Can Isoflurane and Meloxicam Mitigate Pain Associated with Cautery Disbudding of 3-Week-Old Goat Kids?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Experimental Design
2.3. Blood Sampling
2.4. Rectal Temperature
2.5. Body Weight
2.6. Behaviour
2.7. Statistical Analysis
3. Results
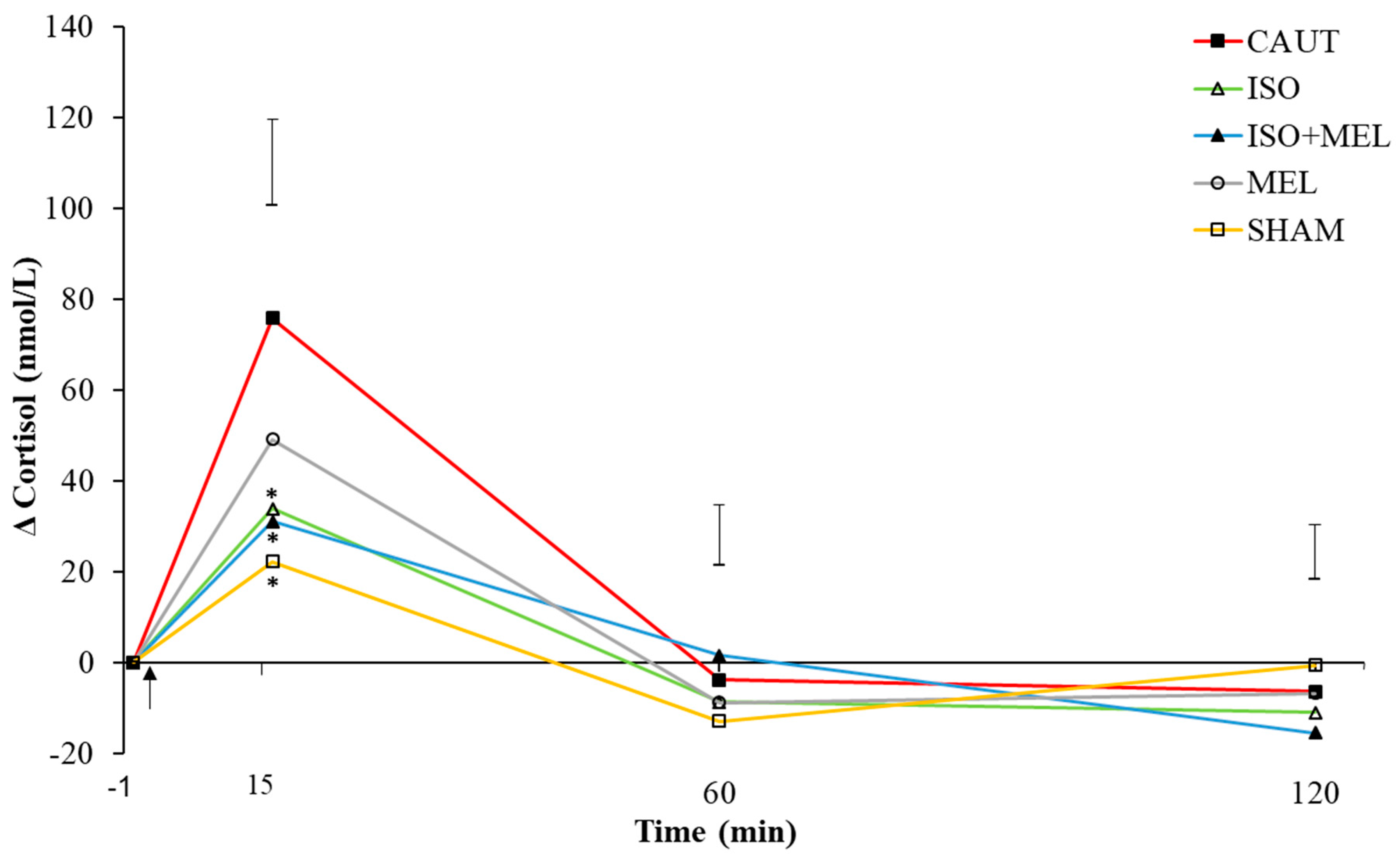
3.1. Cortisol, Lactate and Glucose Concentrations
3.2. Rectal Temperature
3.3. Body Weight
3.4. Behaviour
3.4.1. 1-h Period
3.4.2. 5-min Periods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waiblinger, S.; Schmied-Wagner, C.; Mersmann, D.; Nordmann, E. Social behaviour and injuries in horned and hornless dairy goats. In Proceedings of the XVth International Congress of the International Society for Animal Hygiene, Vienna, Austria, 3–7 July 2011; pp. 421–422. [Google Scholar]
- Smith, M.C.; Sherman, D.M. Dehorning and descenting. In Goat Medicine, 2nd ed.; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 723–731. [Google Scholar] [CrossRef]
- Loretz, C.; Wechsler, B.; Hauser, R.; Rusch, P. A comparison of space requirements of horned and hornless goats at the feed barrier and in the lying area. Appl. Anim. Behav. Sci. 2004, 87, 275–283. [Google Scholar] [CrossRef]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Zobel, G.; Cave, V.M.; Julian, A.F.; Sutherland, M.A. Pain sensitivity and injury associated with three methods of disbudding goat kids: Cautery, cryosurgical and caustic paste. Vet. J. 2018, 239, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; De Luna, J.B.; Gamboa, D.; Reyes, M.; Sánchez, A.; Terrazas, A.; Rojas, S.; Galindo, F. Cortisol and pain-related behavior in disbudded goat kids with and without cornual nerve block. Physiol. Behav. 2015, 138, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.; Gutierrez, J. A first description of the physiological and behavioural responses to disbudding in goat kids. Anim. Welf. 2010, 19, 55–59. [Google Scholar]
- Alvarez, L.; Nava, R.A.; Ramirez, A.; Ramirez, E.; Gutierrez, J. Physiological and behavioural alterations in disbudded goat kids with and without local anaesthesia. Appl. Anim. Behav. Sci. 2009, 117, 190–196. [Google Scholar] [CrossRef]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Cave, V.M.; Sutherland, M.A. Evaluation of alternatives to cautery disbudding of dairy goat kids using physiological measures of immediate and longer-term pain. J. Dairy Sci. 2018, 101, 5374–5387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Dowling, S.K.; Cave, V.M.; Lowe, G.L.; Sutherland, M.A. Effect of isoflurane alone or in combination with meloxicam on the behavior and physiology of goat kids following cautery disbudding. J. Dairy Sci. 2018, 101, 3193–3204. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.M. The scientific assessment of animal welfare. Appl. Anim. Behav. Sci. 1988, 20, 5–19. [Google Scholar] [CrossRef]
