The Presence of D-Penicillamine during the In Vitro Capacitation of Stallion Spermatozoa Prolongs Hyperactive-Like Motility and Allows for Sperm Selection by Thermotaxis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Design
2.3. Semen Collection and Cryopreservation
2.4. Sperm Sample Preparation and Incubation
2.5. Sperm Thermotaxis
2.6. Plasma Membrane Integrity
2.7. Acrosomal Exocytosis
2.8. Motility and Kinetics
2.9. DNA Fragmentation
2.10. Protein Tyrosine Phosphorylation
2.11. Statistical Analysis
3. Results
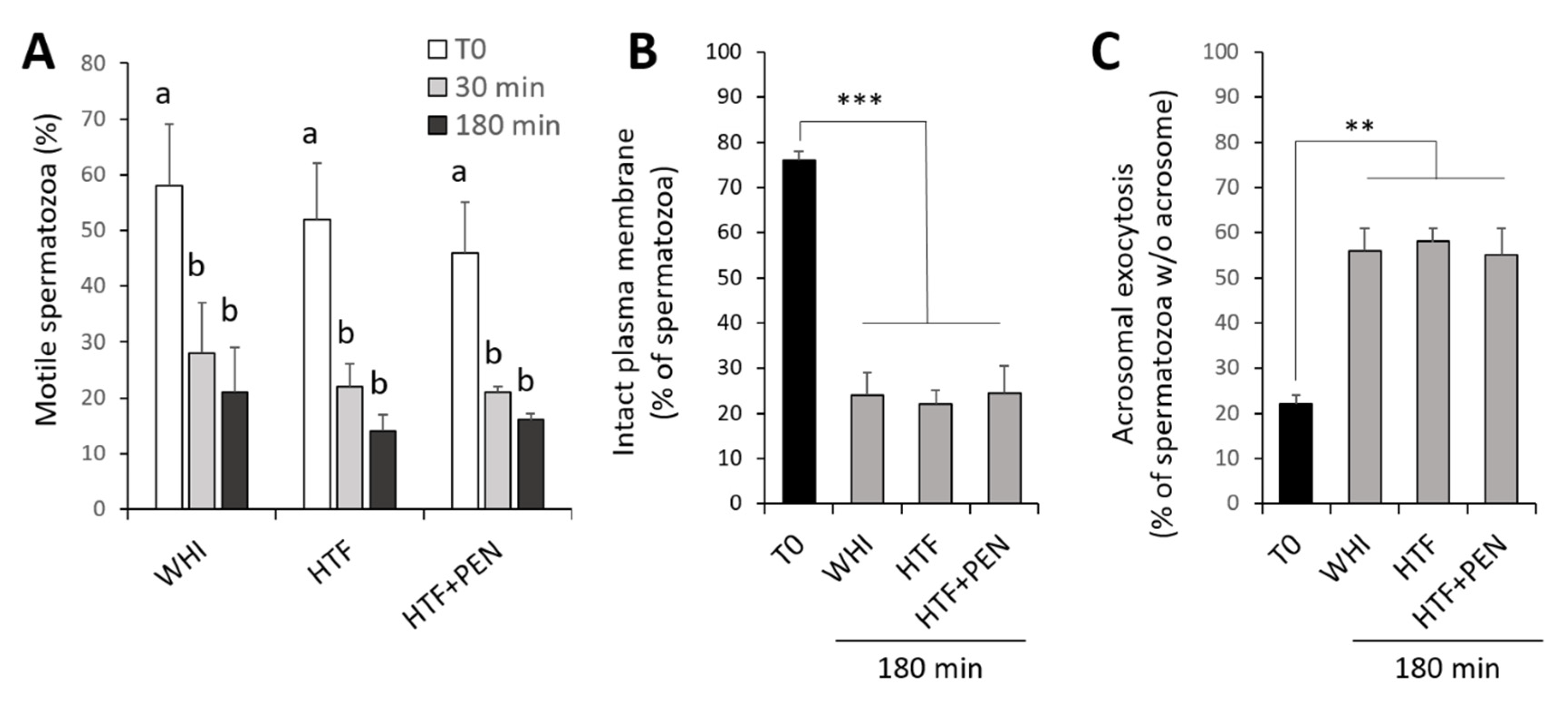
3.1. Effects of the Incubation Medium on Sperm Kinetics and Protein Tyrosine Phosphorylation
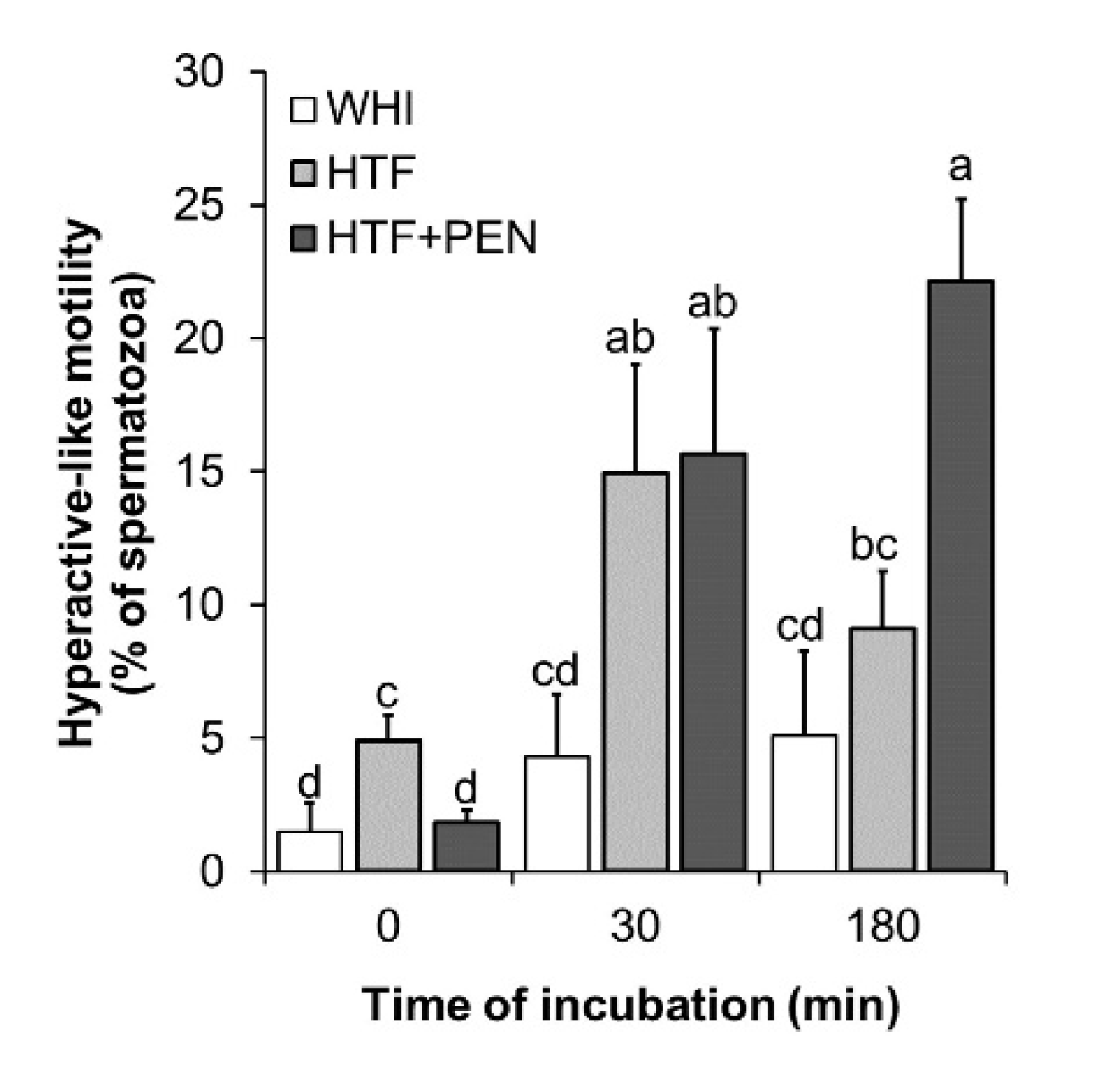
3.2. Effects of HTF Supplementation with Penicillamine on Sperm Kinetics and Protein Tyrosine Phosphorylation
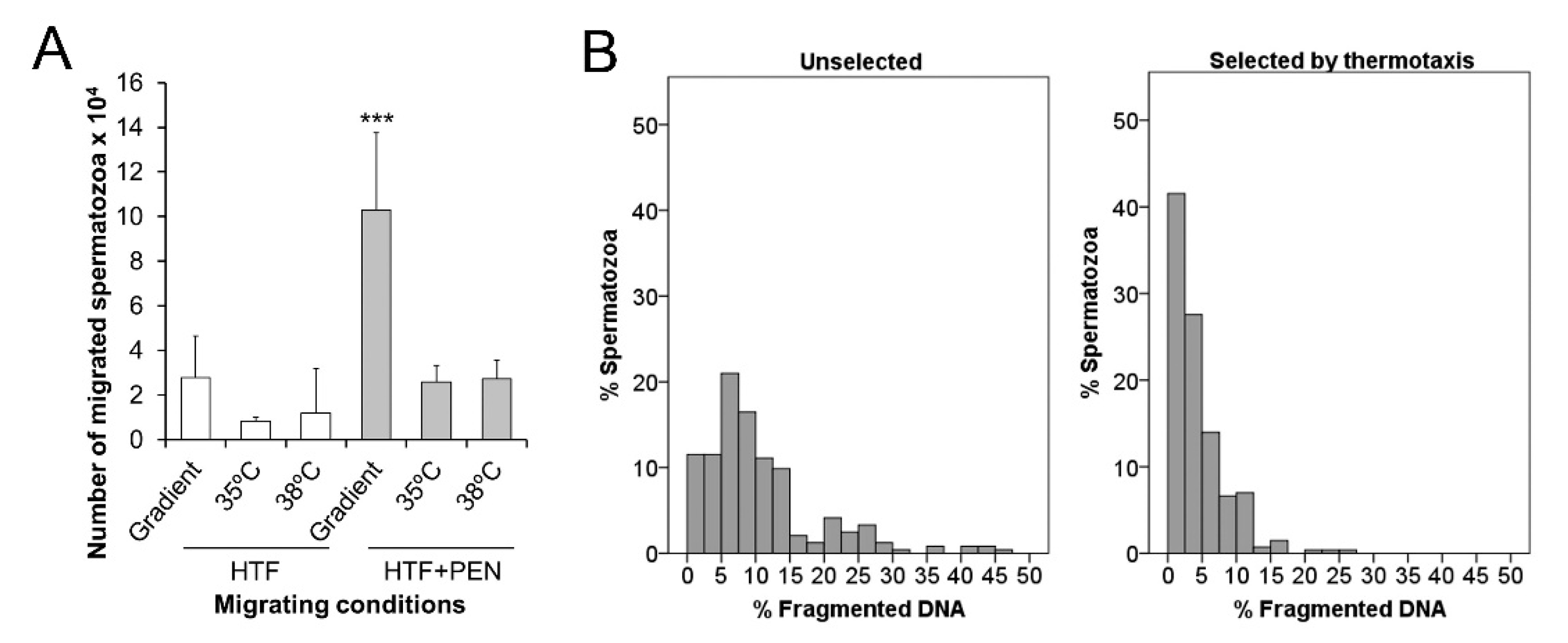
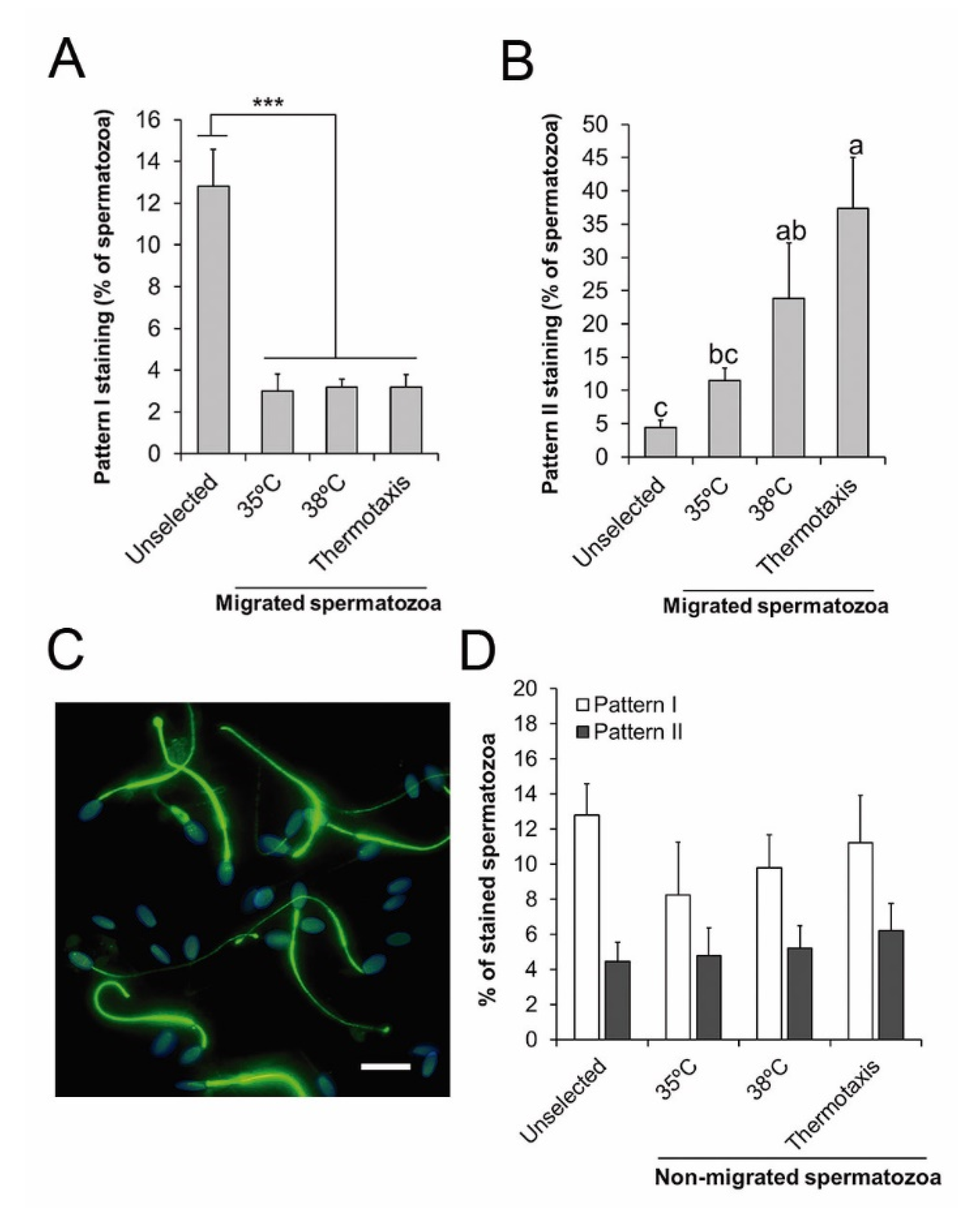
3.3. Sperm Thermotaxis
4. Discussion
4.1. Capacitation-Related Events During Incubation in HTF
4.2. Effect of Penicillamine on Capacitation-Related Events During Incubation with HTF
4.3. Penicillamine Enables Sperm Selection by Thermotaxis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leemans, B.; Gadella, B.M.; Stout, T.A.E.; De Schauwer, C.; Nelis, H.; Hoogewijs, M.; Van Soom, A. Why doesn’t conventional IVF work in the horse? The equine oviduct as a microenvironment for capacitation/fertilization. Reproduction 2016, 152, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.H.; Maclellan, L.J. Update on advanced semen-processing technologies and their application for in vitro embryo production in horses. Reprod. Fertil. Dev. 2019, 31, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, E.G.; Aitken, R.J.; Gibb, Z.; Lambourne, S.R.; Nixon, B. Capacitation in the presence of methyl-β-cyclodextrin results in enhanced zona pellucida-binding ability of stallion spermatozoa. Reproduction 2014, 147, 153–166. [Google Scholar] [CrossRef]
