Effects of Laetiporus sulphureus-Fermented Wheat Bran on Growth Performance, Intestinal Microbiota and Digesta Characteristics in Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Method
2.2. Inoculum Preparation and Solid-State Fermentation (SSF)
2.3. Experimental Birds and Management
2.4. DNA Sample Collection for Metagenomics Analysis
2.5. Polymerase Chain Reaction and Sequencing
2.6. Determination of Total Volatile Fatty Acids in Digesta
2.7. Determination of the Ammonium-Nitrogen Concentration in Digesta
2.8. Determination of the Lactic Acid Concentration in the Intestinal Digest
2.9. Statistical Analysis
3. Results
3.1. Growth Performance
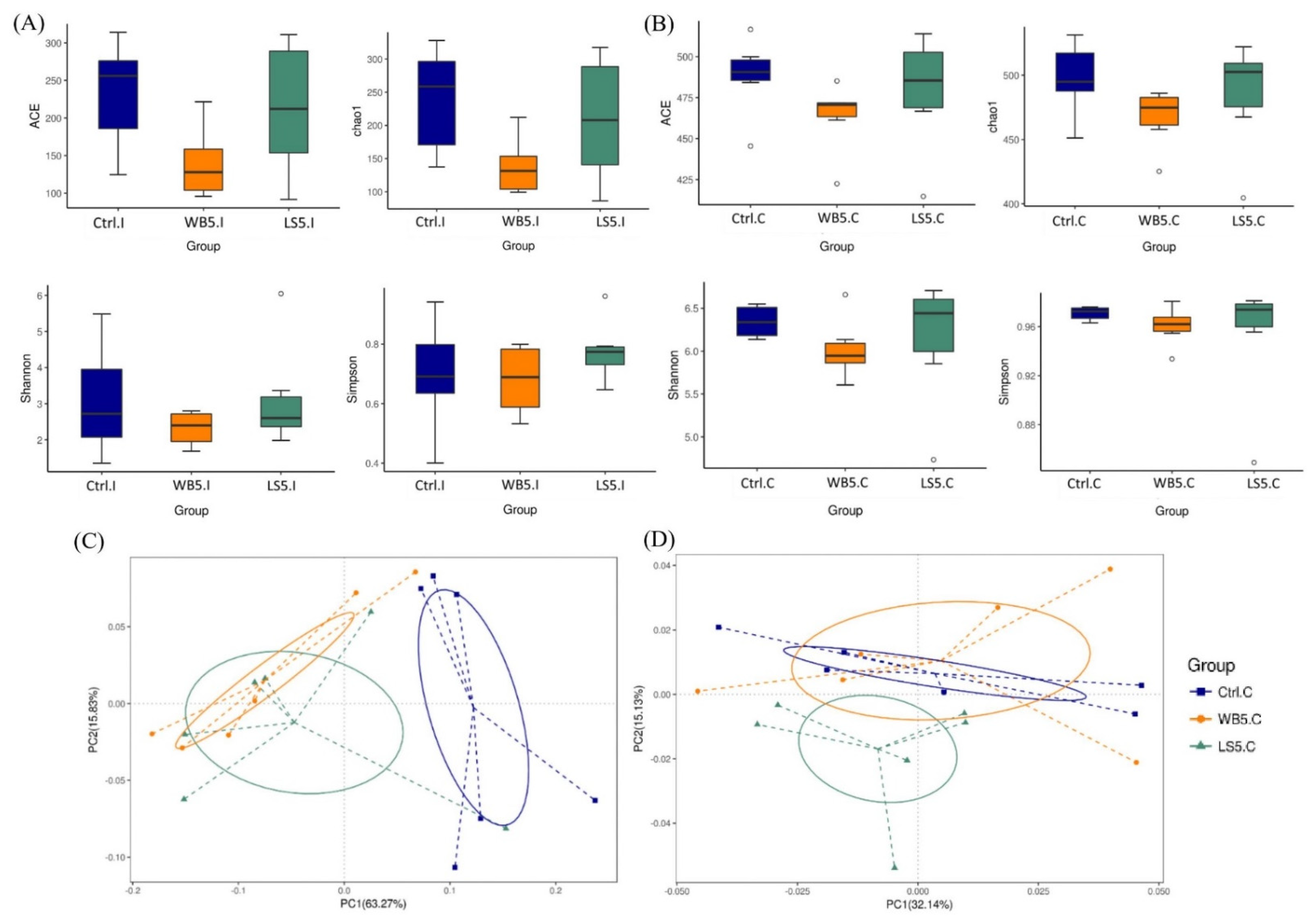
3.2. Effects of LS Supplementation on Diversity of Ileal and Cecal Microbiota
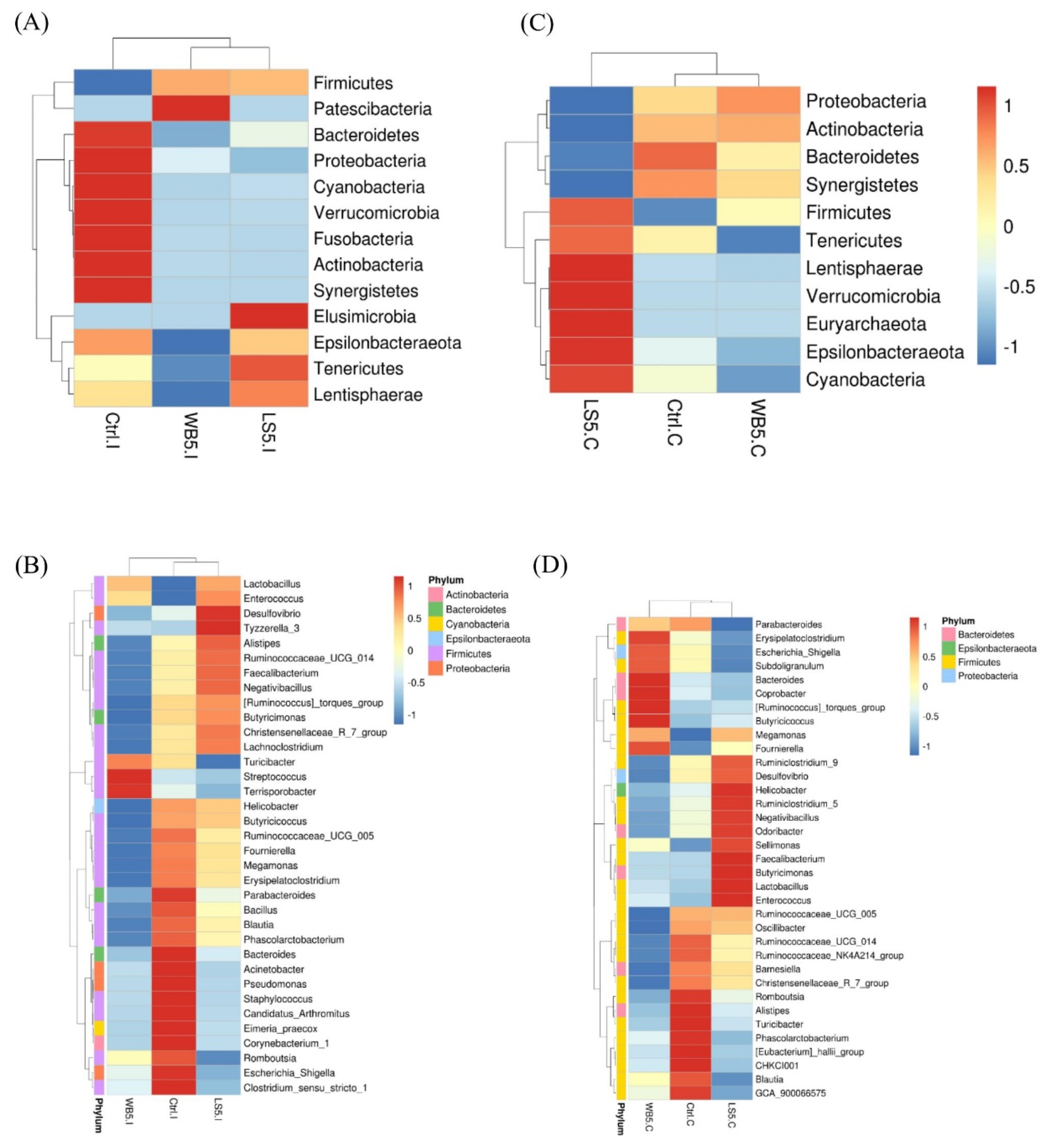
3.3. Heat Map of the Microbiota Composition in the Ileum and Cecum
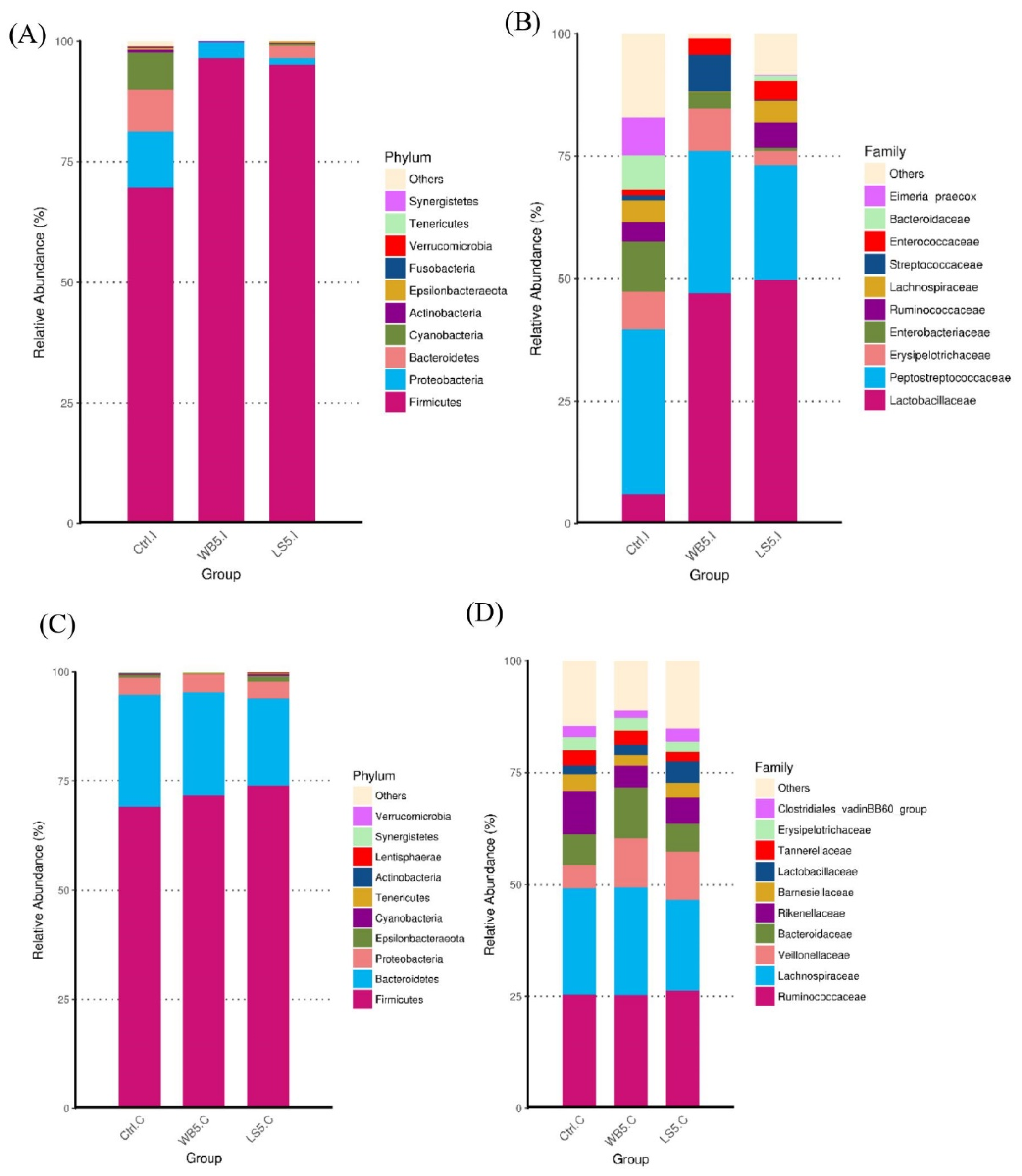
3.4. Mean Differences in Microbiota Composition in the Ileum and Cecum
3.5. Top Ten OTUs of the Ileum and Cecum
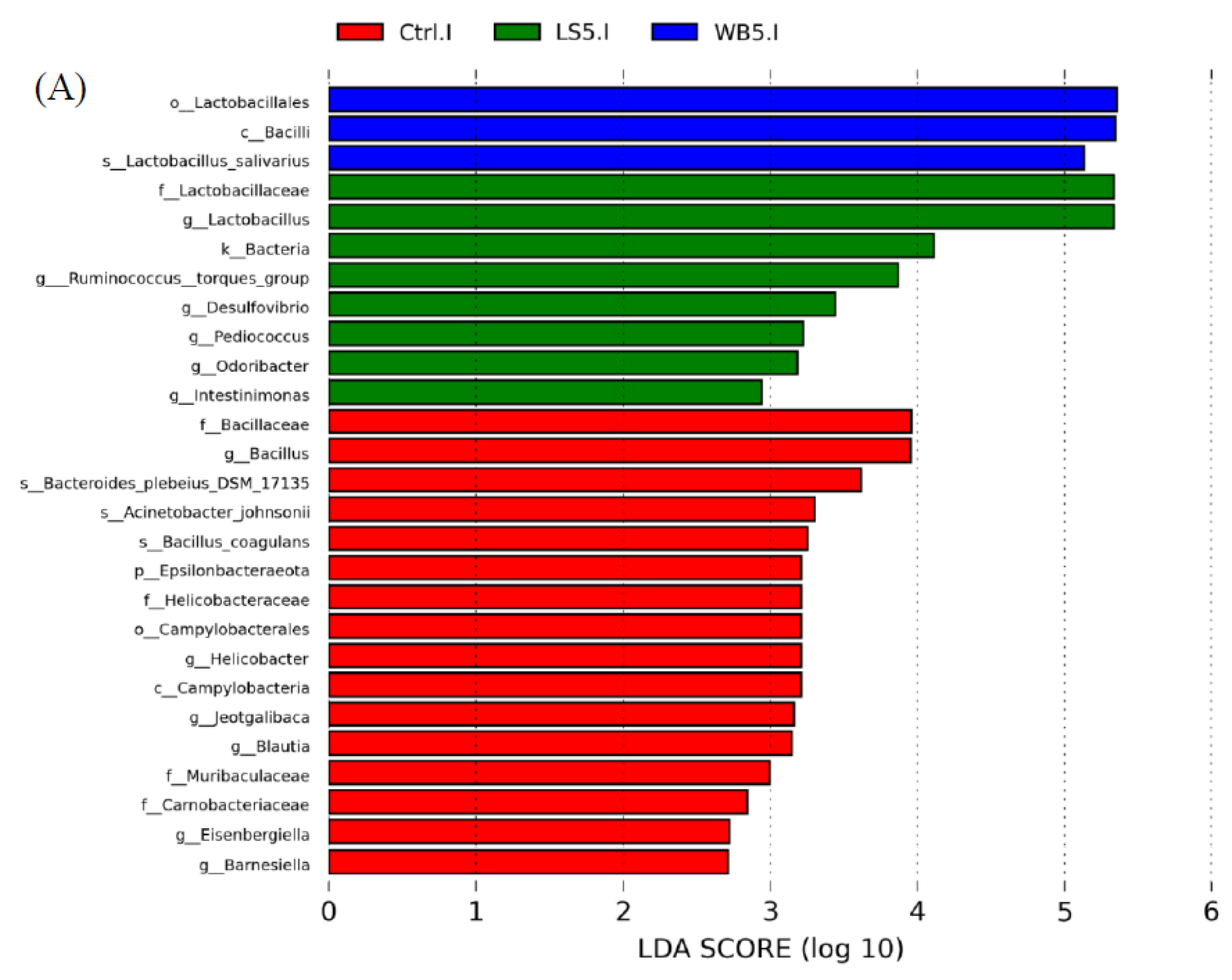
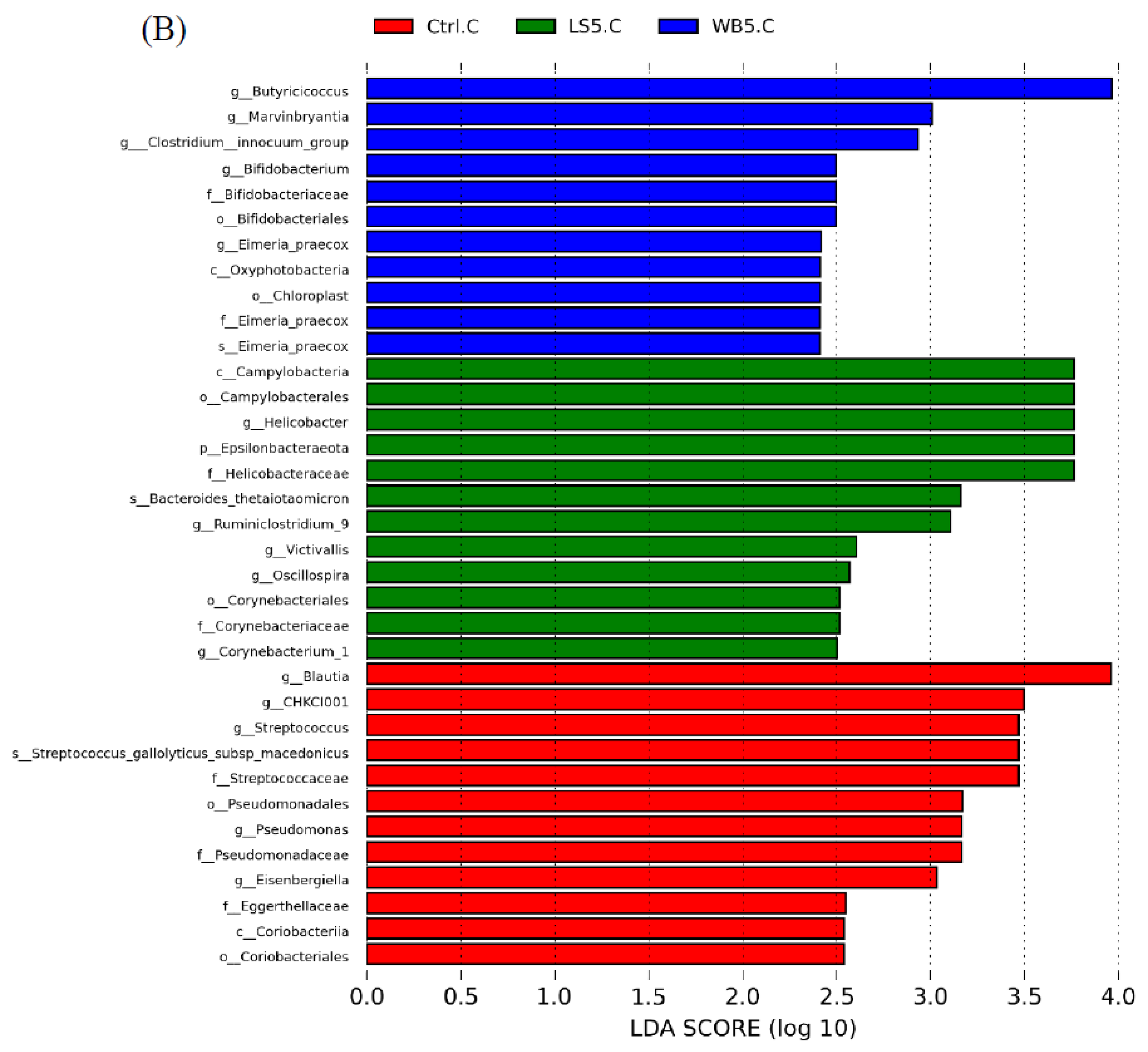
3.6. Linear Discriminant Analysis (LDA) Effect Size (LEfSE)
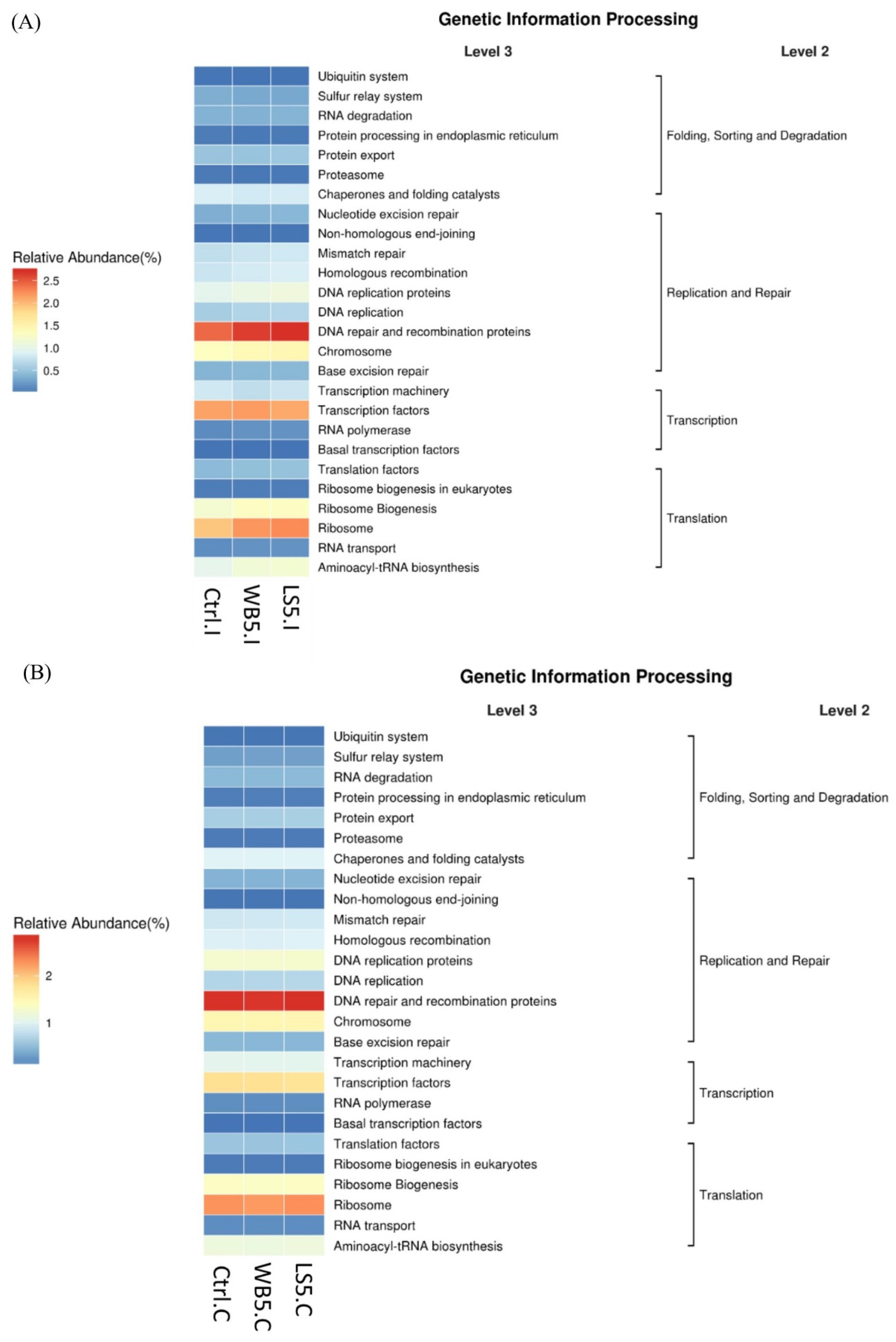
3.7. Effects of LS on the KEGG Pathway in the Ileum and Cecum of the Broilers
3.8. Effects of LS on the Total VFA, pH Value, and Ammonium-Nitrogen of the Ileal and Cecal Digesta in the Broilers
3.9. Effects of LS on the Lactate Concentration of Ieal and Cecal Digesta in Boilers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LS | Laetiporus sulphureus-fermented wheat bran |
| WB | Wheat bran |
| OTU | Operational taxonomic units |
| VFA | Volatile fatty acid |
| FCR | Feed conversion ratio |
| EBI | European Broiler Index |
| NSPs | Non-starch polysaccharides |
| GIT | Gastrointestinal tract |
| BCRC | Bioresource Collection and Research Center |
| MEA | Malt extract agar |
| MEB | Malt extract broth |
| ADG | Average daily weight gains |
| HPLC | High-performance liquid chromatography |
