Effect of Intermittent and Mild Cold Stimulation on the Immune Function of Bursa in Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Experimental Design
2.2. Collecting Samples
2.3. RNA Extraction and Quantitative RT PCR (qPCR) Analysis
2.4. Statistical Analysis
3. Results
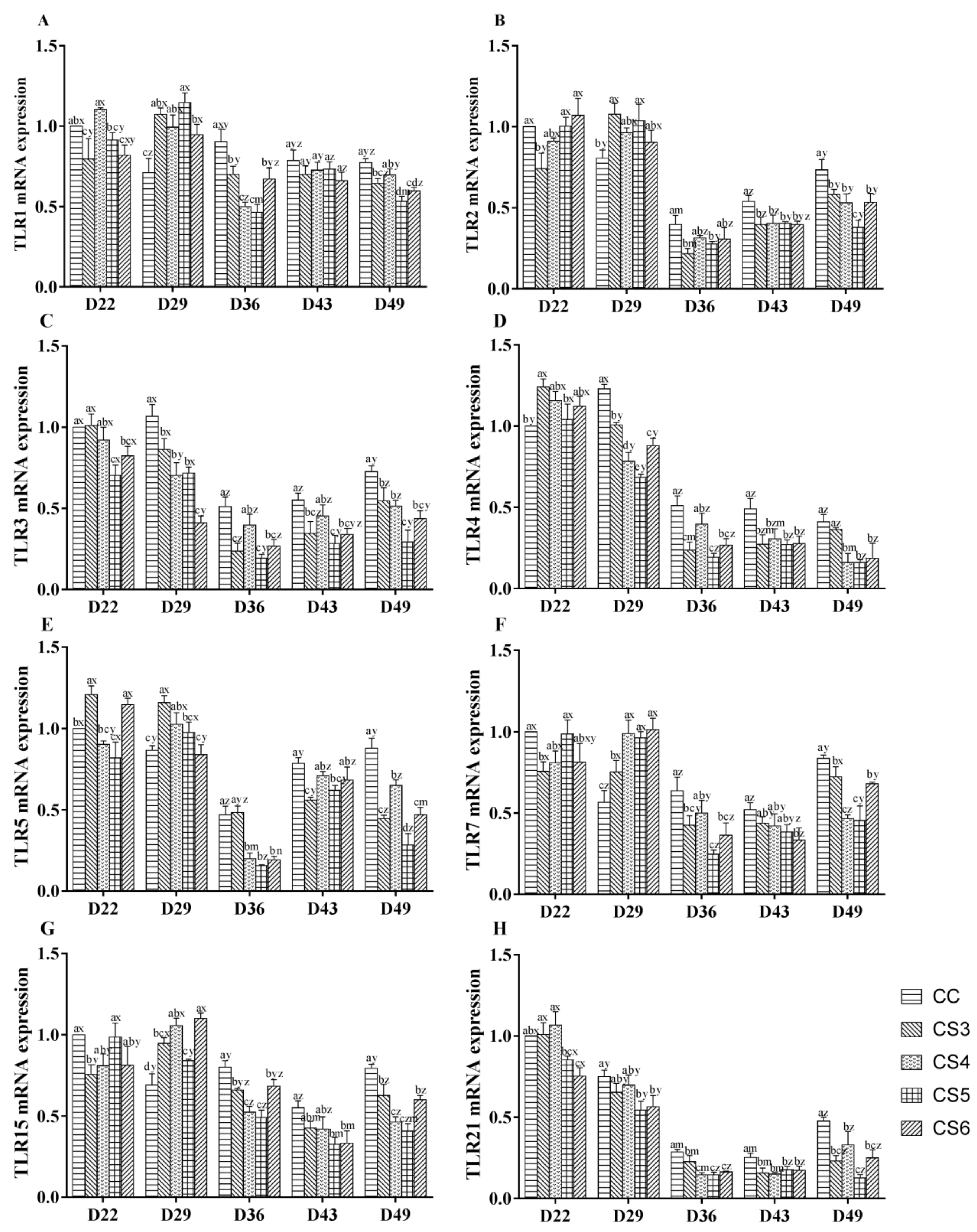
3.1. Relative Expression Levels of Toll-Like Receptors
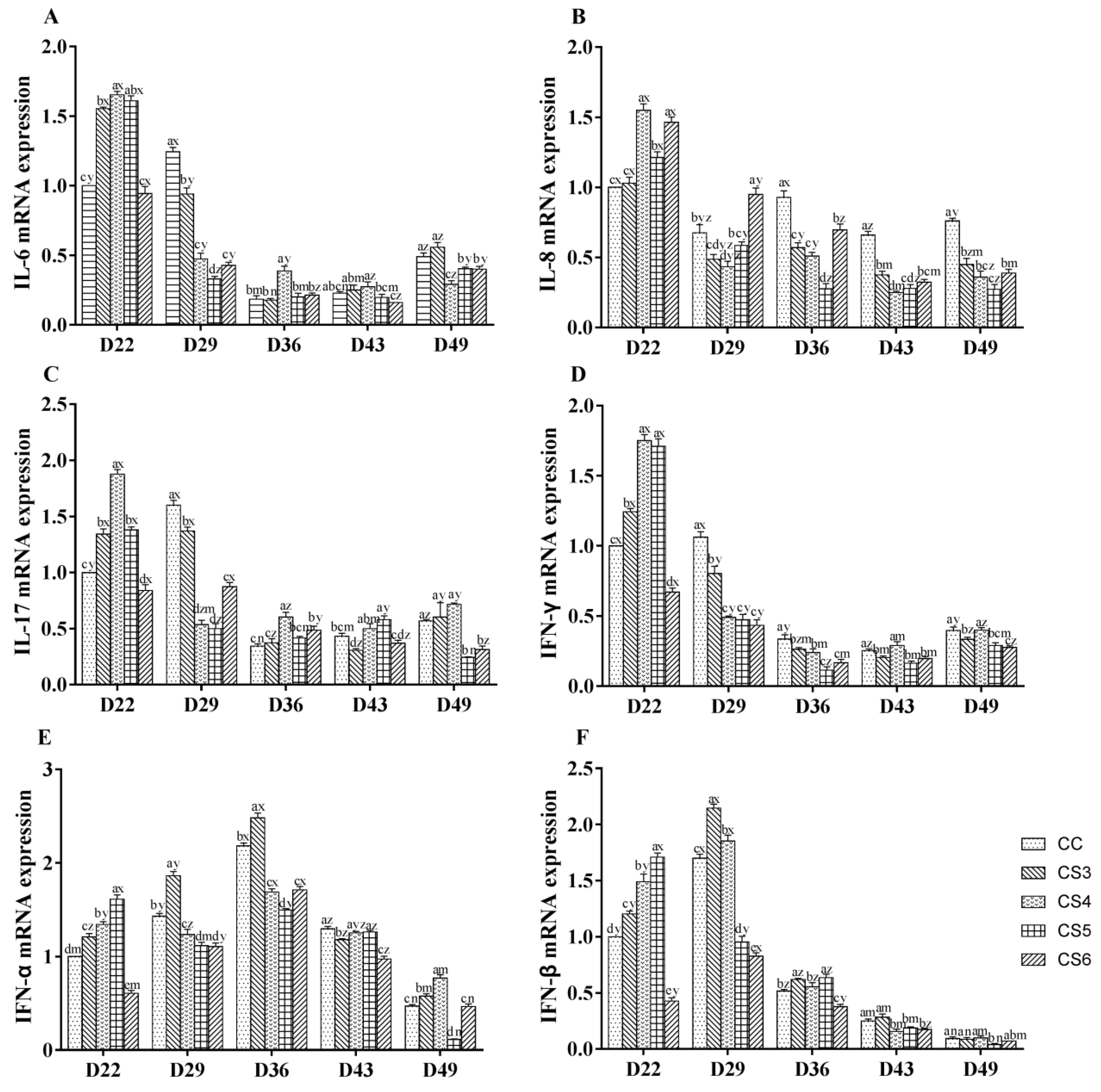
3.2. Relative Expression Levels of Cytokines
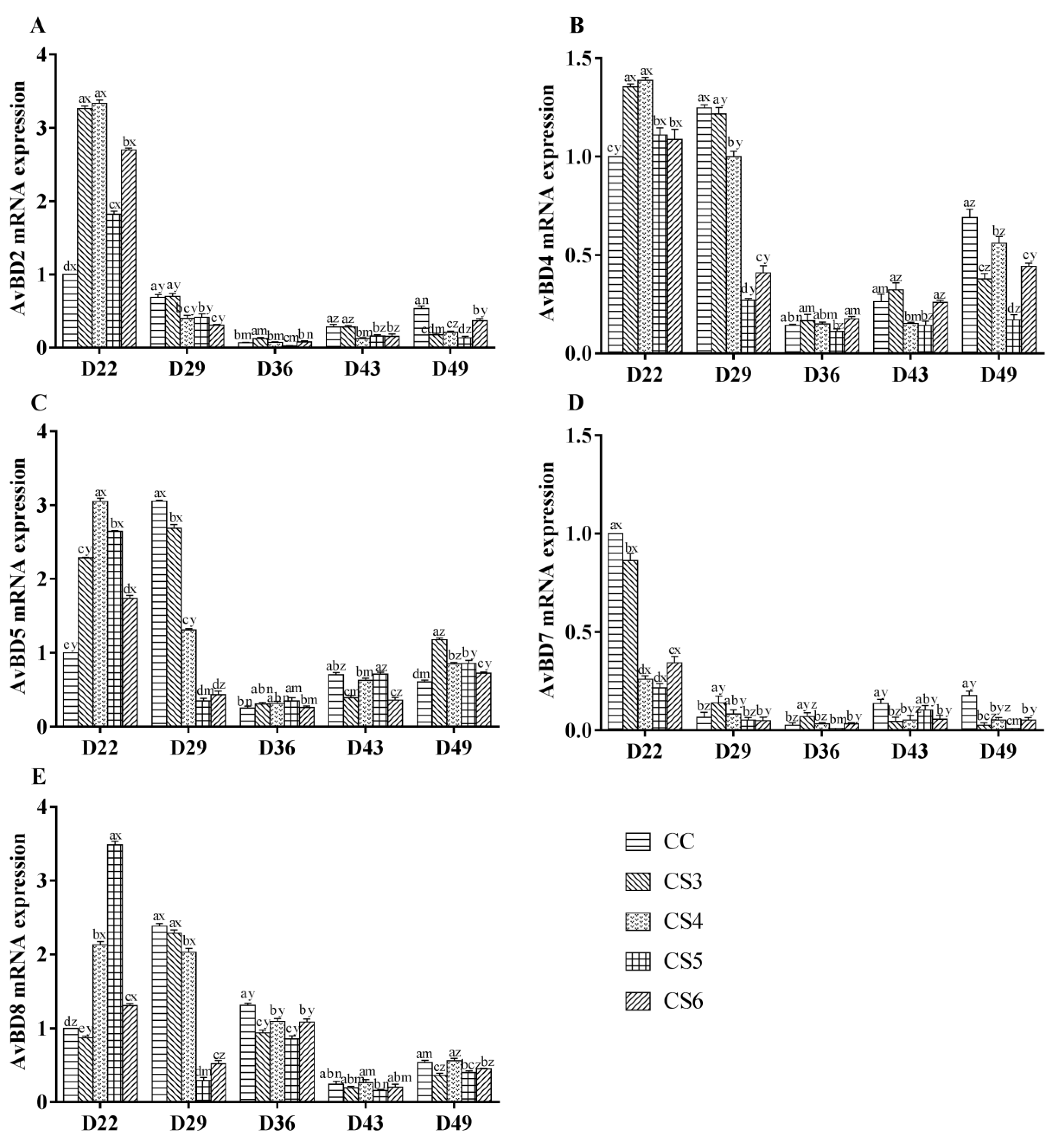
3.3. Relative Expression Levels of Avian β-Defensins (AvBDs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prince, R.P.; Potter, L.M.; Irish, W.W. Response of chickens to temperature and ventilation environments. Poult. Sci. 1961, 40, 102–108. [Google Scholar] [CrossRef]
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. The effect of cold stress on the pathogenesis of necrotic enteritis in broiler chicks. Avian Pathol. 2015, 44, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.W.; Lv, Z.H.; Li, J.L.; Li, S.; Xu, S.W.; Wang, X.L. Effects of cold stress on nitric oxide in duodenum of chicks. Poult. Sci. 2011, 90, 1555–1561. [Google Scholar] [CrossRef]
- Olfati, A.; Mojtahedin, A.; Sadeghi, T.; Akbari, M.; Martinez-Pastor, F. Comparison of growth performance and immune responses of broiler chicks reared under heat stress, cold stress and thermoneutral conditions. Span. J. Agric. Res. 2018, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.S.; Su, H.G.; Zhou, Y.; Li, X.M.; Feng, J.H.; Zhang, M.H. Effects of sustained cold and heat stress on energy intake, growth and mitochondrial function of broiler chickens. J. Integr. Agr. 2016, 15, 2336–2342. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Acclimation, R.S.P. Shock and hardening in the cold. J. Therm. Biol. 2005, 30, 557–562. [Google Scholar] [CrossRef]