- Loux, S.C.; Crawford, K.R.; Ing, N.H.; González-Fernández, L.; Macías-García, B.; Love, C.C.; Varner, D.D.; Velez, I.C.; Choi, Y.H.; Hinrichs, K. CatSper and the Relationship of hyperactivated motility to intracellular calcium andpH kinetics in equine sperm. Biol. Reprod. 2013, 89, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Macías-García, B.; Gonzalez-Fernandez, L.; Loux, S.C.; Rocha, A.M.; Guimarães, T.; Pena, F.J.; Varner, D.D.; Hinrichs, K. Effect of calcium, bicarbonate, and albumin on capacitation-related events in equine sperm. Reproduction 2015, 149, 87–99. [Google Scholar] [CrossRef]
- McPartlin, L.A.; Suarez, S.S.; Czaya, C.A.; Hinrichs, K.; Bedford-Guaus, S.J. Hyperactivation of Stallion Sperm Is Required for Successful In Vitro Fertilization of Equine Oocytes. Biol. Reprod. 2009, 81, 199–206. [Google Scholar] [CrossRef]
- Ortgies, F.; Klewitz, J.; Görgens, A.; Martinsson, G.; Sieme, H. Effect of procaine, pentoxifylline and trolox on capacitation and hyperactivation of stallion spermatozoa. Andrologia 2012, 44, 130–138. [Google Scholar] [CrossRef]
- Arroyo-Salvo, C.; Sanhueza, F.; Fuentes, F.; Treulén, F.; Arias, M.E.; Cabrera, P.; Silva, M.; Felmer, R. Effect of human tubal fluid medium and hyperactivation inducers on stallion sperm capacitation and hyperactivation. Reprod. Domest. Anim. 2018, 54, 184–194. [Google Scholar] [CrossRef]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef]
- Si, Y.; Okuno, M. Role of Tyrosine Phosphorylation of Flagellar Proteins in Hamster Sperm Hyperactivation1. Biol. Reprod. 1999, 61, 240–246. [Google Scholar] [CrossRef]
- Nassar, A.; Mahony, M.; Morshedi, M.; Lin, M.H.; Srisombut, C.; Oehninger, S. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil. Steril. 1999, 71, 919–923. [Google Scholar] [CrossRef]
- Leemans, B.; Stout, T.A.E.; De Schauwer, C.; Heras, S.; Nelis, H.; Hoogewijs, M.; Van Soom, A.; Gadella, B.M. Update on mammalian sperm capacitation: How much does the horse differ from other species? Reproduction 2019, 157, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aguirregomezcorta, J.; Sugrue, E.; Martínez-Fresneda, L.; Newport, D.; Fair, S. Hyperactivated stallion spermatozoa fail to exhibit a rheotaxis-like behaviour, unlike other species. Sci. Rep. 2018, 8, 16897. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Dayag, A.; Tur-Kaspa, I.; Dor, J.; Mashiach, S.; Eisenbach, M. Sperm capacitation in humans is transient and correlates with chemotactic responsiveness to follicular factors. PNAs 1995, 92, 11039–11043. [Google Scholar] [CrossRef]
