Intraspecific Hybrids Versus Purebred: A Study of Hatchery-Reared Populations of Sterlet Acipenser ruthenus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Parental Populations
2.3. Sampling for the Assessment of Population Divergence
2.4. Broodstock Handling and Hormone Induction
2.5. Fertilization and Hatching
2.6. Rearing of Progeny Groups
2.7. Measurement of Performance and Mean Heterosis
2.8. Microsatellite Marker Analysis
2.9. Statistical Analysis
3. Results
3.1. Population Genetic Analysis of Danube and Volga Sterlet
3.2. Performance Comparison of Purebreds and Hybrids
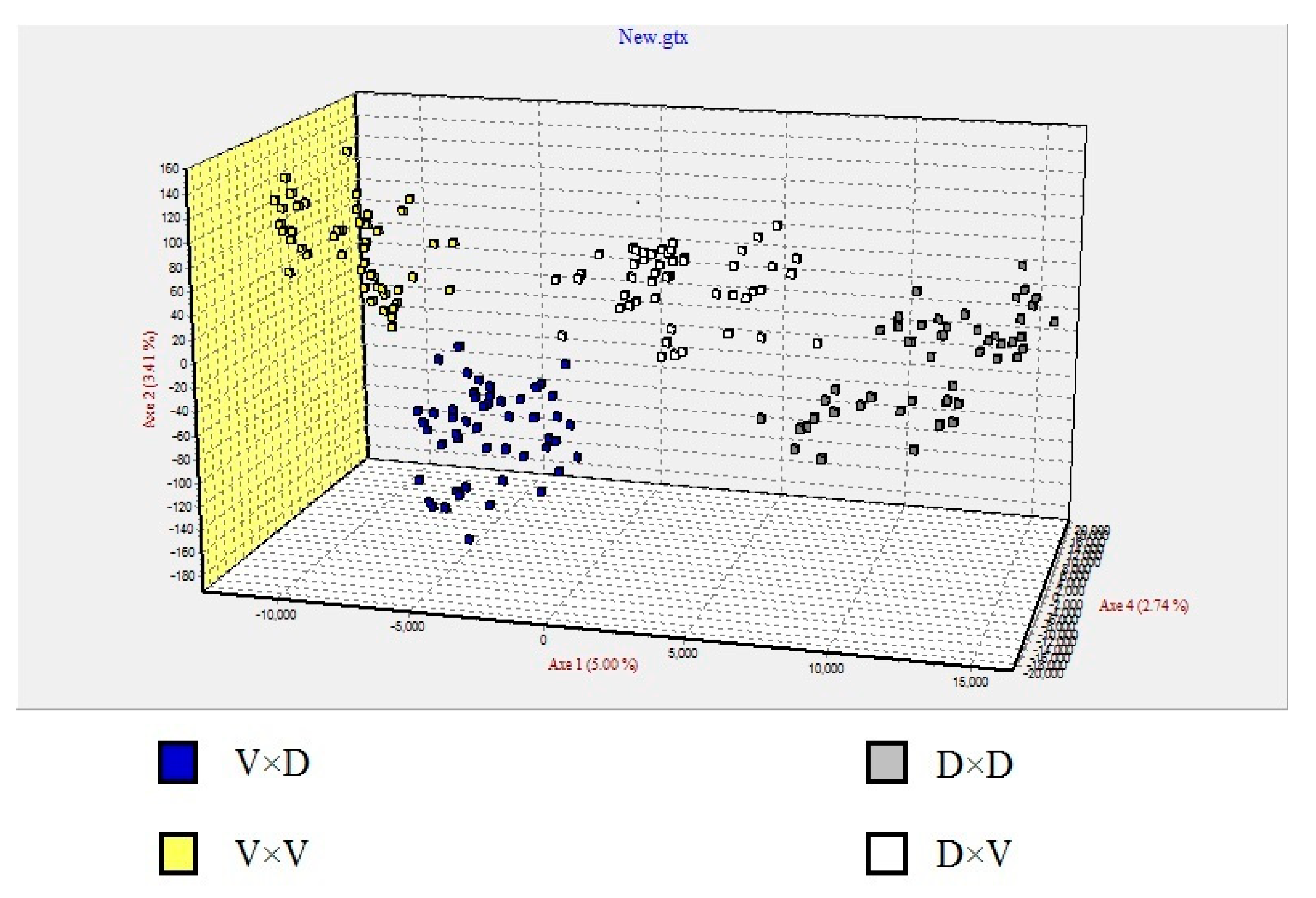
3.3. Microsatellite Marker Analysis
4. Discussion
4.1. Genetic Analysis of Hatchery-Reared Populations of Volga and Danube Sterlet
4.2. Fitness-Related Traits of the Progeny Groups
4.3. Genetic Analysis of the Progeny Groups
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grande, L.; Bemis, W.E. Osteology and Phylogenetic Relationships of Fossil and Recent Paddlefishes (Polyodontidae) with Comments on the Interrelationships of Acipenseriformes. J. Vertebr. Paleontol. 1991, 11, 1–121. [Google Scholar] [CrossRef]
- Bronzi, P.; Chebanov, M.; Michaels, J.T.; Wei, Q.; Rosenthal, H.; Gessner, J. Sturgeon meat and caviar production: Global update 2017. J. Appl. Ichthyol. 2019, 35, 257–266. [Google Scholar] [CrossRef]
- IUCN—The International Union for Conservation of Nature 2013. IUCN Red List of Threatened Species; Version 2013.2; IUCN: Gland, Switzerland, 2013. [Google Scholar]
- Berg, L.S. Ryby Presnykh vod SSSR i Sopredelnykh Stran; AN SSSR; Cz: Moskwa, Russia, 1948; p. 467. [Google Scholar]
- Sokolov, L.I.; Vasil’ev, V.P. Acipenser ruthenus Linneaus, 1758. In The Freshwater Fishes of Europe, Vol. 1, Part II, General Introduction to Fishes, Acipenseriformes; Holčik, J., Ed.; AULA-Verlag: Wiesbaden, Germany, 1989; pp. 227–262. [Google Scholar]
- Fopp-Bayat, D.; Kuzniar, P.; Kolman, R.; Liszewski, T.; Kucinski, M. Genetic analysis of six sterlet (Acipenser ruthenus) populations-recommendations for the plan of restitution in the Dniester River. Iran. J. Fish. Sci. 2015, 14, 634–645. [Google Scholar]
- Chebanov, M.; Billard, R. The culture of sturgeons in Russia: Production of juveniles for stocking and meat for human consumption. Aquat. Living Resour. 2001, 14, 375–381. [Google Scholar] [CrossRef]
- Jarić, I.; Gessner, J. Analysis of publications on sturgeon research between 1996 and 2010. Scientometrics 2011, 90, 715–735. [Google Scholar] [CrossRef]