- Su, Y.; Wei, H.; Bi, Y.; Wang, Y.; Zhao, P.; Zhang, R.; Li, X.; Li, J.; Bao, J. Pre-cold acclimation improves the immune function of trachea and resistance to cold stress in broilers. J. Cell. Physiol. 2019, 234, 7198–7212. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, F.; Li, X.; Su, Y.; Li, H.; Bao, J. Effects of intermittent cold stimulation on antioxidant capacity and mRNA expression in broilers. Livest. Sci. 2017, 204, 110–114. [Google Scholar] [CrossRef]
- Yang, X.; Zetian, Y.; Chengzhi, S. Enhancement of cellular immune function during cold adaptation of BALBc inbred mice. Cryobiology 1992, 20, 422–427. [Google Scholar] [CrossRef]
- Morabito, M.; Crisci, A.; Messeri, A.; Capecchi, V.; Modesti, P.A.; Gensini, G.F.; Orlandini, S. Environmental temperature and thermal indices: What is the most effective predictor of heat-related mortality in different geographical contexts? Sci. World J. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hangalapura, B.N.; Nieuwland, M.G.B.; Buyse, J.; Kemp, B.; Parmentier, H.K. Effect of duration of cold stress on plasma adrenal and thyroid hormone levels and immune responses in chicken lines divergently selected for antibody responses. Poult. Sci. 2004, 83, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, R.; Su, Y.; Bi, Y.; Li, X.; Zhang, X.; Li, J.; Bao, J. Effects of acute cold stress after long-term cold stimulation on antioxidant status, heat shock proteins, inflammation and immune cytokines in broiler heart. Front. Physiol. 2018, 9, 1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.D.; Zhang, F.; Shan, H.; Wang, S.B.; Chen, P.Y. mRNA expression in different developmental stages of the chicken bursa of Fabricius. Poult. Sci. 2016, 95, 1787–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, K.T.; Verma, P.; Reddy, M.R.; Murugesan, S. Molecular characterization of coding sequence and mRNA expression pattern of toll-like receptor 15 in Japanese Quail (Coturnix japonica) and Indigenous Chicken Breeds (Aseel and Kadaknath). J. Poult. Sci. 2011, 48, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Velova, H.; Gutowska-Ding, M.W.; Burt, D.W.; Vinkler, M. Toll-like receptor evolution in birds: Gene duplication, pseudogenisation and diversifying selection. Mol. Biol. Evol. 2018, 35, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef]
