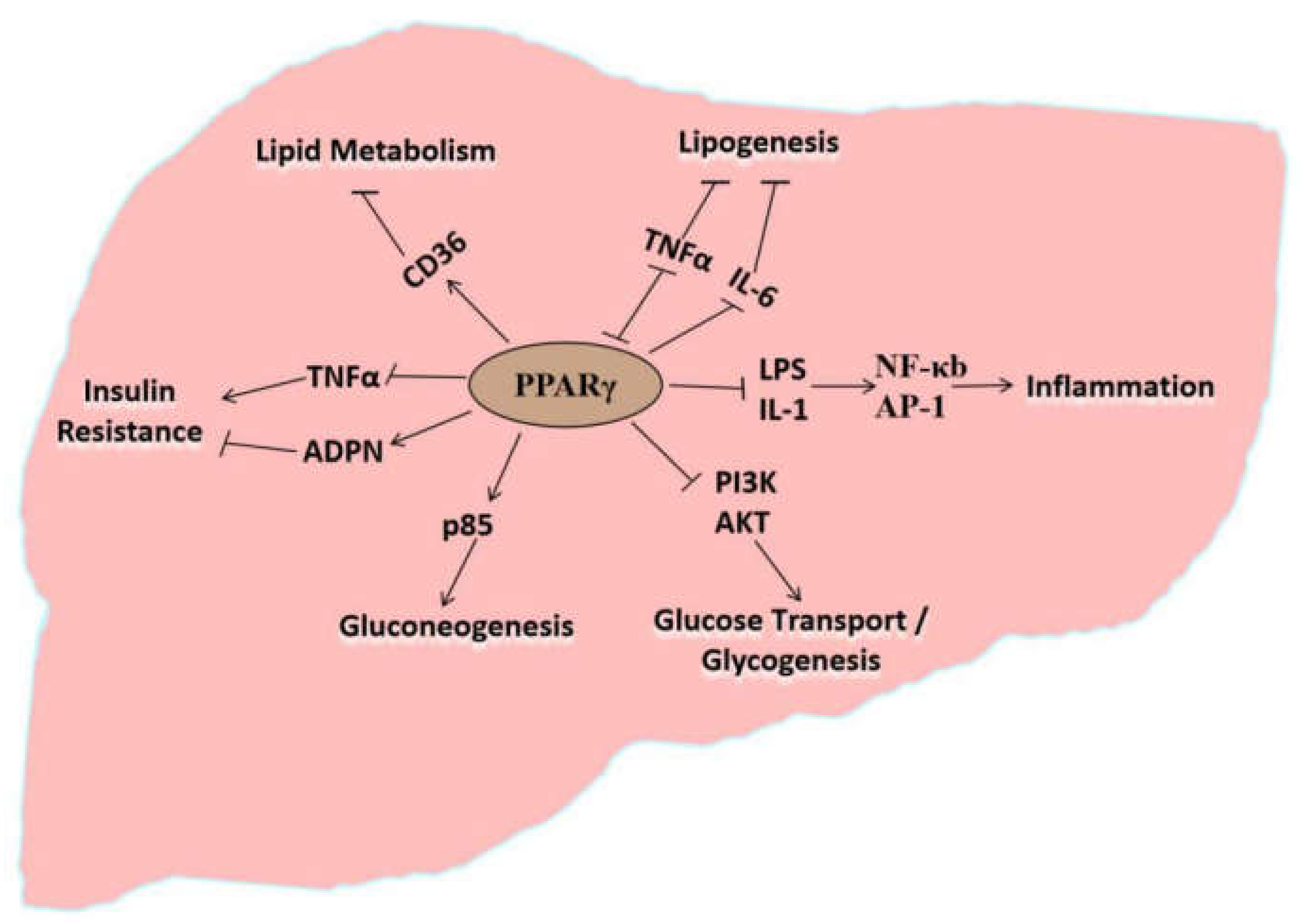
4. Molecular Regulatory Effects of PPARγ on the Pathogenesis of Fatty Liver Disease
5. PPARγ Directly Regulates Lipid Metabolism in the Liver
6. PPARγ Indirectly Participates in Lipid Metabolism via Lipid Oxidation
7. PPARγ Indirectly Participates in Lipid Metabolism via Insulin Resistance
8. PPARγ Indirectly Participates in Lipid Metabolism via Gluconeogenesis
9. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Biological Processes | Proteins or Signaling Factors That Interact with PPARγ | ||
|---|---|---|---|
| Protein Name | Protein Description or Abbreviation | References | |
| Lipid Metabolism | NRF2 | Nuclear factor E2-related factor 2 | [45] |
| CD36 | Fatty acid translocase | [66] | |
| RXR | Retinoid X receptor | [68] | |
| Oxidative Stress | NRF2 | Nuclear factor E2-related factor 2 | [45,46] |
| IFN-γ | Interferon-γ | [74] | |
| IL-1 | Interleukin-1 | [74] | |
| IL-2 | Interleukin-2 | [74] | |
| IL-6 | Interleukin-6 | [74] | |
| LPS | Lipopolysaccharide | [78] | |
| NF-κB | Nuclear factor kappa B | [79] | |
| AP-1 | Activator protein-1 | [78] | |
| STAT-1 | Signal transducers and activators of transcription 1 | [78] | |
| TNF-α | Tumor necrosis factor α | [86] | |
| Insulin Resistance | SREBP-1 | Sterol-regulatory element binding protein-1 | [86] |
| TNF-α | Tumor necrosis factor α | [86] | |
| ADPN | Adiponectin | [88] | |
| CBLB | Casitas B-lineage lymphoma | [88] | |
| SOCS3 | Suppressor of cytokine signaling 3 | [88] | |
| AKT | Protein kinase B | [89] | |
| GLUT4 | Glucose transport protein 4 | [89] | |
| PI3K | Phosphoinositide 3-kinase | [89] | |
| Endoplasmic Reticulum Stress | ATF6 | Activating Transcription Factor 6 | [29] |
| GRP78 | Glucose regulated protein 78 | [30] | |
| IRE1α | Inositol-requiring enzyme-1α | [31] | |
| NRF2 | Nuclear factor E2-related factor 2 | [45] | |
| TNF-α | Tumor necrosis factor α | [86] | |
| Gluconeogenesis | HK | Histinine kinase | [93] |
| PEPCK | Phosphoenolpyruvate carboxykinase | [93] | |
| G6P | Glucose-6-phosphate | [93] | |
| PI3K | Phosphoinositide 3-kinase | [94] | |
References
- Shi, K.R.; Niu, F.; Zhang, Q.; Ning, C.; Yue, S.J.; Hu, C.Z.; Xu, Z.J.; Wang, S.X.; Li, R.R.; Hou, Q.L.; et al. Identification of whole-genome significant single nucleotide polymorphisms in candidate genes associated with serum biochemical traits in Chinese Holstein cattle. Fornt. Genet. 2020, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.G.; Wang, Z.H.; Zhang, Q.; Yue, S.J.; Yin, B.; Jiang, Y.L.; Shi, K.R. Identification of whole-genome significant single nucleotide polymorphisms in candidate genes associated with body conformation traits in Chinese Holstein cattle. Anim Genet. 2020, 51, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Reid, I.M.; Rowlands, G.J.; Dew, A.M.; Collins, R.A.; Roberts, C.J.; Manston, R. The relationship between1 post-parturient fatty liver and blood composition in dairy cows. J. Agric. Sci.-Camb. 1983, 101, 473–480. [Google Scholar] [CrossRef]
- Starke, A.; Schmidt, S.; Haudum, A.; Scholbach, T.; Wohlsein, P.; Beyerbach, M.; Rehage, J. Evaluation of portal blood flow using transcutaneous and intraoperative Doppler ultrasonography in dairy cows with fatty liver. J. Dairy Sci. 2011, 94, 2964–2971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef]