- Reinartz, R. Sturgeons in the Danube River: Biology, Status, Conservation; Literature Study; IAD: Vienna, Austria, 2002. [Google Scholar]
- Birstein, V.J.; Waldman, J.R.; Bemis, W.E. Sturgeon Biodiversity and Conservation; Springer Science & Business Media: Berlin, Germany, 2006; Volume 17. [Google Scholar]
- Bloesch, J.; Jones, T.; Reinartz, R.; Striebel, B. An action plan for the conservation of sturgeons (Acipenseridae) in the Danube River Basin. Österr. Wasser-und Abfallwirtsch. 2006, 58, 81–88. [Google Scholar] [CrossRef]
- Guti, G.; Gaebele, T. Long-term changes of sterlet (Acipenser ruthenus) population in the Hungarian section of the Danube. Opusc. Zool. Budapest. 2009, 40, 17–25. [Google Scholar]
- Bloesch, J. Major obstacles for Danube sturgeon spawning migration: The Iron Gate dams and the navigation project in the lower Danube. Danube News 2016, 33, 11–13. [Google Scholar]
- Reinartz, R.; Peterí, A.; Friedrich, T.; Sandu, C. Ex-situ conservation for Danube River sturgeons—Concept, facts and outlook. Danube News 2016, 33, 6–7. [Google Scholar]
- Friedrich, T. Danube Sturgeons: Past and Future. In Riverine Ecosystem Management: Science for Governing towards a Sustainable Future; Schmutz, S., Sendzimir, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 8, pp. 507–518. [Google Scholar]
- Reinartz, R.; Lippold, S.; Lieckfeldt, D.; Ludwig, A. Population genetic analyses of Acipenser ruthenus as a prerequisite for the conservation of the uppermost Danube population. J. Appl. Ichthyol. 2011, 27, 477–483. [Google Scholar] [CrossRef]
- Robinson, Z.L.; Coombs, J.A.; Hudy, M.; Nislow, K.H.; Letcher, B.H.; Whiteley, A.R. Experimental test of genetic rescue in isolated populations of brook trout. Mol. Ecol. 2017, 26, 4418–4433. [Google Scholar] [CrossRef] [PubMed]
- Drauch, A.M.; Rhodes, O.E., Jr. Genetic evaluation of the lake sturgeon reintroduction program in the Mississippi and Missouri Rivers. N. Am. J. Fish. Manag. 2007, 27, 434–442. [Google Scholar] [CrossRef]
- Schreier, A.D.; Rodzen, J.; Ireland, S.; May, B. Genetic techniques inform conservation aquaculture of the endangered Kootenai River white sturgeon Acipenser transmontanus. Endanger. Species Res. 2012, 16, 65–75. [Google Scholar] [CrossRef]