- Kogut, M.H.; He, H.; Kaiser, P. Lipopolysaccharide binding protein/CD14/ TLR4-dependent recognition of salmonella LPS induces the functional activation of chicken heterophils and up-regulation of pro-inflammatory cytokine and chemokine gene expression in these cells. Anim. Biotechnol. 2005, 16, 165–181. [Google Scholar] [CrossRef]
- St Paul, M.; Mallick, A.I.; Haq, K.; Orouji, S.; Abdul-Careem, M.F.; Sharif, S. In vivo administration of ligands for chicken toll-like receptors 4 and 21 induces the expression of immune system genes in the spleen. Vet. Immunol. Immunopathol. 2011, 144, 228–237. [Google Scholar] [CrossRef]
- St Paul, M.; Paolucci, S.; Read, L.R.; Sharif, S. Characterization of responses elicited by toll-like receptor agonists in cells of the bursa of Fabricius in chickens. Vet. Immunol. Immunopathol. 2012, 149, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Al-Zghoul, M.B.; Saleh, K.M.; Ababneh, M.M.K. Effects of pre-hatch thermal manipulation and post-hatch acute heat stress on the mRNA expression of interleukin-6 and genes involved in its induction pathways in 2 broiler chicken breeds. Poult. Sci. 2019, 98, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, R.; Allan, B. Avian toll-like receptors. Cell Tissue Res. 2011, 343, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.H.; Xu, H.J.; Yong, Y.H.; An, L.L.; Jiao, P.R.; Liao, M. Heat stress upregulation of Toll-like receptors 2/4 and acute inflammatory cytokines in peripheral blood mononuclear cell (PBMC) of Bama miniature pigs: An in vivo and in vitro study. Animal 2014, 8, 1462–1468. [Google Scholar] [CrossRef] [Green Version]
