Oral Supplementation with Ultramicronized Palmitoylethanolamide for Joint Disease and Lameness Management in Four Jumping Horses: A Case Report
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
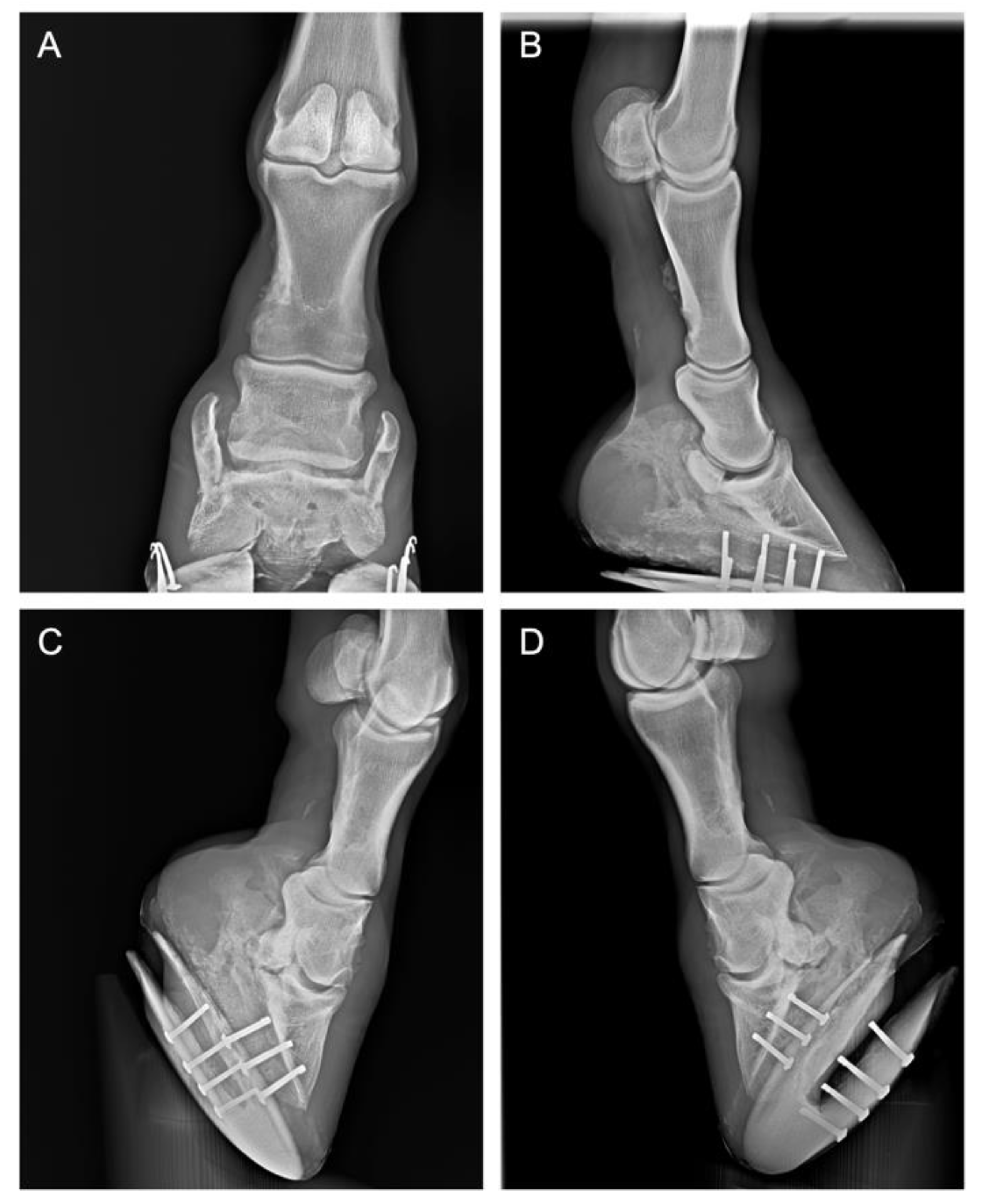
2.1. History and Case Presentation
2.2. Clinical Evaluations
2.3. PEA-un Supplementation
3. Results
Effect of PEA-um Supplementation and Lameness Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trumble, T.N. The use of nutraceuticals for osteoarthritis in horses. Vet. Clin. North Am. Equine Pract. 2005, 21, 575–597. [Google Scholar] [CrossRef] [PubMed]
- Parkes, R.; Newton, R.; Dyson, S. Is there an association between clinical features, response to diagnostic analgesia and radiological findings in horses with a magnetic resonance imaging diagnosis of navicular disease or other injuries of the podotrochlear apparatus? Vet. J. 2015, 204, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Waguespack, R.W.; Hanson, R.R. Treating navicular syndrome in equine patients. Compend. Contin. Educ. Vet. 2011, 33, E2. [Google Scholar] [PubMed]
- Grewal, J.S.; McClure, S.R.; Booth, L.C.; Evans, R.B.; Caston, S.S. Assessment of the ultrasonographic characteristics of the podotrochlear apparatus in clinically normal horses and horses with navicular syndrome. J. Am. Vet. Med. Assoc. 2004, 225, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Neil, K.M.; Caron, J.P.; Orth, M.W. The role of glucosamine and chondroitin sulfate in treatment for and prevention of osteoarthritis in animals. J. Am. Vet. Med. Assoc. 2005, 226, 1079–1088. [Google Scholar] [CrossRef] [Green Version]
- Re, G.; Barbero, R.; Miolo, A.; Di Marzo, V. Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: Potential use in companion animals. Vet. J. 2007, 173, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Stefano, G.D.; Marchettini, P.; Truini, A. Micronized Palmitoylethanolamide: A Post Hoc Analysis of a Controlled Study in Patients with Low Back Pain—Sciatica. CNS Neurol Disord Drug Targets 2019, 18, 491–495. [Google Scholar] [CrossRef]
- Noli, C.; Valle, M.F.D.; Miolo, A.; Medori, C.; Schievano, C.; The Skinalia Clinical Research Group. Efficacy of ultra-micronized palmitoylethanolamide in canine atopic dermatitis: An open-label multi-centre study. Vet. Dermatol. 2015, 26, 432-e101. [Google Scholar] [CrossRef]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br. J. Clin. Pharmacol 2016, 82, 932–942. [Google Scholar] [CrossRef] [Green Version]
- Scarampella, F.; Abramo, F.; Noli, C. Clinical and histological evaluation of an analogue of palmitoylethanolamide, PLR 120 (comicronized Palmidrol INN) in cats with eosinophilic granuloma and eosinophilic plaque: A pilot study. Vet. Dermatol. 2001, 12, 29–39. [Google Scholar] [CrossRef]