References
- Carrasco, J.M.D.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, gut health and chicken productivity: What is the connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, Y.; Verstegen, M.W.A.; Tamminga, S.; Williams, B.A. The role of the commensal gut microbial community in broiler chickens. Worlds Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, B.D.; Klasing, K.C. Modulation of nutrient metabolism and homeostasis by the immune system. Worlds Poult. Sci. J. 2004, 60, 90–100. [Google Scholar] [CrossRef]
- Kabir, S.M.L. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009, 10, 3531–3546. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.D. Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.C.; Lee, M.T.; Lo, C.T.; Chang, S.C.; Lee, T.T. Effects of dietary supplementation of Trichoderma pseudokoningii fermented enzyme powder on growth performance, intestinal morphology, microflora and serum antioxidantive status in broiler chickens. Ital. J. Anim. Sci. 2018, 17, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT Food Sci. Technol. 2014, 56, 211–221. [Google Scholar] [CrossRef]
- Bederska-Łojewska, D.; Świątkiewicz, S.; Arczewska-Włosek, A.; Schwarz, T. Rye non-starch polysaccharides: Their impact on poultry intestinal physiology, nutrients digestibility and performance indices—A review. Ann. Anim. Sci. 2017, 17, 351–369. [Google Scholar] [CrossRef] [Green Version]
- Annison, G. The role of wheat non-starch polysaccharides in broiler nutrition. Aust. J. Agric. Res. 1993, 44, 405–408. [Google Scholar] [CrossRef]
- Lai, L.P.; Lee, M.T.; Chen, C.S.; Yu, B.; Lee, T.T. Effects of co-fermented Pleurotus eryngii stalk residues and soybean hulls by Aureobasidium pullulans on performance and intestinal morphology in broiler chickens. Poult. Sci. 2015, 94, 2959–2969. [Google Scholar] [CrossRef]