- Basu, M.; Paichha, M.; Swain, B.; Lenka, S.S.; Singh, S.; Chakrabarti, R.; Samanta, M. Modulation of TLR2, TLR4, TLR5, NOD1 and NOD2 receptor gene expressions and their downstream signaling molecules following thermal stress in the Indian major carp catla (Catla catla). 3 Biotech 2015, 5, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Zha, Z.; Bucher, F.; Nejatfard, A.; Zheng, T.; Zhang, H.; Yea, K.; Lerner, R.A. Interferon-gamma is a master checkpoint regulator of cytokine-induced differentiation. Proc. Natl. Acad. Sci. USA 2017, 114, E6867–E6874. [Google Scholar] [CrossRef] [Green Version]
- Felten, S.Y.; Madden, K.S.; Bellinger, D.L.; Kruszewska, B.; Moynihan, J.A.; Felten, D.L. The Role Of The Sympathetic Nervous System in The Modulation of Immune Responses. Adv. Paharmacol. 1997, 42, 583–587. [Google Scholar]
- Wigley, P. Avian cytokines in health and disease. Braz. J. Poult. Sci. 2003, 5, 1–14. [Google Scholar] [CrossRef]
- Marsh, B.J.; Williams-Karnesky, R.L.; Stenzel-Poore, M.P. Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience 2009, 158, 1007–1020. [Google Scholar] [CrossRef] [Green Version]
- Hangalapura, B.N.; Kaiser, M.G.; Poel, J.J.; Parmentier, H.K.; Lamont, S.J. Cold stress equally enhances in vivo pro-inflammatory cytokine gene expression in chicken lines divergently selected for antibody responses. Dev. Comp. Immunol. 2006, 30, 503–511. [Google Scholar] [CrossRef]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q.; Zhang, Z.W.; Yao, H.D.; Wang, L.L.; Liu, T.; Yu, X.Y.; Li, S.; Xu, S.W. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res. Vet. Sci. 2013, 95, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Vargovic, P.; Laukova, M.; Ukropec, J.; Manz, G.; Kvetnansky, R. Prior repeated stress attenuates cold-induced immunomodulation associated with "browning" in mesenteric fat of rats. Cell Mol. Neurobiol. 2018, 38, 349–361. [Google Scholar] [CrossRef] [PubMed]
- van Albert Dijk, A.; Veldhuizen, E.J.A.; Haagsman, H.P. Avian defensins. Vet. Immunol. Immunopathol. 2008, 124, 1–18. [Google Scholar] [CrossRef]