- Allen, K.A.; Coetzee, J.F.; Edwards-Callaway, L.N.; Glynn, H.; Dockweiler, J.; KuKanich, B.; Lin, H.; Wang, C.; Fraccaro, E.; Jones, M.; et al. The effect of timing of oral meloxicam administration on physiological responses in calves after cautery dehorning with local anesthesia. J. Dairy Sci. 2013, 96, 5194–5205. [Google Scholar] [CrossRef]
- Faulkner, P.M.; Weary, D.M. Reducing pain after dehorning in dairy calves. J. Dairy Sci. 2000, 83, 2037–2041. [Google Scholar] [CrossRef]
- Graf, B.; Senn, M. Behavioural and physiological responses of calves to dehorning by heat cauterization with or without local anaesthesia. Appl. Anim. Behav. Sci. 1999, 62, 153–171. [Google Scholar] [CrossRef]
- Ingvast-Larsson, C.; Hogberg, M.; Mengistu, U.; Olsen, L.; Bondesson, U.; Olsson, K. Pharmacokinetics of meloxicam in adult goats and its analgesic effect in disbudded kids. J. Vet. Pharmacol. Ther. 2011, 34, 64–69. [Google Scholar] [CrossRef]
- Mellor, D.J.; Cook, C.J.; Stafford, K.J. Quantifying some responses to pain as a stressor. In The Biology of Animal Stress; Moberg, G.P., Mench, J.A., Eds.; CABI: Wallingford, UK, 2000; pp. 173–198. [Google Scholar]
- Brown, S.N.; Warriss, P.D.; Nute, G.R.; Edwards, J.E.; Knowles, T.G. Meat quality in pigs subjected to minimal preslaughter stress. Meat Sci. 1998, 49, 257–265. [Google Scholar] [CrossRef]
- Hambrecht, E.; Eissen, J.J.; Nooijen, R.I.J.; Ducro, B.J.; Smits, C.H.M.; Den Hartog, L.A.; Verstegen, M.W.A. Preslaughter stress and muscle energy largely determine pork quality at two commercial processing plants. J. Anim. Sci. 2004, 82, 1401–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khani, S.; Tayek, J.A. Cortisol increases gluconeogenesis in humans: Its role in the metabolic syndrome. Clin. Sci. 2001, 101, 739–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Cave, V.M.; Sutherland, M.A. Behavioural response of dairy goat kids to cautery disbudding. Appl. Anim. Behav. Sci. 2017, 194, 42–47. [Google Scholar] [CrossRef]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Cave, V.M.; Sutherland, M.A. Evaluation of alternatives to cautery disbudding of dairy goat kids using behavioural measures of post-treatment pain. Appl. Anim. Behav. Sci. 2018, 206, 32–38. [Google Scholar] [CrossRef]
- Stock, M.L.; Baldridge, S.L.; Griffin, D.; Coetzee, J.F. Bovine dehorning: Assessing pain and providing analgesic management. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 103–133. [Google Scholar] [CrossRef]
- Ajuda, I.; Battini, M.; Mattiello, S.; Arcuri, C.; Stilwell, G. Evaluation of Pain Mitigation Strategies in Goat Kids after Cautery Disbudding. Animals 2020, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Nfor, O.N.; Chan, J.P.W.; Kere, M.; Peh, H.C. Disbudding pain: The benefits of disbudding goat kids with dexmedetomidine hydrochloride. Small Rumin. Res. 2016, 139, 60–66. [Google Scholar] [CrossRef]