- Gessner, J.; Arndt, G.M.; Fredrich, F.; Ludwig, A.; Kirschbaum, F.; Bartel, R.; von Nordheim, H. Remediation of Atlantic Sturgeon Acipenser oxyrinchus in the Oder River: Background and First Results. In Biology and Conservation of the European Sturgeon Acipenser sturio L. 1758; Williot, P., Rochard, E., Desse-Berset, N., Kirschbaum, F., Gessner, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; p. 663. [Google Scholar]
- Boscari, E.; Barmintseva, A.; Pujolar, J.M.; Doukakis, P.; Mugue, N.; Congiu, L. Species and hybrid identification of sturgeon caviar: A new molecular approach to detect illegal trade. Mol. Ecol. Resour. 2014, 14, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.W.; Bradburd, G.S.; Kremer, C.T.; Salerno, P.E.; Angeloni, L.M.; Funk, W.C. Genomic and Fitness Consequences of Genetic Rescue in Wild Populations. Curr. Biol. 2020, 30, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Facon, B.; Pointier, J.P.; Jarne, P.; Sarda, V.; David, P. High genetic variance in life-history strategies within invasive populations by way of multiple introductions. Curr. Biol. 2008, 18, 363–367. [Google Scholar] [CrossRef]
- Lippman, Z.B.; Zamir, D. Heterosis: Revisiting the magic. Trends Genet. 2007, 23, 60–66. [Google Scholar] [CrossRef]
- Rius, M.; Darling, J.A. How important is intraspecific genetic admixture to the success of colonising populations? Trends Ecol. Evol. 2014, 29, 233–242. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Eldridge, M.D.B.; Lacy, R.C.; Ralls, K.; Dudash, M.R.; Fenster, C.B. Predicting the Probability of Outbreeding Depression. Conserv. Biol. 2011, 25, 465–475. [Google Scholar] [CrossRef]
- Audet, C.L.; Wilson, C.C.; Pitcher, T.E. Effects of intraspecific hybridisation between two hatchery-reared strains of Atlantic salmon, Salmo salar, on juvenile survival and fitness-related traits. Fish. Manag. Ecol. 2017, 24, 1–9. [Google Scholar] [CrossRef]
- Edmands, S. Hybrid vigour and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evolution 1999, 53, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Edmands, S. Between a rock and a hard place: Evaluating the relative risks of inbreeding and outbreeding for conservation and management. Mol. Ecol. 2007, 16, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Gela, D.; Rodina, M.; Linhart, O. Řízená Reprodukce Jeseterů [The Artificial Reproduction of the Sturgeons (Acipenser)]; Methodology Edition (Technology Series); Research Institute of Fish Culture and Hydrobiology University of South Bohemia: Vodňany, Czech Republic, 2008; p. 24. ISBN 978-80-85887-62-4. [Google Scholar]