- Ma, D.Y.; Zhou, C.Y.; Zhang, M.Y.; Han, Z.X.; Shao, Y.H.; Liu, S.W. Functional analysis and induction of four novel goose (anser cygnoides) avian beta-defensins in response to salmonella enteritidis infection. Comp. Immunol. Microb. 2012, 35, 197–207. [Google Scholar] [CrossRef]
- Hong, Y.H.; Song, W.; Lee, S.H.; Lillehoj, H.S. Differential gene expression profiles of beta-defensins in the crop, intestine, and spleen using a necrotic enteritis model in 2 commercial broiler chicken lines. Poult. Sci. 2012, 91, 1081–1088. [Google Scholar] [CrossRef]
- Hangalapura, B.N.; Nieuwland, M.G.; de Vries Reilingh, G.; Heetkamp, M.J.; van den Brand, H.; Kemp, B.; Parmentier, H.K. Effects of cold stress on immune responses and body weight of chicken lines divergently selected for antibody responses to sheep red blood cells. Poult. Sci. 2003, 82, 1692–1700. [Google Scholar] [CrossRef]
- St Paul, M.; Brisbin, J.T.; Abdul-Careem, M.F.; Sharif, S. Immunostimulatory properties of toll-like receptor ligands in chickens. Vet. Immunol. Immunopathol. 2013, 152, 191–199. [Google Scholar] [CrossRef]
- Huang, S. Upregulation of TLR4 mRNA expression levels in broiler chickens under acute heat stress. Revista Brasileira De Ciência Avícola 2017, 19, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, L.; Kong, X.; Wu, F.; Zhou, C.; Nie, G.; Li, X. Expression patterns of toll-like receptors in natural triploid carassius auratus after infection with aeromonas hydrophila. Vet. Immunol. Immunopathol. 2015, 168, 77–82. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Calefi, A.S.; Cruz, D.S.G.; Aloia, T.P.A.; Zager, A.; Astolfi-Ferreira, C.S.; Piantino Ferreira, J.A.; Sharif, S.; Palermo-Neto, J. Heat stress decreases expression of the cytokines, avian beta-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017, 186, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Brenner, I.K.M.; Castellani, J.W.; Gabaree, C.; Young, A.J.; Zamecnik, J.; Shephard, R.J.; Shek, P.N. Immune changes in humans during cold exposure: Effects of prior heating and exercise. J. Appl. Physiol. 