- Steel, C.M.; Hopper, B.J.; Richardson, J.L.; Alexander, G.R.; Robertson, I.D. Clinical findings, diagnosis, prevalence and predisposing factors for lameness localised to the middle carpal joint in young Standardbred racehorses. Equine Vet. J. 2006, 38, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Marques-Smith, P.; Kallerud, A.S.; Johansen, G.M.; Boysen, P.; Jacobsen, A.M.; Reitan, K.M.; Henriksen, M.M.; Lofgren, M.; Fjordbakk, C.T. Is clinical effect of autologous conditioned serum in spontaneously occurring equine articular lameness related to ACS cytokine profile? BMC Vet. Res. 2020, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Dobenecker, B.; Reese, S.; Jahn, W.; Schunck, M.; Hugenberg, J.; Louton, H.; Oesser, S. Specific bioactive collagen peptides (PETAGILE((R))) as supplement for horses with osteoarthritis: A two-centred study. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018, 102 (Suppl. 1), 16–23. [Google Scholar] [CrossRef] [PubMed]
- De Clifford, L.T.; Lowe, J.N.; McKellar, C.D.; Bolwell, C.; David, F. Use of a 2.5% Cross-Linked Polyacrylamide Hydrogel in the Management of Joint Lameness in a Population of Flat Racing Thoroughbreds: A Pilot Study. J. Equine Vet. Sci. 2019, 77, 57–62. [Google Scholar] [CrossRef]
- De Grauw, J.C.; van Loon, J.P. Systematic pain assessment in horses. Vet. J. 2016, 209, 14–22. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [Green Version]
- McDougall, J.J.; Albacete, S.; Schuelert, N.; Mitchell, P.G.; Lin, C.; Oskins, J.L.; Bui, H.H.; Chambers, M.G. Lysophosphatidic acid provides a missing link between osteoarthritis and joint neuropathic pain. Osteoarthr. Cartil. 2017, 25, 926–934. [Google Scholar] [CrossRef] [Green Version]
- Rijkenhuizen, A.B. Navicular disease: A review of what’s new. Equine Vet. J. 2006, 38, 82–88. [Google Scholar] [CrossRef]
- Contino, E.K. Management and Rehabilitation of Joint Disease in Sport Horses. Vet. Clin. North Am. Equine Pract. 2018, 34, 345–358. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Fusco, M.; Della Valle, M.F.; Zusso, M.; Costa, B.; Giusti, P. Palmitoylethanolamide, a naturally occurring disease-modifying agent in neuropathic pain. Inflammopharmacology 2014, 22, 79–94. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Peritore, A.F.; Piras, C.; Cuzzocrea, S.; Crupi, R. Palmitoylethanolamide and Related ALIAmides: Prohomeostatic Lipid Compounds for Animal Health and Wellbeing. Vet. Sci. 2020, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M.K.; Kopsky, D.J. Palmitoylethanolamide, a neutraceutical, in nerve compression syndromes: Efficacy and safety in sciatic pain and carpal tunnel syndrome. J. Pain Res. 2015, 8, 729–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faig-Marti, J.; Martinez-Catassus, A. Use of palmitoylethanolamide in carpal tunnel syndrome: A prospective randomized study. J. Orthop. Traumatol. 2017, 18, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Gugliandolo, E.; Campolo, M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. Correction: Effect of a new formulation of micronized and ultramicronized N-palmitoylethanolamine in a tibia fracture mouse model of complex regional pain syndrome. PLoS ONE 2018, 13, e0201501. [Google Scholar] [CrossRef] [PubMed]
- Britti, D.; Crupi, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Schievano, C.; Morittu, V.M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017, 13, 229. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Di Paola, R.; Cordaro, M.; Gugliandolo, E.; Casili, G.; Morittu, V.M.; Britti, D.; Esposito, E.; Cuzzocrea, S. Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 2016, 119, 27–41. [Google Scholar] [CrossRef]