- Štěch, L.; Linhart, O.; Shelton, W.L.; Mims, S.D. Minimally invasive surgical removal of ovulated eggs of paddlefish (Polyodon spathula). Aquac. Int. 1999, 7, 129–133. [Google Scholar] [CrossRef]
- Dettlaff, T.A.; Ginzburg, A.S.; Schmalhausen, O.I. Sturgeon Fishes: Developmental Biology and Aquaculture; Springer Science & Business Media: London, UK, 1993; p. 313. [Google Scholar]
- Börk, K.; Drauch, A.; Israel, J.A.; Pedroia, J.; Rodzen, J.; May, B. Development of new microsatellite primers for green sturgeon and white sturgeon. Conserv. Genet. 2008, 9, 973–979. [Google Scholar] [CrossRef]
- Welsh, A.B.; Blumberg, M.; May, B. Identification of microsatellite loci in lake sturgeon, Acipenser fulvescens, and their variability in green sturgeon, A. medirostris. Mol. Ecol. Notes 2003, 3, 47–55. [Google Scholar] [CrossRef]
- King, T.L.; Lubinski, B.A.; Spidle, A.P. Microsatellite DNA variation in Atlantic sturgeon (Acipenser oxyrinchus oxyrinchus) and cross-species amplification in the Acipenseridae. Conserv. Genet. 2001, 2, 103–119. [Google Scholar] [CrossRef]
- McQuown, E.C.; Sloss, B.L.; Sheehan, R.J.; Rodzen, J.; Tranah, G.J.; May, B. Microsatellite analysis of genetic variation in sturgeon: New primer sequences for Scaphirhynchus and Acipenser. Trans. Am. Fish. Soc. 2000, 129, 1380–1388. [Google Scholar] [CrossRef]
- Havelka, M.; Hulák, M.; Rodina, M.; Flajšhans, M. First evidence of autotriploidization in sterlet (Acipenser ruthenus). J. Appl. Genet. 2013, 54, 201–207. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. Genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Kaczmarczyk, D.; Fopp-Bayat, D. Assemblage of spawning pairs based on their individual genetic profiles—As tool for maintaining genetic variation within sturgeon populations. Aquac. Res. 2013, 44, 677–682. [Google Scholar] [CrossRef]
- Bartley, D.M.; Rana, K.; Immink, A.J. The use of inter-specific hybrids in aquaculture and fisheries. Rev. Fish Biol. Fish. 2001, 10, 325–337. [Google Scholar] [CrossRef]
- Shivaramu, S.; Vuong, D.T.; Havelka, M.; Šachlová, H.; Lebeda, I.; Kašpar, V.; Flajšhans, M. Influence of interspecific hybridization on fitness-related traits in Siberian sturgeon and Russian sturgeon. Czech. J. Anim. Sci. 2019, 64, 78–88. [Google Scholar] [CrossRef]
- Memis, D.; Ercan, E.; Çelikkale, M.S.; Timur, M.; Zarkua, Z. Growth and Survival Rate of Russian Sturgeon (Acipenser gueldenstaedtii) Larvae from Fertilized Eggs to Artificial Feeding. Turkish J. Fish. Aquat. Sci. 2009, 9, 47–52. [Google Scholar]