1999, 87, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.R.; Li, S.Z.; Fang, H.G.; Zhang, X.; Wang, J.F.; Guo, S.; Ji, H.; Zang, L.; Guo, L.; Zhen, L.H.; et al. Different duration of cold stress enhances pro-inflammatory cytokines profile and alterations of Th1 and Th2 type cytokines secretion in serum of wistar rats. J. Anim. Vet. Adv. 2012, 11, 1538–1545. [Google Scholar] [CrossRef]
- Loizzo, A.; Loizzo, S.; Lopez, L.; d′Amore, A.; Renzi, P.; Spampinato, S.; di Carlo, S.; Bacosi, A.; Zuccaro, P.; Roberta, P.; et al. Naloxone prevents cell-mediated immune alterations in adult mice following repeated mild stress in the neonatal period. Br. J. Pharmacol. 2002, 135, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, P.; Chen, Y.F.; Chen, Y.F.; Chung, L.C.; Tamilselvi, S.; Shen, C.Y.; Day, C.H.; Chen, R.J.; Viswanadha, V.P.; Kuo, W.W.; et al. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018, 233, 6458–6471. [Google Scholar] [CrossRef]
- Bagchi, A.K.; Akolkar, G.; Mandal, S.; Ayyappan, P.; Yang, X.; Singal, P.K. Toll-like receptor 2 dominance over Toll-like receptor 4 in stressful conditions for its detrimental role in the heart. Am. J. Physiol. Heart C 2017, 312, H1238–H1247. [Google Scholar] [CrossRef] [Green Version]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- de Ruiz Morales, J.M.G.; Puig, L.; Dauden, E.; Canete, J.D.; Pablos, J.L.; Martin, A.O.; Juanatey, C.G.; Adan, A.; Montalban, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Monin, L.; Gaffen, S.L. Interleukin 17 family cytokines: Signaling mechanisms, biological activities, and therapeutic implications. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef]
- van Dijk, A.; Veldhuizen, E.J.; Haagsman, H.P. The role of antimicrobial peptides in birds. researchgate.net/publication/267938436_The_Role_of_Antimicrobial_Peptides_in_Birds.