- Eisenbach, M. Mammalian sperm chemotaxis and its association with capacitation. Dev. Genet. 1999, 25, 87–94. [Google Scholar] [CrossRef]
- Giojalas, L.C.; Rovasio, R.A.; Fabro, G.; Gakamsky, A.; Eisenbach, M. Timing of sperm capacitation appears to be programmed according to egg availability in the female genital tract. Fertil. Steril. 2004, 82, 247–249. [Google Scholar] [CrossRef]
- Harrison, R.A.P. Capacitation mechanisms, and the role of capacitation as seen in eutherian mammals. Reprod. Fertil. Dev. 1996, 8, 581–594. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef]
- Petrunkina, A.M.; Waberski, D.; Günzel-Apel, A.R.; Töpfer-Petersen, E. Determinants of sperm quality and fertility in domestic species. Reproduction 2007, 134, 3–17. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Mitchell, L.A.; Lambourne, S.R.; Connaughton, H.S.; De Iuliis, G.N. Sperm Motility Is Lost In Vitro as a Consequence of Mitochondrial Free Radical Production and the Generation of Electrophilic Aldehydes but Can Be Significantly Rescued by the Presence of Nucleophilic Thiols. Biol. Reprod. 2012, 87, 110. [Google Scholar] [CrossRef]
- Pavlok, A. D-penicillamine and granulosa cells can effectively extend the fertile life span of bovine frozen-thawed spermatozoa in vitro: Effect on fertilization and polyspermy. Theriogenology 2000, 53, 1135–1146. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Laguna-Barraza, R.; De Castro, A.C.; Sánchez-Calabuig, M.J.; Cano-Oliva, E.; De Castro-Pita, F.J.; Montoro-Buils, L.; Pericuesta, E.; Fernández-González, R.; Gutiérrez-Adán, A. Sperm selection by thermotaxis improves ICSI outcome in mice. Sci. Rep. 2018, 8, 2902. [Google Scholar] [CrossRef] [PubMed]