- Chebanov, M.; Galich, E. Sturgeon Hatchery Manual; FAO Fisheries and Aquaculture Technical Paper 558; Food and Agriculture Organisation of the United Nations: Ankara, Turkey, 2011; p. 325. [Google Scholar]
- Gjerde, B.; Reddy, P.V.; Mahapatra, K.D.; Saha, J.N.; Jana, R.K.; Meher, P.K.; Sahoo, M.; Lenka, S.; Govindassamy, P.; Rye, M. Growth and survival in two complete diallele crosses with five stocks of Rohu carp (Labeo rohita). Aquaculture 2002, 209, 103–115. [Google Scholar] [CrossRef]
- Panase, P.; Mengumphan, K. Growth performance, length-weight relationship and condition factor of backcross and reciprocal hybrid catfish reared in net cages. Int. J. Zool. Res. 2015, 11, 57–64. [Google Scholar]
- Liu, X.; Liang, H.; Li, Z.; Liang, Y.; Lu, C.; Li, C.; Chang, Y.; Zou, G.; Hu, G. Performances of the hybrid between CyCa nucleocytplasmic hybrid fish and scattered mirror carp in different culture environments. Sci. Rep. 2017, 7, 46329. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Pearson Education, Ltd.: Essex, UK, 1996. [Google Scholar]
- Glogowski, J.; Kolman, R.; Szczepkowski, M.; Horvath, A.; Urbanyi, B.; Sieczynski, P.; Rzemieniecki, A.; Domagala, J.; Demianowicz, W.; Kowalski, R.; et al. Fertilization rate of Siberian sturgeon (Acipenser baeri, Brandt) milt cryopreserved with methanol. Aquaculture 2002, 211, 367–373. [Google Scholar] [CrossRef]
- Wei, Q.W.; Zou, Y.; Li, P.; Li, L. Sturgeon aquaculture in China: Progress, strategies and prospects assessed on the basis of nation-wide surveys (2007–2009). J. Appl. Ichthyol. 2011, 27, 162–168. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, W.; Li, L.; Ma, X.; Chen, J. Genetic variation and relationships of seven sturgeon species and ten interspecific hybrids. Genet. Sel. Evol. 2013, 45, 21. [Google Scholar] [CrossRef]
- Myrvold, K.M.; Kennedy, B.P. Density dependence and its impact on individual growth rates in an age-structured stream salmonid population. Ecosphere 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Losos, J.B.; Ricklefs, R.E. Adaptation and diversification on islands. Nature 2009, 457, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Leary, R.F.; Allendorf, F.W.; Knudsen, K.L. Developmental stability and enzyme heterozygosity in rainbow trout. Nature 1983, 301, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.G.K. Genetic Resources of Indian Major Carps; FAO Fisheries and Aquaculture Technical Paper 387; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2000; p. 76. [Google Scholar]