- Godden, S.; Rapnicki, P.; Stewart, S.; Fetrow, J.; Johnson, A.; Bey, R.; Farnsworth, R. Effectiveness of an internal teat seal in the prevention of new intramammary infections during the dry and early-lactation periods in dairy cows when used with a dry cow intramammary antibiotic. J. Dairy Sci. 2003, 86, 3899–3911. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Peñagaricano, F.; Santos, J.E.P.; DeVries, T.J.; McBride, B.W.; Ribeiro, E.S. Long-term effects of postpartum clinical disease on milk production, reproduction, and culling of dairy cows. J. Dairy Sci. 2019, 102, 11701–11717. [Google Scholar] [CrossRef]
- Quenon, J.; Ingrand, S.; Magne, M. Managing the transition from purebred to rotational crossbred dairy cattle herds: Three technical pathways from a retrospective case-study analysis. Animal 2020, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Huang, W.M.; Tian, Y.J.; Cao, Z.J. Energy metabolism and its regulation of perinatal dairy cows. Prog. J. Nutr. 2012, 170–176. [Google Scholar]
- Vries, A.B.; Huang, H.W.; Zou, Y.; Cao, Z.J. Economic benefit analysis of prolonging the service life of dairy cattle. China Dairy Cattle 2014, 12, 68–75. [Google Scholar]
- Carpenter, A.J.; Ylioja, C.M.; Vargas, C.F.; Mamedova, L.K.; Mendonca, L.G.; Coetzee, J.F.; Hollis, L.C.; Gehring, R.; Bradford, B.J. Hot topic: Early postpartum treatment of commercial dairy cows with nonsteroidal antiinflammatory drugs increases whole-lactation milk yield. J. Dairy Sci. 2016, 99, 672–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, S.J.; Zhao, Y.Q.; Gu, X.R.; Yin, B.; Jiang, Y.L.; Wang, Z.H.; Shi, K.R. A genome-wide association study suggests new candidate genes for milk production traits in Chinese Holstein cattle. Anim. Genet. 2017, 48, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef]
- Katoh, N. Relevance of apolipoproteins in the development of fatty liver and fatty liver-related peripartum diseases in dairy cows. J. Vet. Med. Sci. 2002, 64, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Jorritsma, R.; Jorritsma, H.; Schukken, Y.H. Relationships between fatty liver and fertility and some periparturient diseases in commercial Dutch dairy herds. Theriogenology 2000, 54, 1065–1074. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Yin, Z.Y.; Lin, X.Y.; Yan, Z.G.; Wang, Z.H. Effects of feeding fatty acid calcium and the interaction of forage quality on production performance and biochemical indexes in early lactation cow. J. Anim. Physiol. Anim. Nutr. 2015, 99, 899–904. [Google Scholar] [CrossRef]
- Farid, A.S.; Honkawa, F.; Fath, E.M. Serum paraoxonase-1 as biomarker for improved diagnosis of fatty liver in dairy cows. BMC Vet. Res. 2013, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Pullen, D.L.; Liesman, J.S.; Emery, R.S. A species comparison of liver slice synthesis and secretion of triacylglycerol from nonesterified fatty acids in media. J. Anim. Sci. 1990, 68, 1395–1399. [Google Scholar] [CrossRef]
- Nasr, P.; Ignatova, S.; Kechagias, S.; Ekstedt, M. Natural history of nonalcoholic fatty liver diasease: A prospective follow-up study with serial biopsies. Hepatol. Res. 2018, 2, 199–210. [Google Scholar]
- Demir, M.; Lang, S.; Steffen, H.M. Nonalcoholic fatty liver disease—Current status and future directions. J. Digest Dis. 2015, 16, 541–557. [Google Scholar] [CrossRef]