- Bahat, A.; Tur-Kaspa, I.; Gakamsky, A.; Giojalas, L.C.; Breitbart, H.; Eisenbach, M. Thermotaxis of mammalian sperm cells: A potential navigation mechanism in the female genital tract. Nat. Med. 2003, 9, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Bahat, A.; Caplan, S.R.; Eisenbach, M. Thermotaxis of human sperm cells in extraordinarily shallow temperature gradients over a wide range. PLoS ONE 2012, 7, e41915. [Google Scholar] [CrossRef]
- Garner, D.L.; Johnson, L.A. Viability Assessment of Mammalian Sperm Using SYBR-14 and Propidium Iodide1. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef]
- Lybaert, P.; Danguy, A.; Leleux, F.; Meuris, S.; Lebrun, P. Improved methodology for the detection and quantification of the acrosome reaction in mouse spermatozoa. Histol. Histopathol. 2009, 24, 999–1007. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Giaretta, E.; Munerato, M.; Yeste, M.; Galeati, G.; Spinaci, M.; Tamanini, C.; Mari, G.; Bucci, D. Implementing an open-access CASA software for the assessment of stallion sperm motility: Relationship with other sperm quality parameters. Anim. Reprod. Sci. 2017, 176, 11–19. [Google Scholar] [CrossRef]
- Mortimer, D.; Serres, C.; Mortimer, S.T.; Jouannet, P. Influence of image sampling frequency on the perceived movement characteristics of progressively motile human spermatozoa. Gamete Res. 1988, 20, 313–327. [Google Scholar] [CrossRef]
- Su, T.W.; Choi, I.; Feng, J.; Huang, K.; McLeod, E.; Ozcan, A. Sperm trajectories form chiral ribbons. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef]
- Końca, K.; Lankoff, A.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Koza, Z.; Wojcik, A. A cross-platform public domain PC image-analysis program for the comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 534, 15–20. [Google Scholar] [CrossRef]
- De Andrade, A.F.C.; Zaffalon, F.G.; Celeghini, E.C.C.; Nascimento, J.; Bressan, F.F.; Martins, S.M.M.K.; de Arruda, R.P. Post-thaw addition of seminal plasma reduces tyrosine phosphorylation on the surface of cryopreserved equine sperm, but does not reduce lipid peroxidation. Theriogenology 2012, 77, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Naresh, S.; Atreja, S.K. The protein tyrosine phosphorylation during in vitro capacitation and cryopreservation of mammalian spermatozoa. Cryobiology 2015, 70, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Pommer, A.C.; Rutllant, J.; Meyers, S.A. Phosphorylation of Protein Tyrosine Residues in Fresh and Cryopreserved Stallion Spermatozoa under Capacitating Conditions. Biol. Reprod. 