- Chu, Y.T.; Lo, C.T.; Chang, S.C.; Lee, T.T. Effects of Trichoderma fermented wheat bran on growth performance, intestinal morphology and histological findings in broiler chickens. Ital. J. Anim. Sci. 2017, 16, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, S.; Seidavi, A.R.; Rahati, M.; Nieto, J.A.G. Effect of mushroom powder and flavophospholipol on carcass in broiler chickens. Rev. Mex. Cienc. Pecu. 2015, 6, 469–481. [Google Scholar] [CrossRef] [Green Version]
- Kutlu, H.R.; Saber, S.N.; Kutay, H.; Celik, L.; Yusuf, U.Z.U.N.; Nurten, T.O.Y.; Kutlu, M.; Yucelt, O.; Burgut, A.; Thiery, P.; et al. Effect of multi-enzyme produced by a single fungus on growth performance and some carcass parameters of broiler chicks fed on maize-soya based diets. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 221–230. [Google Scholar]
- Nasehi, M.; Torbatinejad, N.M.; Zerehdaran, S.; Safaei, A.R. Effect of (Pleurotus florida) fungi on chemical composition and rumen degradability of wheat and barley straw. Iran. J. Appl. Anim. Sci. 2019, 4, 257–261. [Google Scholar]
- Pan, Y.G.; Lin, W.C.; Lo, C.T.; Chang, S.C.; Yu, B.; Lee, T.T. Effects of substitution of Bermuda grass hay with Trichoderma fermented rice straw on growth, blood and rumen fluid parameters in Barbados sheep. J. Appl. Anim. Res. 2018, 46, 1162–1168. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Lu, C.C.; Lin, C.S.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Wu, T.R.; Tsai, Y.H.; Yeh, T.S.; Lu, J.J.; et al. Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int. J. Obes. 2018, 42, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhuo, C.; Teng, C.; Yu, S.; Wang, X.; Hu, Y.; Ren, G.; Yu, M.; Qu, J. Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int. J. Biol. Macromol. 2016, 93, 904–912. [Google Scholar] [CrossRef]
- Adams, S.; Che, D.; Hailong, J.; Zhao, B.; Rui, H.; Danquah, K.; Qin, G. Effects of pulverized oyster mushroom (Pleurotus ostreatus) on diarrhea incidence, growth performance, immunity, and microbial composition in piglets. J. Sci. Food Agric. 2019, 99, 3616–3627. [Google Scholar] [CrossRef] [Green Version]