- Dzikiti, T.B.; Stegmann, G.F.; Dzikiti, L.N.; Hellebrekers, L.J. Effects of midazolam on isoflurane minimum alveolar concentration in goats. Small Rumin. Res. 2011, 97, 104–109. [Google Scholar] [CrossRef] [Green Version]
- McEwen, M.M.; Gleed, R.D.; Ludders, J.W.; Stokol, T.; Del Piero, F.; Erb, H.N. Hepatic effects of halothane and isoflurane anesthesia in goats. J. Am. Vet. Med. Assoc. 2000, 217, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Dugdale, A. Veterinary Anaesthesia: Principles to Practice; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Riebold, T.W. Ruminants. In Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones, 5th ed.; Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A., Robertson, S.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 912–924. [Google Scholar]
- Dahl, J.B.; Kehlet, H. Non-steroidal anti-inflammatory drugs: Rationale for use in severe postoperative pain. Br. J. Anaesth. 1991, 66, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Del Tacca, M.; Colucci, R.; Fornai, M.; Blandizzi, C. Efficacy and tolerability of meloxicam, a COX-2 preferential nonsteroidal anti-inflammatory drug—A review. Clin. Drug Investig. 2002, 22, 799–818. [Google Scholar] [CrossRef]
- Dove, W.F. The physiology of horn growth: A study of the morphogenesis, the interaction of tissues, and the evolutionary processes of a mendelian recessive character by means of transplantation of tissues. J. Exp. Zool. 1935, 69, 347–405. [Google Scholar] [CrossRef]
- Hull, B.L. Dehorning the adult goat. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 183–185. [Google Scholar] [CrossRef]
- McMeekan, C.M.; Stafford, K.J.; Mellor, D.J.; Bruce, R.A.; Ward, R.N.; Gregory, N.G. Effects of regional analgesia and/or a non-steroidal anti-inflammatory analgesic on the acute cortisol response to dehorning in calves. Res. Vet. Sci. 1998, 64, 147–150. [Google Scholar] [CrossRef]
- Sylvester, S.P.; Mellor, D.J.; Stafford, K.J.; Bruce, R.A.; Ward, R.N. Acute cortisol responses of calves to scoop dehorning using local anaesthesia and/or cautery of the wound. Aust. Vet. J. 1998, 76, 118–122. [Google Scholar] [CrossRef]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Cave, V.M.; Turner, A.R.; Sutherland, M.A. The effectiveness of clove oil and two different cautery disbudding methods on preventing horn growth in dairy goat kids. PLoS ONE 2018, 13, e0198229. [Google Scholar] [CrossRef] [Green Version]
- Sanford, S.E. Meningoencephalitis caused by thermal disbudding in goat kids. Can. Vet. J. 1989, 30, 832. [Google Scholar]
- Thompson, K.G.; Bateman, R.S.; Morris, P.J. Cerebral infarction and meningoencephalitis following hot-iron disbudding of goat kids. N. Zea. Vet. J. 2005, 53, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.J.; Adams, D.S.; Trigo, F.J. Meningoencephalitis after hot-iron disbudding of goat kids. Vet. Med. Small Anim. Clin. 1983, 78, 599–601. [Google Scholar]