2003, 68, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Sakase, M.; Fukushima, M.; Harayama, H. Changes of IZUMO1 in bull spermatozoa during the maturation, acrosome reaction, and cryopreservation. Theriogenology 2016, 86, 2179–2188.e3. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Bioenergetics of mammalian sperm capacitation. BioMed Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef]
- Darr, C.R.; Varner, D.D.; Teague, S.; Cortopassi, G.A.; Datta, S.; Meyers, S.A. Lactate and Pyruvate Are Major Sources of Energy for Stallion Sperm with Dose Effects on Mitochondrial Function, Motility, and ROS Production. Biol. Reprod. 2016, 95, 34. [Google Scholar] [CrossRef]
- Leemans, B.; Gadella, B.M.; Stout, T.A.E.; Nelis, H.; Hoogewijs, M.; Van Soom, A. An alkaline follicular fluid fraction induces capacitation and limited release of oviduct epithelium-bound stallion sperm. Reproduction 2015, 150, 193–208. [Google Scholar] [CrossRef]
- Orsi, N.M.; Gopichandran, N.; Leese, H.J.; Picton, H.M.; Harris, S.E. Fluctuations in bovine ovarian follicular fluid composition throughout the oestrous cycle. Reproduction 2005, 129, 219–228. [Google Scholar] [CrossRef]
- Zeilmaker, G.H.; Verhamme, C.M.P.M. Lactate concentrations in pre-ovulatory follicles of pro-oestrous rats before and after onset of oocyte maturation. Acta Endocrinol. 1977, 86, 380–383. [Google Scholar] [CrossRef]
- Nandi, S.; Girish Kumar, V.; Manjunatha, B.M.; Ramesh, H.S.; Gupta, P.S.P. Follicular fluid concentrations of glucose, lactate and pyruvate in buffalo and sheep, and their effects on cultured oocytes, granulosa and cumulus cells. Theriogenology 2008, 69, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Gull, I.; Geva, E.; Lerner-Geva, L.; Lessing, J.B.; Wolman, I.; Amit, A. Anaerobic glycolysis. The metabolism of the preovulatory human oocyte. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 85, 225–228. [Google Scholar] [CrossRef]