- Ríos, J.L.; Andújar, I.; Recio, M.C.; Giner, R.M. Lanostanoids from fungi: A group of potential anticancer compounds. J. Nat. Prod. 2012, 75, 2016–2044. [Google Scholar] [CrossRef]
- Lin, W.C.; Lee, T.T. Laetiporus sulphureus-fermented wheat bran enhanced the broiler growth performance by improving the intestinal microflora and inflammation status. Poult. Sci. 2020, 99, 3606–3616. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Marcu, A.; Vacaru-Opriș, I.; Gabi, D.; Liliana, P.C.; Marcu, A.; Marioara, N.; Ioan, P.; Dorel, D.; Bartolomeu, K.; Cosmin, M. The influence of genetics on economic efficiency of broiler chickens growth. Anim. Sci. Biotech. 2013, 46, 339–346. [Google Scholar]
- Kromann, R.P.; Meyer, J.H.; Stielau, W.J. Steam distillation of volatile fatty acids in rumen ingesta. J. Dairy Sci. 1967, 50, 73. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Yan, W.; Sun, C.; Yuan, J.; Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017, 7, 45308. [Google Scholar] [CrossRef]
- Ocejo, M.; Oporto, B.; Hurtado, A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free–range slow–growing chickens throughout their productive lifespan. Sci. Rep. 2019, 9, 2506. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, T.; Guo, Y. Dietary fiber and chicken microbiome interaction: Where will it lead to? Anim. Nutr. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Wanzenböck, E.; Zitz, U.; Steinbauer, C.; Kneifel, W.; Domig, K.J.; Schedle, K. A diet containing native or fermented wheat bran does not interfere with natural microbiota of laying hens. Animal 2020, 14, 1147–1155. [Google Scholar] [CrossRef]
- Han, G.G.; Kim, E.B.; Lee, J.; Lee, J.Y.; Jin, G.; Park, J.; Huh, C.S.; Kwon, I.K.; Kil, D.Y.; Choi, Y.J.; et al. Relationship between the microbiota in different sections of the gastrointestinal tract, and the body weight of broiler chickens. Springerplus 2016, 5, 911. [Google Scholar] [CrossRef] [Green Version]