- Li, J.; Lu, X.; Ma, J. Characterization and expression analysis of attacins, antimicrobial peptide-encoding genes, from the desert beetle microdera punctipennis in response to low temperatures. CryoLetters 2017, 38, 65–74. [Google Scholar]
| Gene | Reference Sequence | Primer Sequences (5′-3′) |
|---|---|---|
| TLR1 | NM_001081709 | Forward: AGTCCATCTTTGTGTTGTCGCC Reverse: ATTGGCTCCAGCAAGATCAGG |
| TLR2 | XM_001232192 | Forward: GATTGTGGACAACATCATTGACTC Reverse: AGAGCTGCTTTCAAGTTTTCCC |
| TLR3 | NM_001011691 | Forward: TCAGTACATTTGTAACACCCCGCC Reverse: GGCGTCATAATCAAACACTCC |
| TLR4 | NM_001030693.1 | Forward: AGTCTGAAATTGCTGAGCTCAAAT Reverse: GCGACGTTAAGCCATGGAAG |
| TLR5 | NM_001024586 | Forward: CCTTGTGCTTTGAGGAACGAGA Reverse: CACCCATCTTTGAGAAACTGCC |
| TLR7 | NM_001011688 | Forward: TTCTGGCCACAGATGTGACC Reverse: CCTTCAACTTGGCAGTGCAG |
| TLR15 | NM_001037835 | Forward: GTTCTCTCTCCCAGTTTTGTAAATAGC Reverse: GTGGTTCATTGGTTGTTTTTAGGAC |
| TLR21 | NM_001030558 | Forward: TGCCCCTCCCACTGCTGTCCACT Reverse: AAAGGTGCCTTGACATCCT |
| IL-6 | NM_204628.1 | Forward: AAATCCCTCCTCGCCAATCT Reverse: CCCTCACGGTCTTCTCCATAAA |
| IL-8 | NM_205018.1 | Forward: GGCTTGCTAGGGGAAATGA Reverse: AGCTGACTCTGACTAGGAAACTGT |
| IFN-γ | NM_205149.1 | Forward: GAACTGGACAGGGAGAAATGAGA Reverse: ACGCCATCAGGAAGGTTGTT |
| IFN-α | XM_004937097.1 | Forward: GGACATGGCTCCCACACTAC Reverse: GGCTGCTGAGGATTTTGAAGA |
| IFN-β | NM_001024836.1 | Forward: CACCACCACCTTCTCCT Reverse: TGTGCGGTCAATCCAGT |
| AvBD2 | NM_204992 | Forward: GGTTGTCTTCGCCCCGGCGGGA Reverse: TTATGCATTCCAAGGCCATTTG |
| AvBD4 | NM_001001610 | Forward: TCATCGTGCTCCTCTTTGTG Reverse: AATACTTGGGACGGCATAGC |
| AvBD5 | NM_001001608 | Forward: GCTGTCCCTTGCTCGAGGATT Reverse: GGAATACCATCGGCTCCGGC |
| AvBD7 | NM_001001194 | Forward: ACCTGCTGCTGTCTGTCCTC Reverse: TGCACAGCAAGAGCCTATT |
| AvBD8 | NM_001001781 | Forward: TTCTCCTCACTGTGCTCCAA Reverse: AAGGCTCTGGTATGGAGGTG |
| β-actin | NM_205518.1 | Forward: CACCACAGCCGAGAGAGAAAT Reverse: TGACCATCAGGGAGTTCATAGC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xue, G.; Li, S.; Fu, Y.; Yin, J.; Zhang, R.; Li, J. Effect of Intermittent and Mild Cold Stimulation on the Immune Function of Bursa in Broilers. Animals 2020, 10, 1275. https://doi.org/10.3390/ani10081275
Liu Y, Xue G, Li S, Fu Y, Yin J, Zhang R, Li J. Effect of Intermittent and Mild Cold Stimulation on the Immune Function of Bursa in Broilers. Animals. 2020; 10(8):1275. https://doi.org/10.3390/ani10081275
Chicago/Turabian StyleLiu, Yanhong, Ge Xue, Shuang Li, Yajie Fu, Jingwen Yin, Runxiang Zhang, and Jianhong Li. 2020. "Effect of Intermittent and Mild Cold Stimulation on the Immune Function of Bursa in Broilers" Animals 10, no. 8: 1275. https://doi.org/10.3390/ani10081275