- Caray, D.; de Boyer des Roches, A.; Frouja, S.; Andanson, S.; Veissier, I. Hot-iron disbudding: Stress responses and behavior of 1- and 4-week-old calves receiving anti-inflammatory analgesia without or with sedation using xylazine. Livest. Sci. 2015, 179, 22–28. [Google Scholar] [CrossRef]
- Mirra, A.; Spadavecchia, C.; Bruckmaier, R.; Gutzwiller, A.; Casoni, D. Acute pain and peripheral sensitization following cautery disbudding in 1- and 4-week-old calves. Physiol. Behav. 2018, 184, 248–260. [Google Scholar] [CrossRef]
- Casoni, D.; Mirra, A.; Suter, M.R.; Gutzwiller, A.; Spadavecchia, C. Can disbudding of calves (one versus four weeks of age) induce chronic pain? Physiol. Behav. 2019, 199, 47–55. [Google Scholar] [CrossRef]
- Ting, S.T.L.; Earley, B.; Veissier, I.; Gupta, S.; Crowe, M.A. Effects of age of Holstein-Friesian calves on plasma cortisol, acute-phase proteins, immunological function, scrotal measurements and growth in response to Burdizzo castration. Anim. Sci. 2005, 80, 377–386. [Google Scholar] [CrossRef]
- Offinger, J.; Meyer, H.; Fischer, J.; Kastner, S.B.; Piechotta, M.; Rehage, J. Comparison of isoflurane inhalation anaesthesia, injection anaesthesia and high volume caudal epidural anaesthesia for umbilical surgery in calves; metabolic, endocrine and cardiopulmonary effects. Vet. Anaesth. Analg. 2012, 39, 123–136. [Google Scholar] [CrossRef]
- Antognini, J.F.; Eisele, P.H. Anesthetic potency and cardiopulmonary effects of enflurane, halothane, and isoflurane in goats. Lab. Anim. Sci. 1993, 43, 607–610. [Google Scholar]
- Lin, H.-C. Comparative anesthesia and analgesia of ruminants and swine. In Veterinary Anesthesia and Analgesia: The Fifth Edition of Lumb and Jones; Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A., Robertson, S.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 743–753. [Google Scholar]
- Heinrich, A.; Duffield, T.F.; Lissemore, K.D.; Millman, S.T. The effect of meloxicam on behavior and pain sensitivity of dairy calves following cautery dehorning with a local anesthetic. J. Dairy Sci. 2010, 93, 2450–2457. [Google Scholar] [CrossRef] [Green Version]
- Theurer, M.E.; White, B.J.; Coetzee, J.F.; Edwards, L.N.; Mosher, R.A.; Cull, C.A. Assessment of behavioral changes associated with oral meloxicam administration at time of dehorning in calves using a remote triangulation device and accelerometers. BMC Vet. Res. 2012, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Todd, C.G.; Millman, S.T.; McKnight, D.R.; Duffield, T.F.; Leslie, K.E. Nonsteroidal anti-inflammatory drug therapy for neonatal calf diarrhea complex: Effects on calf performance. J. Anim. Sci. 2010, 88, 2019–2028. [Google Scholar] [CrossRef] [PubMed]

| Behaviour | Description |
|---|---|