- Muir, W.I.; Bryden, W.L.; Husband, A.J. Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 2000, 24, 325–342. [Google Scholar] [CrossRef]
- Rinttila, T.; Apajalahti, J. Intestinal microbiota and metabolites–implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013, 22, 647–658. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Juda, M.; Malm, A. The preliminary study of prebiotic potential of Polish wild mushroom polysaccharides: The stimulation effect on Lactobacillus strains growth. Eur. J. Nutr. 2018, 57, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiater, A.; Paduch, R.; Pleszczyńska, M.; Próchniak, K.; Choma, A.; Kandefer-Szerszeń, M.; Szczodrak, J. α-(1–3)-D-glucans from fruiting bodies of selected macromycetes fungi and the biological activity of their carboxymethylated products. Biotechnol. Lett. 2011, 33, 787–795. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.J.; Kang, B.W.; Park, J.U.; Kim, M.J.; Lee, H.H.; Choi, Y.H.; Jeong, Y.K. Biochemical characterization of the exopolysaccharide purified from Laetiporus sulphureus mycelia. J. Microbiol. Biotechnol. 2011, 21, 1287–1293. [Google Scholar] [CrossRef] [Green Version]
- Seong, H.; Bae, J.H.; Seo, J.; Kim, S.A.; Kim, T.J.; Han, N. Comparative analysis of prebiotic effects of seaweed polysaccharides laminaran, porphyran, and ulvan using in vitro human fecal fermentation. J. Funct. Foods 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Yan, Q.; You, X.; Yang, S.; Jiang, Z. In vitro digestibility and prebiotic potential of curdlan (1→ 3)-β-d-glucan oligosaccharides in Lactobacillus species. Carbohydr. Polym. 2018, 188, 17–26. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Juda, M.; Malm, A.; Lemieszek, M.; Rzeski, W.; Kaczyński, Z. New biological activity of the polysaccharide fraction from Cantharellus cibarius and its structural characterization. Food Chem. 2018, 268, 355–361. [Google Scholar] [CrossRef]