- Arnold, M.L. Natural Hybridization and Evolution; Oxford Series in ecology and Evolution; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
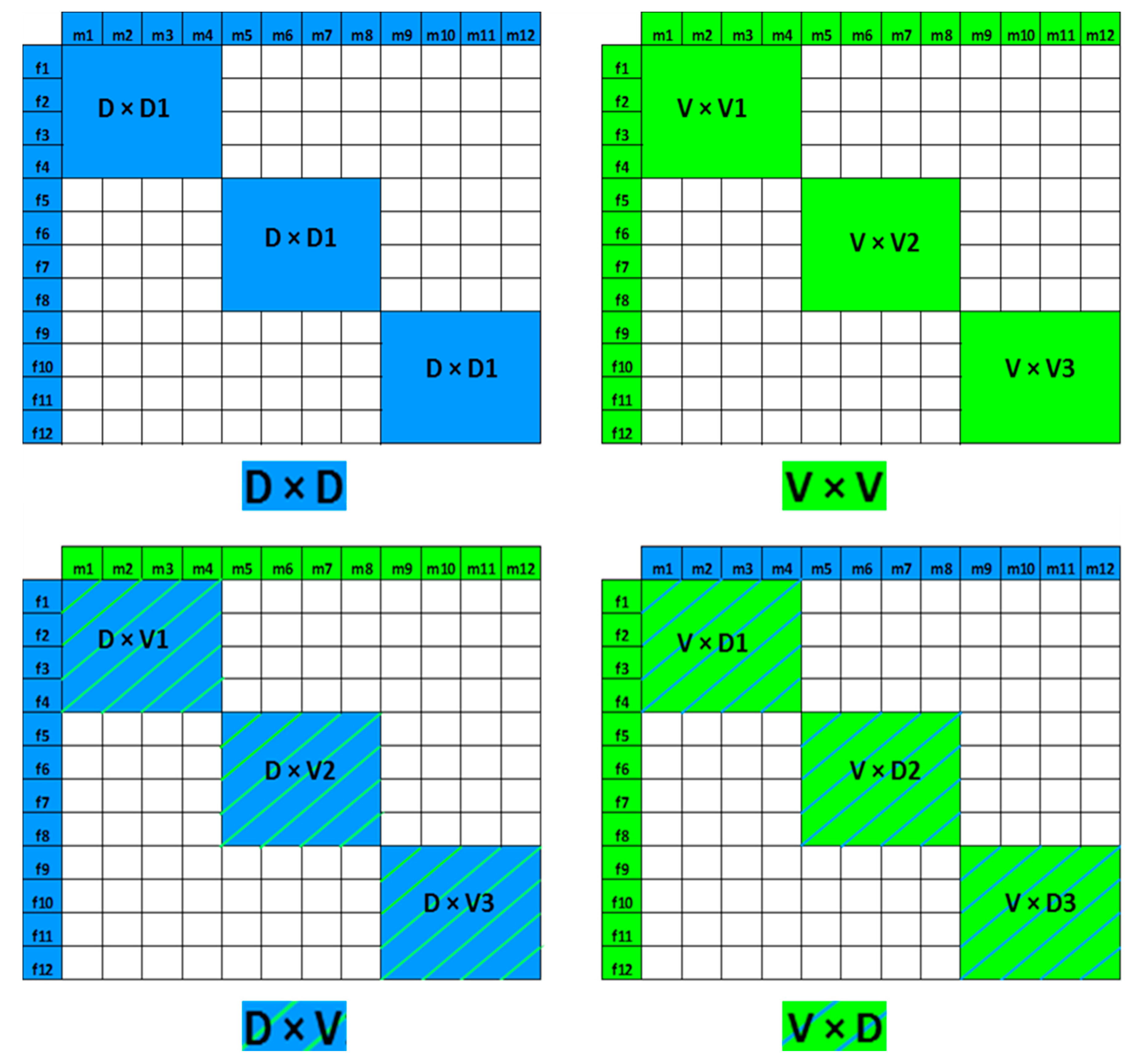



| Fish Weight (g) | Rearing System | Days Post-Hatching | Life Stage | Feed | Feed Size (mm) | Protein (%) | Fat (%) | Crude Fiber (%) | Ash (%) | Total p (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.2–0.5 | Indoor troughs | 1–11 | Larvae | Alltech Coppens® Advance | 0.2–0.5 | 56 | 15 | 0.1 | 12 | 1.99 |
| 0.5–1.5 | Indoor troughs | 12–29 | Fry | Alltech Coppens® Advance | 0.5–0.8 | 56 | 15 | 0.1 | 12.0 | 1.99 |
| 1.5–5.0 | Indoor circular tanks | 30–58 | Early fingerling | Alltech Coppens® Start Premium | 1.0 | 54 | 15 | 0.3 | 10.3 | 1.73 |
| 5.0–10 | Indoor circular tanks | 59–85 | Fingerling | Alltech Coppens® Start Premium | 1.0/1.5 | 54 | 15 | 0.3 | 10.3 | 1.73 |
| 10–50 | Indoor circular tanks | 86–228 | Early juvenile | Alltech Coppens® Alevin | 2.0 | 54 | 15 | 1.1 | 9.0 | 1.32 |
| 50–100 | Outdoor circular tanks | 229–325 | Early juvenile | Alltech Coppens® Alevin | 2.0 | 54 | 15 | 1.1 | 9.0 | 1.32 |
| 100–200 | Outdoor circular tanks | 326–504 | Early Juvenile | Alltech Coppens® Supreme-15 | 3.0 | 49 | 10 | 1.5 | 7.9 | 1.27 |
| Locus | Danube | Volga |
|---|---|---|
| Spl 163 | 0.811 | 0.772 |
| Spl 101 | 0.743 | 0.831 |
| Spl 173 | 0.589 | 0.650 |
| AfuG 135 | 0.605 | 0.763 |
| Aox 45 | 0.882 | 0.578 |
| AciG 35 | 0.722 | 0.602 |
| Locus | Ho | Ho SD | He | He SD | NA | F |
|---|---|---|---|---|---|---|
| Danube | 0.7346 * | 0.0133 | 0.7231 | 0.0482 | 5.7 * | 0.165 |
| Volga | 0.6862 * | 0.0287 | 0.7018 | 0.0197 | 5.2 * | 0.132 |
| 58 dph (n = 240) | 175 dph (n = 240) | 229 dph (n = 240) | 325 dph (n = 240) | 386 dph (n = 240) | 504 dph (n = 240) | |
|---|---|---|---|---|---|---|
| Body weight (g) | ||||||
| V × D | 5.28 ± 2.38 b | 42.7 ± 20.50 a | 61.40 ± 26.11 a | 80.20 ± 36.62 a | 107.17 ± 36.97 ab | 137.73 ± 58.45 b |
| V × V | 5.09 ± 2.05 b | 43.21 ± 17.23 a | 63.55 ± 22.99 a | 84.96 ± 33.29 b | 98.56 ± 40.47 a | 124.82 ± 57.65 c |
| D × D | 4.48 ± 1.97 b | 47.85 ± 20.08 b | 67.63 ± 25.13 a | 86.90 ± 27.21 b | 112.42 ± 35.49 ab | 142.66 ± 45.58 ab |
| D × V | 1.31 ± 0.88 a | 49.36 ± 20.85 c | 70.79 ± 28.32 b | 92.68 ± 33.76 c | 116.78 ± 50.53 b | 144.98 ± 59.51 a |
| Heterosis (%) | ||||||
| V × D growth | 10.34 | −6.22 | −6.39 | −6.66 | 1.59 | 2.98 |