| Head shaking | Rapid continuous tilting of the head from side to side concluding with a return to neutral position. Head shakes separated by >1 s were considered separate events. |
| Head scratching | The rear foot touches any part of the head or neck (including collar). Scratches separated by >1 s were considered separate events. |
| Body shaking | Hackles on the back are raised and body shakes from side to side concluding at a return to neutral position. Body shakes separated by >1 s were considered separate events. |
| Self-grooming | The kid’s muzzle contacts any part of the body or legs (excluding hoof) with a rhythmic back and forth motion. A separate grooming event was considered to occur after a pause of >1 s. |
| Feeding | The mouth covers at least half of the nipple of the feeding bucket for >3 s, usually followed by suckling motions. Repetitions following separation of the mouth from the nipple of >3 s were considered separate events. |
| Plasma Concentrations | Time (min) | ||
|---|---|---|---|
| 15 | 60 | 120 | |
| a Δ Glucose (mmol/L) | |||
| CAUT | <−0.1 | <−0.1 | 0.2 |
| ISO | <0.1 | 0.2 | 0.5 |
| ISO + MEL | −0.8 | −0.7 | −0.1 |
| MEL | 0.5 | 0.2 | 0.5 |
| SHAM | 0.6 | 0.3 | 0.8 |
| Max SED | 0.4 | 0.5 | 0.4 |
| b Δ Lactate (mmol/L) | |||
| CAUT | 0.2 | <−0.1 | −0.1 |
| ISO | −0.2 | 0.1 | −0.2 |
| ISO + MEL | −0.3 | −0.2 | −0.2 |
| MEL | 0.4 | 0.1 | 0.3 |
| SHAM | −0.1 | −0.6 | −0.5 |
| Max SED | 0.4 | 0.4 | 0.3 |
| Behaviours | CAUT | ISO | ISO + MEL | MEL | SHAM | SED |
|---|---|---|---|---|---|---|
| Head shaking (No./h) | 24.4 | 12.6 | 15.7 | 20.5 | 18.0 | 5.1 |
| Body shaking (No./h) | 1.9 | 1.1 | 2.9 | 1.6 | 2.7 | 0.9 |
| Head scratching (No./h) | 10.6 | 9.8 | 7.1 | 7.0 | 4.9 | 4.5 |
| Self-grooming (No./h) | 2.9 | −0.8 | 3.0 | 4.1 | 5.2 | 3.3 |
| Feeding (No./h) | 4.0 a,b | −1.7 a | 5.8 a,b | 9.9 b | 0.7a | 3.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hempstead, M.N.; Waas, J.R.; Stewart, M.; Cave, V.M.; Sutherland, M.A. Can Isoflurane and Meloxicam Mitigate Pain Associated with Cautery Disbudding of 3-Week-Old Goat Kids? Animals 2020, 10, 878. https://doi.org/10.3390/ani10050878
Hempstead MN, Waas JR, Stewart M, Cave VM, Sutherland MA. Can Isoflurane and Meloxicam Mitigate Pain Associated with Cautery Disbudding of 3-Week-Old Goat Kids? Animals. 2020; 10(5):878. https://doi.org/10.3390/ani10050878
Chicago/Turabian StyleHempstead, Melissa N., Joseph R. Waas, Mairi Stewart, Vanessa M. Cave, and Mhairi A. Sutherland. 2020. "Can Isoflurane and Meloxicam Mitigate Pain Associated with Cautery Disbudding of 3-Week-Old Goat Kids?" Animals 10, no. 5: 878. https://doi.org/10.3390/ani10050878
APA StyleHempstead, M. N., Waas, J. R., Stewart, M., Cave, V. M., & Sutherland, M. A. (2020). Can Isoflurane and Meloxicam Mitigate Pain Associated with Cautery Disbudding of 3-Week-Old Goat Kids? Animals, 10(5), 878. https://doi.org/10.3390/ani10050878