- Nian, F.; Guo, Y.; Ru, Y.; Péron, A.; Li, F. Effect of xylanase supplementation on the net energy for production, performance and gut microflora of broilers fed corn/soy-based diet. Asian-Australas. J. Anim. Sci. 2011, 24, 1282–1287. [Google Scholar] [CrossRef]
- Falck, P.; Precha-Atsawanan, S.; Grey, C.; Immerzeel, P.; Stålbrand, H.; Adlercreutz, P.; Nordberg Karlsson, E. Xylooligosaccharides from hardwood and cereal xylans produced by a thermostable xylanase as carbon sources for Lactobacillus brevis and Bifidobacterium adolescentis. J. Agric. Food Chem. 2013, 61, 7333–7340. [Google Scholar] [CrossRef]
- Karlsson, N.E.; Schmitz, E.; Linares-Pastén, J.A.; Adlercreutz, P. Endo-xylanases as tools for production of substituted xylooligosaccharides with prebiotic properties. Appl. Microbiol. Biotechnol. 2018, 102, 9081–9088. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.B.; Wagschal, K.; Grigorescu, A.A.; Braker, J.D. Highly active β-xylosidases of glycoside hydrolase family 43 operating on natural and artificial substrates. Appl. Microbiol. Biotechnol. 2013, 97, 4415–4428. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi1, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef]
- Barroso, E.; Muñoz-González, I.; Jiménez, E.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; Del Carmen Martínez-Cuesta, M.; Requena, T. Phylogenetic profile of gut microbiota in healthy adults after moderate intake of red wine. Mol. Nutr. Food Res. 2017, 61, 1600620. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.P. Green and black tea phenolics: Bioavailability, transformation by colonic microbiota, and modulation of colonic microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef]
- Muñoz, R.; Rivas, B.; Toledano, F.; Reverón, I.; Santamaría Rubio, L.; Esteban-Torres, M.; Curiel, J.A.; Rodríguez, H.; Landete, J.M. Biotransformation of phenolics by Lactobacillus plantarum in fermented foods. In Fermented Foods in Health and Disease Prevention, 1st ed.; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 63–83. [Google Scholar]