- González-Fernández, L.; Sánchez-Calabuig, M.J.; Calle-Guisado, V.; García-Marín, L.J.; Bragado, M.J.; Fernández-Hernández, P.; Gutiérrez-Adán, A.; Macías-García, B. Stage-specific metabolomic changes in equine oviductal fluid: New insights into the equine fertilization environment. Theriogenology 2020, 143, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pesini, E.; Díez-Sánchez, C.; López-Pérez, M.J.; Enríquez, J.A. The Role of the Mitochondrion in Sperm Function: Is There a Place for Oxidative Phosphorylation or Is This a Purely Glycolytic Process? Curr. Top. Dev. Biol. 2007, 77, 3–19. [Google Scholar]
- Pérez-Cerezales, S.; Ramos-Ibeas, P.; Acuña, O.S.; Avilés, M.; Coy, P.; Rizos, D.; Gutiérrez-Adán, A. The oviduct: From sperm selection to the epigenetic landscape of the embryo. Biol. Reprod. 2018, 98, 262–276. [Google Scholar] [CrossRef]
- Leclerc, P.; De Lamirande, E.; Gagnon, C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic. Biol. Med. 1997, 22, 643–656. [Google Scholar] [CrossRef]
- Gadella, B.M.; Harrison, R.A.P. The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development 2000, 127, 2407–2420. [Google Scholar]
- Kerns, K.; Sharif, M.; Zigo, M.; Xu, W.; Hamilton, L.E.; Sutovsky, M.; Ellersieck, M.; Drobnis, E.Z.; Bovin, N.; Oko, R.; et al. Sperm cohort-specific zinc signature acquisition and capacitation-induced zinc flux regulate sperm-oviduct and sperm-zona pellucida interactions. Int. J. Mol. Sci. 2020, 21, 2121. [Google Scholar] [CrossRef]
- Ruiz-Díaz, S.; Grande-Pérez, S.; Arce-López, S.; Tamargo, C.; Hidalgo, C.O.; Pérez-Cerezales, S. Changes in the Cellular Distribution of Tyrosine Phosphorylation and Its Relationship with the Acrosomal Exocytosis and Plasma Membrane Integrity during In Vitro Capacitation of Frozen/Thawed Bull Spermatozoa. Int. J. Mol. Sci. 2020, 21, 2725. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum. Reprod. 1995, 10, 15–24. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int. J. Androl. 1993, 16, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; Alsalamat, H.A.; Bashatwah, R.M. Reactive oxygen species: The dual role in physiological and pathological conditions of the human body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Boryshpolets, S.; Afanzar, O.; Brandis, A.; Nevo, R.; Kiss, V.; Eisenbach, M. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 2015, 5, 16146. [Google Scholar] [CrossRef]