- Du, H.T.; Wang, C.Y.; Wang, X.P.; Ma, M.W.; Li, F.C. The effects of dietary α-linolenic acid on growth performance, meat quality, fatty acid composition, and liver relative enzyme mRNA expression of growing meat rabbits. J. Anim. Feed Sci. 2013, 22, 122–129. [Google Scholar] [CrossRef]
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37, 81–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu. Rev. Pathol.-Mech. 2010, 5, 145–171. [Google Scholar] [CrossRef] [Green Version]
- Day, C.P. Non-alcoholic fatty liver disease: A massive problem. J. Clin. Med. 2011, 11, 176–178. [Google Scholar] [CrossRef]
- Clavien, P.; Camargo, C.; Gorczynski, R.; Washington, M.; Levy, G.; Langer, B.; Greig, P. Reasctant cytokines and neutrophil adhesion after warm ischemia in cirrhotic and noncirrhotic human livers. Hepatology 1996, 23, 1456. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak, M.; Lerotić, I.; Barsić, N.; Tomasić, V.; Virović, J.L.; Velagić, V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J. Gastroenterol. 2007, 13, 4539–4550. [Google Scholar] [CrossRef]
- Jou, J.; Choi, S.S.; Diehl, A.M. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin. Liver Dis. 2008, 28, 370–379. [Google Scholar] [CrossRef]
- Dela Peña, A.; Leclercq, I.; Field, J.; George, J.; Jones, B.; Farrell, G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology 2005, 129, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Winnay, J.N.; Boucher, J.; Mori, M.A.; Ueki, K.; Kahn, C.R. A regulatory subunit of phosphoinositide 3-kinaseincreases the nuclear accumulation of X-box binding protein-1 to modulate the unfolded protein response. Nat. Med. 2010, 16, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Bjornsson, E.; Angulo, P. Non-alcoholic fatty liver disease. Gastroenterology 2007, l42, 1023–1130. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.; Lam, V.; Han, J.; Hassler, J.; Finck, B.N.; Davidson, N.O.; Kaufman, R.J. IRE1α-XBP1S induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metabolism. 2012, 16, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, P.J.; Gaspers, L.D.; Pierobon, N.; Thomas, A.P. Calcium-dependent regulation of glucose homeostasis in the liver. Cell Calcium. 2014, 55, 306–316. [Google Scholar] [CrossRef]
- Tacer, K.F.; Rozman, D. Nonalcoholic fatty liver disease: Focus on lipoprotein and lipid deregulation. J. Lipid Res. 2011, 2011, 783976. [Google Scholar]
- Ferramosca, A.D.; Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insight and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Adams, L.A.; Angulo, P.; Lindor, K.D. Nonalcoholic fatty liver disease. Can. Med. Assoc. J. 2005, 172, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowman, J.K.; Tomlinson, J.W.; Newsome, P.N. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010, 103, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Fu, C.; Li, F. Acetate affects the process of lipid metabolism in rabbit liver, skeletal muscle and adipose tissue. Animals 2019, 9, 799. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.J.; Liu, X.F.; Zhao, X.C.; Guo, X.Q.; Kang, M.J.; Xu, B.H. Identification and antioxidant characterisation of thioredoxin-like1 from Apis cerana cerana. Apidologie 2012, 43, 737–752. [Google Scholar] [CrossRef] [Green Version]