- Valastro, C.; Campanile, D.; Marinaro, M.; Franchini, D.; Piscitelli, F.; Verde, R.; Di Marzo, V.; Di Bello, A. Characterization of endocannabinoids and related acylethanolamides in the synovial fluid of dogs with osteoarthritis: A pilot study. BMC Vet. Res. 2017, 13, 309. [Google Scholar] [CrossRef] [Green Version]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-analysis. Pain Physician 2016, 19, 11–24. [Google Scholar]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018, 15, 264. [Google Scholar] [CrossRef] [Green Version]
- Bartolucci, M.L.; Marini, I.; Bortolotti, F.; Impellizzeri, D.; Di Paola, R.; Bruschetta, G.; Crupi, R.; Portelli, M.; Militi, A.; Oteri, G.; et al. Micronized palmitoylethanolamide reduces joint pain and glial cell activation. Inflamm. Res. 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Steels, E.; Venkatesh, R.; Steels, E.; Vitetta, G.; Vitetta, L. A double-blind randomized placebo controlled study assessing safety, tolerability and efficacy of palmitoylethanolamide for symptoms of knee osteoarthritis. Inflammopharmacology 2019, 27, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Noli, C.; Della Valle, M.F.; Miolo, A.; Medori, C.; Schievano, C.; The Skinalia Clinical Research Group. Effect of dietary supplementation with ultramicronized palmitoylethanolamide in maintaining remission in cats with nonflea hypersensitivity dermatitis: A double-blind, multicentre, randomized, placebo-controlled study. Vet. Dermatol. 2019, 30, 387-e117. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Case | Diagnosis | Parameter | Baseline | 30 Days after PEA-um | 60 Days after PEA-um | 90 Days after PEA-um | 120 Days after PEA-um |
|---|---|---|---|---|---|---|---|
| (1) 7-year-old S.I. gelding jumping horse | Navicular sindrome | Lameness Flexion test | 1 3 | 1 2 | 0 1 | 0 0 | 0 0 |
| (2) 16-year-old NRPS gelding jumping horse | Navicular syndrome and distal interphalangeal joint arthrosis of the right anterior limb | Lameness Flexion test | 3 3 | 2 3 | 2 2 | 1 1 | 0 0 |
| (3) 14-year-old Holst. mare jumping horse | Distal intertarsal joint arthritis | Lameness Flexion test | 2 3 | 1 2 | 0 1 | 0 0 | 0 0 |
| (4) 15-year-old NRPS mare jumping horse | Distal intertarsal joint arthritis | Lameness Flexion test | 3 3 | 2 2 | 1 1 | 0 1 | 0 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugliandolo, E.; Barbagallo, A.; Peritore, A.F.; Cuzzocrea, S.; Crupi, R. Oral Supplementation with Ultramicronized Palmitoylethanolamide for Joint Disease and Lameness Management in Four Jumping Horses: A Case Report. Animals 2020, 10, 1469. https://doi.org/10.3390/ani10091469
Gugliandolo E, Barbagallo A, Peritore AF, Cuzzocrea S, Crupi R. Oral Supplementation with Ultramicronized Palmitoylethanolamide for Joint Disease and Lameness Management in Four Jumping Horses: A Case Report. Animals. 2020; 10(9):1469. https://doi.org/10.3390/ani10091469
Chicago/Turabian StyleGugliandolo, Enrico, Alfio Barbagallo, Alessio Filippo Peritore, Salvatore Cuzzocrea, and Rosalia Crupi. 2020. "Oral Supplementation with Ultramicronized Palmitoylethanolamide for Joint Disease and Lameness Management in Four Jumping Horses: A Case Report" Animals 10, no. 9: 1469. https://doi.org/10.3390/ani10091469