- Johnson, T.J.; Youmans, B.P.; Noll, S.; Cardona, C.; Evans, N.P.; Karnezos, T.P.; Ngunjiri, J.M.; Abundo, M.C.; Lee, C.W. A consistent and predictable commercial broiler chicken bacterial microbiota in antibiotic-free production displays strong correlations with performance. Appl. Environ. Microbiol. 2018, 84, e00362-18. [Google Scholar] [CrossRef] [Green Version]
- Siegerstetter, S.C.; Schmitz-Esser, S.; Magowan, E.; Wetzels, S.U.; Zebeli, Q.; Lawlor, P.G.; O’Connell, N.E.; Metzler-Zebeli, B.U. Intestinal microbiota profiles associated with low and high residual feed intake in chickens across two geographical locations. PLoS ONE 2017, 12, e0187766. [Google Scholar] [CrossRef]
- Stanley, D.; Hughes, R.J.; Geier, M.S.; Moore, R.J. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: Challenges presented for the identification of performance enhancing probiotic bacteria. Front. Microbiol. 2016, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Rubio, L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2019, 98, 695–706. [Google Scholar] [CrossRef]
- Oakley, B.B.; Kogut, M.H. Spatial and temporal changes in the broiler chicken cecal and fecal microbiomes and correlations of bacterial taxa with cytokine gene expression. Front. Vet. Sci. 2016, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of shortchain fatty acids on production of pro-inflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Gilbert, M.S.; Ijssennagger, N.; Kies, A.K.; van Mil, S.W.C. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G159–G170. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]

| Items | Experimental Diets | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | 5% WB | 5% LS | |||
| FBW (g) | 2393.94 | 2407.64 | 2499.46 | 17.12 | 0.11 |
| ADG (g/chick/d) | 67.05 | 67.44 | 69.65 | 0.50 | 0.25 |
| FCR (kg feed/kg gain) | 1.47 b | 1.45 b | 1.41 a | 0.01 | 0.04 |
| Viability (%) | 93.75 | 91.25 | 93.75 | 0.72 | 0.31 |
| EBI 2 | 424.28 b | 413.89 b | 468.72 a | 4.21 | 0.02 |
| Items | Experimental Diets | SEM | p-Value | ||