- Rotman, Y.; Sanyal, A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 2017, 66, 180–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Loomba, R.; Rinella, A.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; Ratziu, V.; et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018, 68, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Cortez-Pinto, H. Non-alcoholic fatty liver disease and insulin resistance. Eur. J. Gastroenterol. Hepat. 2005, 17, 823–826. [Google Scholar] [CrossRef]
- Fraenkel, E.; Lazurova, I.; Feher, J. Role of lipid peroxidation in non-alcoholic steatohepatitis. Orvosi Hetilap. 2004, 145, 611–618. [Google Scholar] [PubMed]
- He, X.W.; Gao, J.; Hou, H.; Qi, Z.; Chen, H.; Zhang, X.X. Inhibition of mitochondrial fatty acid oxidation contributes to development of nonalcoholic fatty acid liver disease induced by environmental cadmium exposure. Environ. Sci. Technol. 2019, 53, 13992–14000. [Google Scholar] [CrossRef]
- Wang, S.; Dougherty, E.J.; Danner, R.L. PPARγ signaling and emerging opportunities for improved therapeutics. Pharmacol. Res. 2016, 111, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Yu, W.; Li, X.; Zhou, F.; Zhang, W.; Shen, Q.; Li, J.; Zhang, C.; Shen, P. Apigenin, a modulator of PPARgamma, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 2017, 136, 136–149. [Google Scholar] [CrossRef]
- Cheng, Q.; Jiang, S.; Huang, L.; Wang, Y.; Yang, W.; Yang, Z.; Ge, J. Effects of zearalenone-induced oxidative stress and Keap1-Nrf2 signaling pathway-related gene expression in the ileum and mesenteric lymph nodes of post-weaning gilts. Toxicology 2020, 429, 152337. [Google Scholar] [CrossRef]
- Zhu, Y.; Kan, L.; Qi, C.; Kanwar, Y.S.; Yeldandi, A.V.; Rao, M.S.; Reddy, J.K. Isolation and characterization of peroxisome proliferator-activated receptor (PPAR) interacting protein (PRIP) as a coactivator for PPAR. J. Biol. Chem. 2020, 275, 13510–13516. [Google Scholar] [CrossRef] [Green Version]
- Latruffe, N.; Vamecq, J. Peroxisome proliferators and peroxisome proliferator activated receptors (PPARs) as regulators of lipid metabolism. Biochimie 1997, 79, 81–94. [Google Scholar] [CrossRef]
- Mano, H.; Kimura, C.; Fujisawa, Y.; Kameda, T.; Watanabe-Mano, M.; Kaneko, H.; Kumegawa, M. Cloning and function of rabbit peroxisome proliferator-activated receptor delta/beta in mature osteoclasts. J. Biol. Chem. 2020, 275, 8126–8132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vamecq, J.; Latruffe, N. Medical of significance peroxisome proliferator-activated receptors. Lancet 1999, 354, 141–148. [Google Scholar] [CrossRef]
- Fu, C.Y.; Liu, L.; Li, F.C. Acetate alters the process of lipid metabolism in rabbits. Animal 2018, 12, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhang, Y.; Breyer, M.D. The Role of PPARs in the Transcriptional Control of Cellular Processes. Drug News Perspect. 2002, 15, 147–154. [Google Scholar] [CrossRef]
- Bordji, K.; Grillasca, J.P.; Gouze, J.N.; Magdalou, J.; Schohn, H.; Keller, J.M.; Terlain, B. Evidence for the presence of peroxisome proliferator-activated receptor (PPAR) alpha and gamma and retinoid Z receptor in cartilage. J. Biol. Chem. 2000, 275, 12243–12250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications a review. J. Nutr. 2014, 13, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: The “future” in dermatology therapeutics? Arch. Dermatol. Res. 2015, 307, 767–780. [Google Scholar] [CrossRef]