| D × V growth | −72.62 | 8.41 | 7.91 | 7.86 | 10.70 | 13.40 |
| V × D survival | 11.35 | −0.28 | −4.02 | 6.08 | 8.48 | 10.53 |
| D × V survival | −19.47 | −43.81 | −38.71 | −34.73 | −19.01 | −10.37 |
| Days Post Hatching | V × D (n = 240) | V × V (n = 240) | D × D (n = 240) | D × V (n = 240) |
|---|---|---|---|---|
| Specific growth rate (% day−1) | ||||
| 58–175 | 1.77 ± 0.15 a | 1.82 ± 0.05 b | 2.02 ± 0.04 c | 3.10 ± 0.02 d |
| 176–229 | 0.69 ± 0.16 a | 0.72 ± 0.18 a | 0.64 ± 0.13 a | 0.67 ± 0.08 a |
| 230–325 | 0.28 ± 0.08 a | 0.30 ± 0.09 a | 0.26 ± 0.06 a | 0.28 ± 0.04 a |
| 326–386 | 0.49 ± 0.11 a | 0.23 ± 0.12 a | 0.43 ± 0.13 a | 0.37 ± 0.16 a |
| 387–504 | 0.23 ± 0.02 a | 0.21 ± 0.09 a | 0.20 ± 0.03 a | 0.18 ± 0.04 a |
| Growth heterogeneity | ||||
| 58–175 | 1.07 ± 0.10 a | 1.01 ± 0.02 b | 0.94 ± 0.05 c | 0.59 ± 0.04 d |
| 176–229 | 0.89 ± 0.09 a | 0.91 ± 0.02 a | 0.90 ± 0.08 a | 1.02 ± 0.01 a |
| 230–325 | 1.08 ± 0.07 a | 1.09 ± 0.17 a | 1.06 ± 0.17 a | 1.14 ± 0.16 a |
| 326–386 | 0.82 ± 0.07 a | 0.99 ± 0.19 a | 0.82 ± 0.12 a | 1.10 ± 0.19 b |
| 387–504 | 1.24 ± 0.10 a | 1.10 ± 0.10 ab | 1.02 ± 0.16 ab | 0.83 ± 0.10 b |
| Locus | Ho | Ho SD | He | He SD | NA | NA SD |
|---|---|---|---|---|---|---|
| D × D | 0.6553 | 0.0442 | 0.7536 | 0.0482 | 4.5 | 1.44 |
| V × V | 0.5919 * | 0.0311 | 0.6250 * | 0.0327 | 4.3 * | 1.52 |
| D × V | 0.6962 * | 0.0498 | 0.7589 * | 0.0685 | 4.6 * | 1.65 |
| V × D | 0.6398 | 0.0523 | 0.7462 | 0.0279 | 4.5 | 1.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivaramu, S.; Lebeda, I.; Kašpar, V.; Flajšhans, M. Intraspecific Hybrids Versus Purebred: A Study of Hatchery-Reared Populations of Sterlet Acipenser ruthenus. Animals 2020, 10, 1149. https://doi.org/10.3390/ani10071149
Shivaramu S, Lebeda I, Kašpar V, Flajšhans M. Intraspecific Hybrids Versus Purebred: A Study of Hatchery-Reared Populations of Sterlet Acipenser ruthenus. Animals. 2020; 10(7):1149. https://doi.org/10.3390/ani10071149
Chicago/Turabian StyleShivaramu, Sahana, Ievgen Lebeda, Vojtěch Kašpar, and Martin Flajšhans. 2020. "Intraspecific Hybrids Versus Purebred: A Study of Hatchery-Reared Populations of Sterlet Acipenser ruthenus" Animals 10, no. 7: 1149. https://doi.org/10.3390/ani10071149
APA StyleShivaramu, S., Lebeda, I., Kašpar, V., & Flajšhans, M. (2020). Intraspecific Hybrids Versus Purebred: A Study of Hatchery-Reared Populations of Sterlet Acipenser ruthenus. Animals, 10(7), 1149. https://doi.org/10.3390/ani10071149