|---|---|---|---|---|---|
| Control | 5% WB | 5% LS | |||
| Ileum | |||||
| Ace | 139.66 | 212.56 | 232.67 | 16.93 | 0.09 |
| Chao1 | 138.29 | 208.83 | 238.80 | 18.03 | 0.10 |
| Simpson | 0.70 | 0.68 | 0.78 | 0.03 | 0.44 |
| Shannon | 3.08 | 2.32 | 3.15 | 0.30 | 0.47 |
| Cecum | |||||
| Ace | 487.91 | 463.67 | 478.53 | 6.55 | 0.34 |
| Chao1 | 497.03 | 467.15 | 484.96 | 7.74 | 0.31 |
| Simpson | 0.97 | 0.96 | 0.95 | 0.01 | 0.59 |
| Shannon | 6.34 | 6.02 | 6.14 | 0.12 | 0.53 |
| Items | Experimental Diets | SEM | p-Values | ||
|---|---|---|---|---|---|
| Control | 5% WB | 5% LS | |||
| Total VFA | μmole/g | ||||
| Ileum | 11.25 | 11.73 | 11.36 | 0.09 | 0.03 |
| Cecum | 32.74 b | 34.01 a | 35.20 a | 0.34 | 0.02 |
| NH3-N | |||||
| Ileum | 151.32 a | 152.27 a | 140.24 b | 0.0004 | 1.02 |
| Cecum | 190.02 a | 189.60 a | 174.57 b | 0.011 | 1.74 |
| pH value | |||||
| Ileum | 5.54 a | 5.55 a | 5.01 b | 0.07 | 0.03 |
| Cecum | 7.25 b | 7.40 a | 7.08 c | 0.02 | 0.004 |
| Items | Experimental Diets | SEM | p-Values | ||
|---|---|---|---|---|---|
| Control | 5% WB | 5% LS | |||
| μM | |||||
| Ileum | 43.16 b | 43.82 b | 58.02 a | 0.87 | 0.002 |
| Cecum | 90.88 b | 92.73 b | 108.12 a | 1.07 | 0.0003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.C.; Lee, T.T. Effects of Laetiporus sulphureus-Fermented Wheat Bran on Growth Performance, Intestinal Microbiota and Digesta Characteristics in Broiler Chickens. Animals 2020, 10, 1457. https://doi.org/10.3390/ani10091457
Lin WC, Lee TT. Effects of Laetiporus sulphureus-Fermented Wheat Bran on Growth Performance, Intestinal Microbiota and Digesta Characteristics in Broiler Chickens. Animals. 2020; 10(9):1457. https://doi.org/10.3390/ani10091457
Chicago/Turabian StyleLin, Wei Chih, and Tzu Tai Lee. 2020. "Effects of Laetiporus sulphureus-Fermented Wheat Bran on Growth Performance, Intestinal Microbiota and Digesta Characteristics in Broiler Chickens" Animals 10, no. 9: 1457. https://doi.org/10.3390/ani10091457