- Cui, J.X.; Zeng, Y.Q.; Wang, H.; Chen, W.; Du, J.F.; Chen, Q.M.; Hu, Y.X.; Yang, L. The effects of DGAT1 and DGAT2 mRNA expression on fat deposition in fatty and lean breeds of pig. Livest Sci. 2011, 140, 292–296. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Yang, D.D.; Liu, Z.L.; Zeng, Y.Q.; Chen, W. Expression of lipid metabolism genes provides new insights into intramuscular fat deposition in Laiwu pigs. Asian Aystral. J. Anim. 2020, 33, 390–397. [Google Scholar] [CrossRef]
- Filip-Ciubotaru, F.; Foia, L.; Manciuc, C.; Grigore, C. PPARs: Structure, mechanisms of action and control. Note I. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2011, 115, 477–484. [Google Scholar]
- Yu, X.L.; Guo, X.Q.; Kang, M.J.; Liu, L.; Xu, B.H. Identification and expression analysis of a putative fatty acid-binding protein gene in the Asian honeybee, Apis cerana cerana. J. Insect Sci. 2013, 13, 101. [Google Scholar] [CrossRef] [Green Version]
- Kurosaki, E.; Nakano, R.; Shimaya, A.; Yoshida, S.; Ida, M.; Suzuki, T.; Shibasaki, M.; Shikama, H. Differential effects of YM440 a hypoglycemic agent on binding to a peroxisome proliferator-activated receptor gamma and its transactivation. Biochem. Pharmacol. 2003, 65, 795–805. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.Z.; Yang, W.X.; Muhantay, G.; Chen, Y.; Xing, J.M.; Liu, J.Z. Correlation between heart-type fatty acid-binding protein gene polymorphism and mrna expression with intramuscular fat in baicheng-oil chicken. Asian Austral. J. Anim. 2015, 28, 1380. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhu, L. Effect of 24 h fasting on gene expression of AMPK, appetite regulation peptides and lipometabolism related factors in the hypothalamus of broiler chicks. Asian Austral. J. Anim. 2012, 25, 1300–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ji, R.; Sun, H.; Peng, J.; Ma, X.; Wang, C.; Fu, Y.; Bao, L.; Jin, Y. Scutellarin ameliorates nonalcoholic fatty liver disease through the PPARγ/PGC-1α-Nrf2 pathway. Free Radical. Res. 2018, 52, 198–211. [Google Scholar] [CrossRef]
- Moran-Salvador, E. Role for PPARgamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. Faseb. J. 2011, 25, 2538–2550. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.H.; Wang, X.J.; Jiao, H.C.; Lin, H. Peripheral insulin doesn’t alter appetite of broiler chicks. Asian Austral. J. Anim. 2016, 29, 1294–1299. [Google Scholar] [CrossRef] [Green Version]
- Rangwala, S.M.; Lazar, M.A. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol. Sci. 2004, 25, 331–336. [Google Scholar] [CrossRef]
- Larter, C.Z.; Yeh, M.M.; Williams, J.; Bell-Anderson, K.S.; Farrell, G.C. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J. Hepatol. 2008, 49, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Shiraishi, S.; Kishimoto, K.; Miura, S.; Ezaki, O. An increase in liver PPARgamma2 is an initial event to induce fatty liver in response to a diet high in butter: PPARgamma2 knockdown improves fatty liver induced by high-saturated fat. J. Nutr. Biochem. 2011, 22, 543–553. [Google Scholar] [CrossRef]
- Gavrilova, O.; Haluzik, M.; Matsusue, K.; Cutson, J.J.; Johnson, L.; Dietz, K.R.; Nicol, C.J.; Vinson, C.; Gonzalez, F.J.; Reitman, M.L. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 2003, 278, 34268–34276. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.R.; Meng, F.; Jia, H.H.; Guo, X.Q.; Xu, B.H. The identification and oxidative stress response of a zeta class glutathione s-transferase (GSTZ1) gene from apis cerana cerana. J. Insect Physiol. 