- Roy, D.; Levi, K.; Kiss, V.; Nevo, R.; Eisenbach, M. Rhodopsin and melanopsin coexist in mammalian sperm cells and activate different signaling pathways for thermotaxis. Sci. Rep. 2020, 10, 112. [Google Scholar] [CrossRef]
- De Toni, L.; Garolla, A.; Menegazzo, M.; Magagna, S.; Di Nisio, A.; Šabović, I.; Rocca, M.S.; Scattolini, V.; Filippi, A.; Foresta, C. Heat Sensing Receptor TRPV1 Is a Mediator of Thermotaxis in Human Spermatozoa. PLoS ONE 2016, 11, e0167622. [Google Scholar] [CrossRef]
- Si, Y. Hyperactivation of hamster sperm motility by temperature-dependent tyrosine phosphorylation of an 80-kDa protein. Biol. Reprod. 1999, 61, 247–252. [Google Scholar] [CrossRef]
- Boryshpolets, S.; Pérez-Cerezales, S.; Eisenbach, M. Behavioral mechanism of human sperm in thermotaxis: A role for hyperactivation. Hum. Reprod. 2015, 30, 884–892. [Google Scholar] [CrossRef]
- Leemans, B.; Stout, T.A.E.; Van Soom, A.; Gadella, B.M. pH-dependent effects of procaine on equine gamete activation. Biol. Reprod. 2019, 101, 1056–1074. [Google Scholar] [CrossRef]
- Colás, C.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Caffeine induces ram sperm hyperactivation independent of cAMP-dependent protein kinase. Int. J. Androl. 2010, 33, 187–197. [Google Scholar] [CrossRef]

| Kinetics | T0 | 30 min | 180 min | ||||||
|---|---|---|---|---|---|---|---|---|---|
| WHI | HTF | HTF + PEN | WHI | HTF | HTF + PEN | WHI | HTF | HTF + PEN | |
| VCL (µm/s) | 80 ± 6 | 96 ± 5 * | 97 ± 2 a * | 87 ± 7 | 109 ± 7 * | 114 ± 5 b * | 81 ± 7 | 107 ± 4 * | 122 ± 3 b ** |
| VAP (µm/s) | 36 ± 1 | 40 ± 2 | 40 ± 0.8 a | 37 ± 1 | 44 ± 3 | 45 ± 2 b | 35 ± 2 | 41 ± 2 | 47 ± 1 b * |
| VSL (µm/s) | 28 ± 2 | 30 ± 1 | 29 ± 1 | 27 ± 1 | 26 ± 3 | 27 ± 1 | 24 ± 2 | 25 ± 2 | 25 ± 2 |
| LIN (%) | 37 ± 4 | 33 ± 2 a | 31 ± 2 a | 34 ± 2 | 25 ± 3 b | 25 ± 1 b | 30 ± 1 | 24 ± 2 b * | 21 ± 1 c * |