2012, 58, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Dubaisi, S.; Fang, H.; Kocarek, T.A. Transcriptional regulation of human cytosolic sulfotransferase 1C3 by peroxisome proliferator-activated receptor gamma in LS180 human colorectal adenocarcinoma cells. Mol. Pharmacol. 2016, 90, 562–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Li, H.P.; Li, X.L.; Jiao, H.C.; Lin, H.; Ardashir, S.; Wang, Y.F.; Song, Z.G. Effects of acute heat stress on gene expression of brain-gut neuropeptides in broiler chickens (Gallus gallus domesticus). Anim. Sci. J. 2013, 91, 5194–5201. [Google Scholar]
- Khazaei, M. Effect of peroxisome proliferator-activated receptor gamma on inflammatory markers. ARYA Atheroscler 2015, 11, 261–262. [Google Scholar]
- Liu, L.; Fu, C.; Li, F.C. Dietary niacin supplementation suppressed hepatic lipid accumulation in rabbits. Asian Austral. J. Anim. 2016, 29, 1748–1755. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.S.; Zhai, R.N.; Liu, Z.L.; Lin, X.Y.; Wang, Z.H.; Hu, Z.Y. The effect of intravenous infusions of glutamine on duodenal cell autophagy and apoptosis in early-weaned calves. Animals 2019, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.N.; Dong, X.S.; Feng, L.; Li, S.L.; Hu, Z.Y. The effect of heat stress on autophagy and apotosis of rumen, abomasum, duodenum, liver and kidney cells in calves. Animals 2019, 9, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef]
- Genini, D.; Carbone, G.M.; Catapano, C.V. Multiple interactions between peroxisome proliferators-activated receptors and the ubiquitin-proteasome system and implications for cancer pathogenesis. PPAR Res. 2008, 2008, 195065. [Google Scholar] [CrossRef] [Green Version]
- Li, R.G.; Wang, X.P.; Wang, C.Y.; Ma, M.W.; Li, F.C. Growth performance, meat quality and fatty acid metabolism response of growing meat rabbits to dietary linoleic acid. Asian Austral. J. Anim. 2012, 25, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, X.J.; Jiao, H.C.; Zhao, J.P.; Lin, H. Glucocorticoids inhibited hypothalamic target of rapamycin in high fat diet-fed chicks. Poultry Sci. 2015, 94, 2221–2227. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, H.; Niu, Y.; Zhang, W.; Zhu, L.; Li, X.; Lu, S.; Fan, J.; Li, X.; Ning, G.; et al. Circulating periostin in relation to insulin resistance and nonalcoholic fatty liver disease among overweight and obese subjects. Sci. Rep. UK 2016, 6, 37886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, A.; Fairlie, D.P.; Brown, L. Lysine acetylation in obesity, diabetes and metabolic disease. Immunol. Cell Biol. 2012, 90, 39–46. [Google Scholar] [CrossRef]
- Garcia-Roche, M.; Casal, A.; Mattiauda, D.A.; Ceriani, M.; Jasinsky, A.; Mastrogiovanni, M.; Trostchansky, A.; Carriquiry, M.; Cassina, A.; Quijiano, C. Impaired hepatic mitochondrial function during early lactation in dairy cows: Association with protein lysine acetylation. PLoS ONE 2019, 14, e0213780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Q.; Zhou, F.Y.; Bai, M.Y.; Liu, Y.; Zhang, L.; Zhu, Q.; Bi, Y.; Ning, G.; Zhou, L.; Wang, X. The pivotal role of protein acetylation in linking glucose and fatty acid metabolism to β-cell function. Cell Death Dis. 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalloyer, F.; Staels, B. Fibrates, glitazones, and peroxisome proliferator-activated receptors. Arterioscl. Throm. Vas. 2010, 30, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.J. Expression of androgen receptor and estrogen receptor-alpha in the developing pituitary gland of male sheep lamb. Anim. Reprod Sci. 2011, 127, 164–168. [Google Scholar]
- Shi, H.; Cave, B.; Inouye, K.; Bjorbaek, C.; Flier, J.S. Overexpression of suppressor of cytokine signaling 3 in adipose tissue causes local but not systemic insulin resistance. Diabetes 2006, 55, 699–707. [Google Scholar] [CrossRef] [Green Version]
- Armoni, M.; Kritz, N.; Harel, C.; Bar-Yoseph, F.; Chen, H.; Quon, M.J.; Karnieli, E. Peroxisome proliferator-activated receptor-gamma represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. J. Biol. Chem. 2003, 278, 30614–30623. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.J.; Zhou, M.; Huang, L.B.; Yang, W.R.; Yang, Z.B.; Jiang, S.Z.; Ge, J.S. Zearalenone promotes follicle growth through modulation of Wnt-1/β-catenin signaling pathway and expression of estrogen receptor genes in ovaries of post-weaning piglets. J. Agr. Food Chem. 2018, 66, 7899–7906. [Google Scholar] [CrossRef]
- Moller, D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2011, 414, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.G.; Lin, X.Y.; Yan, Z.G.; Hu, Z.Y.; Wang, Y.; Wang, Z.H. Effects of forage source and particle size on feed sorting, milk production and nutrient digestibility in lactating dairy cows. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1472–1481. [Google Scholar] [CrossRef]
- Goto, M.; Yoshioka, T.; Battelino, T.; Ravindranath, T.; Zeller, W.P. TNFalpha decreases gluconeogenesis in hepatocytes isolated from 10-day-old rats. Pediatr. Res. 2001, 49, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Bo, Q.F.; Sun, X.M.; Liu, J.; Sui, X.M.; Li, G.X. Antitumor action of the peroxisome proliferator-activated receptor-γ agonist rosiglitazone in hepatocellular carcinoma. Oncol. Lett. 2015, 10, 3554. [Google Scholar] [CrossRef] [Green Version]
- Tong, E.K.; England, L.; Glantz, S.A. Changing conclusions on secondhand smoke in a sudden infant death syndrome review funded by the tobacco industry. Pediatrics 2005, 115, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Lou, X.M.; Li, J.; Zhang, X.X.; Wang, J.M.; Wang, C.F. Variations in fatty acid composition of Laoshan goat milk from partum to 135 days postpartum. Anim. Sci. J. 2018, 89, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Anania, C.; Perla, F.M.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean diet and nonalcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 2083–2094. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Li, R.; Xu, Z.; Zhang, Q. Identification of Crucial Genetic Factors, Such as PPARγ, that Regulate the Pathogenesis of Fatty Liver Disease in Dairy Cows Is Imperative for the Sustainable Development of Dairy Industry. Animals 2020, 10, 639. https://doi.org/10.3390/ani10040639
Shi K, Li R, Xu Z, Zhang Q. Identification of Crucial Genetic Factors, Such as PPARγ, that Regulate the Pathogenesis of Fatty Liver Disease in Dairy Cows Is Imperative for the Sustainable Development of Dairy Industry. Animals. 2020; 10(4):639. https://doi.org/10.3390/ani10040639
Chicago/Turabian StyleShi, Kerong, Ranran Li, Zhongjin Xu, and Qin Zhang. 2020. "Identification of Crucial Genetic Factors, Such as PPARγ, that Regulate the Pathogenesis of Fatty Liver Disease in Dairy Cows Is Imperative for the Sustainable Development of Dairy Industry" Animals 10, no. 4: 639. https://doi.org/10.3390/ani10040639