| STR (%) | 78 ± 4 | 75 ± 3 a | 71 ± 3 a | 74 ± 2 | 59 ± 7 b * | 61 ± 3 b * | 64 ± 4 | 61 ± 4 b | 55 ± 3 b * |
| WOB (%) | 46 ± 2 | 43 ± 1 a | 43 ± 1 a | 44 ± 3 | 41 ± 1 a | 40 ± 1 a b | 46 ± 2 | 39 ± 1 b | 39 ± 1 b |
| Beat Cross (Hz) | 24.2 ± 1.4 | 24 ± 0.5 a | 23.7 ± 0.5 | 25.3 ± 1 | 26 ± 0.5 a b | 24.2 ± 0.7 | 26.1 ± 0.9 | 27 ± 1 b | 24.7 ± 1.1 |
| ALH (µm) | 3 ± 0.2 | 3.5 ± 0.2 * | 3.5 ± 0.1 a * | 3.2 ± 0.2 | 3.9 ± 0.3 * | 4.1 ± 0.2 b * | 2.9 ± 0.2 | 3.8 ± 0.2 * | 4.4 ± 0.1 b ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Díaz, S.; Oseguera-López, I.; De La Cuesta-Díaz, D.; García-López, B.; Serres, C.; Sanchez-Calabuig, M.J.; Gutiérrez-Adán, A.; Perez-Cerezales, S. The Presence of D-Penicillamine during the In Vitro Capacitation of Stallion Spermatozoa Prolongs Hyperactive-Like Motility and Allows for Sperm Selection by Thermotaxis. Animals 2020, 10, 1467. https://doi.org/10.3390/ani10091467
Ruiz-Díaz S, Oseguera-López I, De La Cuesta-Díaz D, García-López B, Serres C, Sanchez-Calabuig MJ, Gutiérrez-Adán A, Perez-Cerezales S. The Presence of D-Penicillamine during the In Vitro Capacitation of Stallion Spermatozoa Prolongs Hyperactive-Like Motility and Allows for Sperm Selection by Thermotaxis. Animals. 2020; 10(9):1467. https://doi.org/10.3390/ani10091467
Chicago/Turabian StyleRuiz-Díaz, Sara, Ivan Oseguera-López, David De La Cuesta-Díaz, Belén García-López, Consuelo Serres, Maria José Sanchez-Calabuig, Alfonso Gutiérrez-Adán, and Serafin Perez-Cerezales. 2020. "The Presence of D-Penicillamine during the In Vitro Capacitation of Stallion Spermatozoa Prolongs Hyperactive-Like Motility and Allows for Sperm Selection by Thermotaxis" Animals 10, no. 9: 1467. https://doi.org/10.3390/ani10091467
APA StyleRuiz-Díaz, S., Oseguera-López, I., De La Cuesta-Díaz, D., García-López, B., Serres, C., Sanchez-Calabuig, M. J., Gutiérrez-Adán, A., & Perez-Cerezales, S. (2020). The Presence of D-Penicillamine during the In Vitro Capacitation of Stallion Spermatozoa Prolongs Hyperactive-Like Motility and Allows for Sperm Selection by Thermotaxis. Animals, 10(9), 1467. https://doi.org/10.3390/ani10091467







