Prepartum Fat Mobilization in Dairy Cows with Equal Body Condition and Its Impact on Health, Behavior, Milk Production and Fertility during Lactation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Diets
2.2. Group Allocation
2.3. Blood Sample Collection and Analysis
2.4. Health Status and Herd Retention
2.5. Behavioral Data
2.6. Milk Production and Reproductive Data
2.7. Statistical Analysis
3. Results
3.1. Blood Biomarkers
3.2. Health Status and Herd Retention
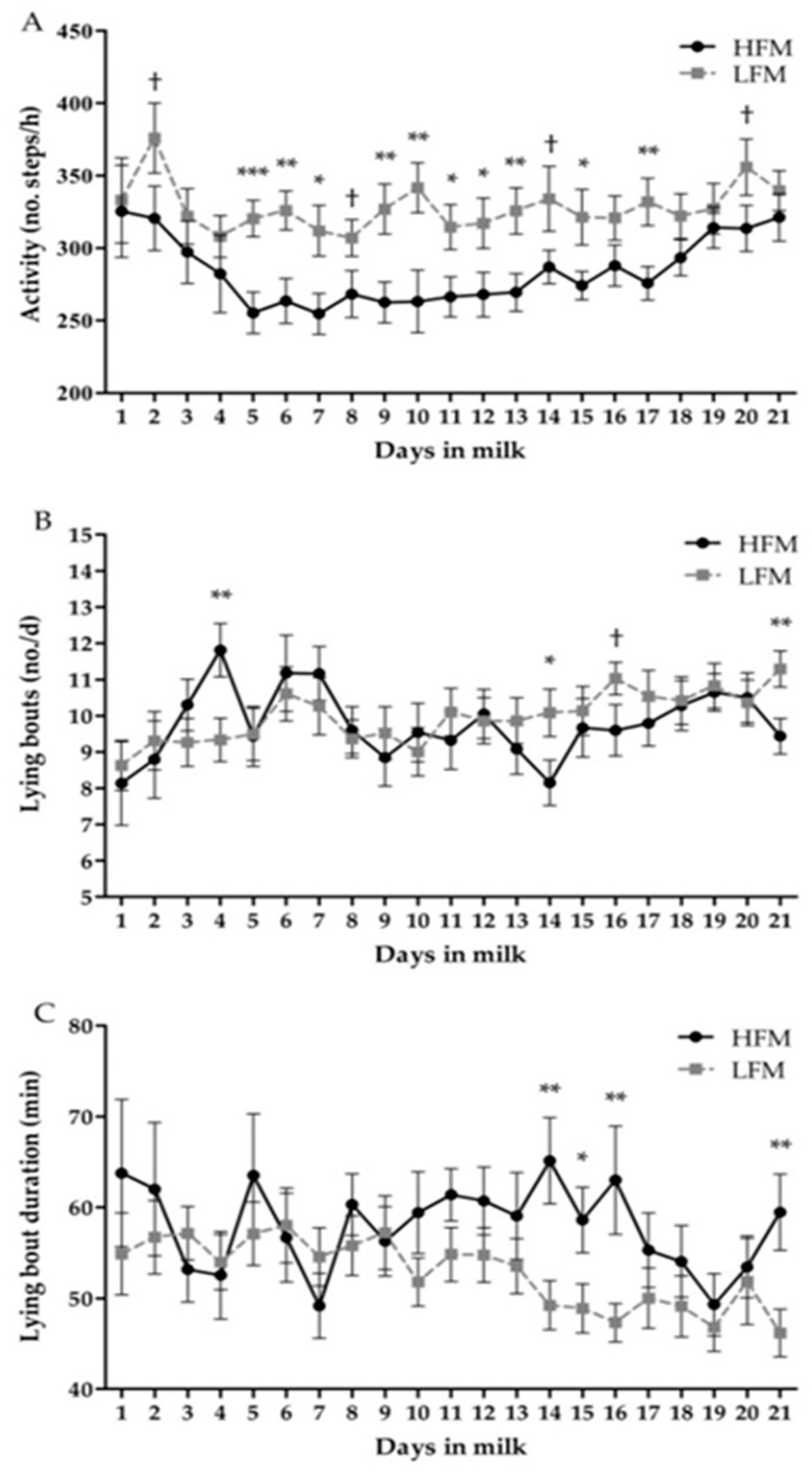
3.3. Behavior
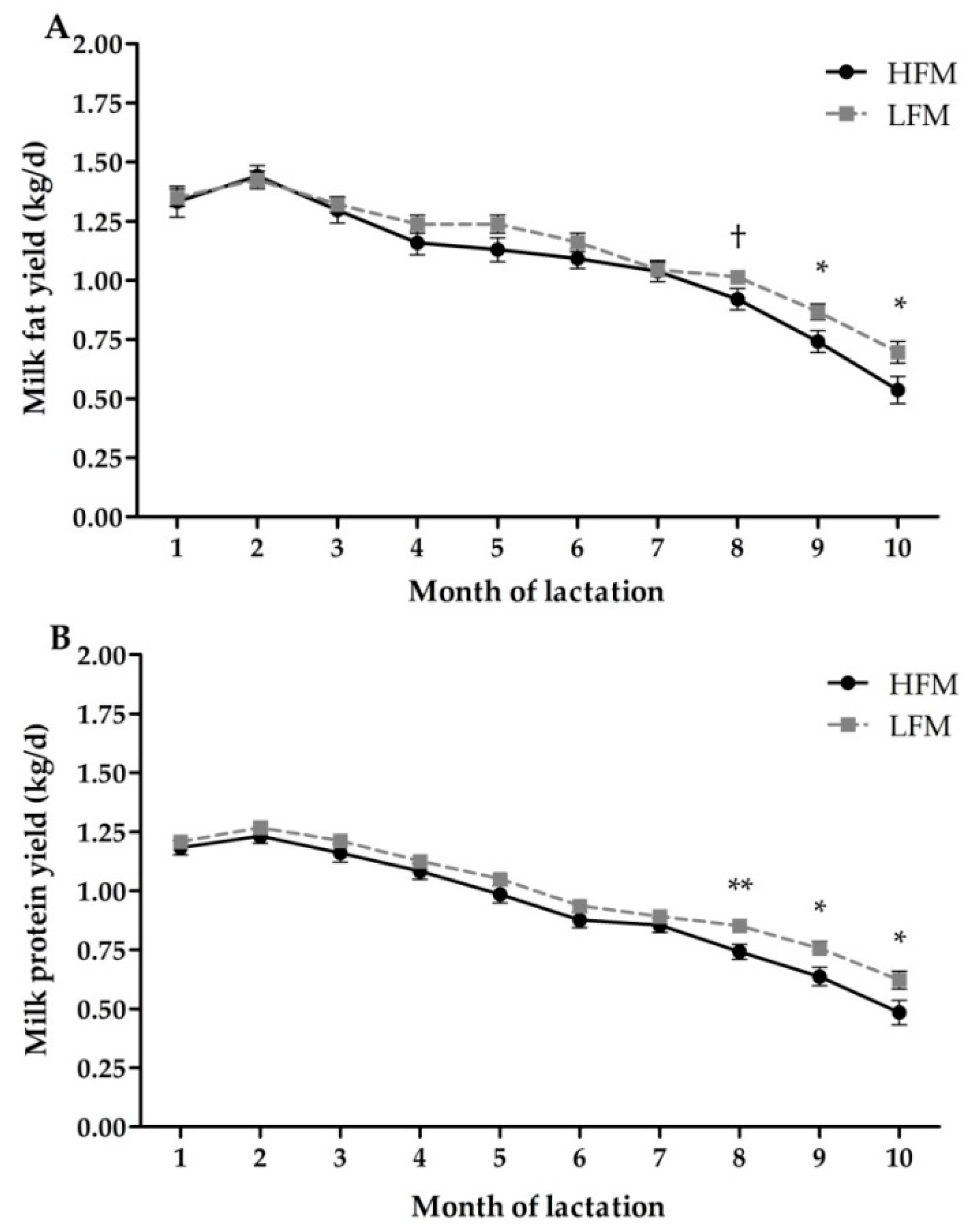
3.4. Milk Production and Composition
3.5. Reproduction
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Contreras, G.A.; Sordillo, L. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef]
- Drackley, J.K.; Andersen, J.B. Splanchnic metabolism of long-chain fatty acids in ruminants. In Ruminant Physiology: Digestion, Metabolism and Impact of Nutrition on Gene Expression, Immunology and Stress; Sejrsen, K., Hvelplund, T., Nielsen, M.O., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; Part 3; pp. 199–224. [Google Scholar]
- Huzzey, J.; Nydam, D.; Grant, R.; Overton, T. Associations of prepartum plasma cortisol, haptoglobin, fecal cortisol metabolites, and nonesterified fatty acids with postpartum health status in Holstein dairy cows. J. Dairy Sci. 2011, 94, 5878–5889. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Loor, J.; Bertoni, G.; Hosseini, A.; Roche, J.R.; Trevisi, E. Functional welfare—Using biochemical and molecular technologies to understand better the welfare state of peripartal dairy cattle. Anim. Prod. Sci. 2013, 53, 931–953. [Google Scholar] [CrossRef]
- Roche, J.R.; Kay, J.K.; Friggens, N.C.; Loor, J.; Berry, D. Assessing and Managing Body Condition Score for the Prevention of Metabolic Disease in Dairy Cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.; Nydam, D.; Stokol, T.; Overton, T. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef]
- Barletta, R.; Filho, M.M.; Carvalho, P.; Del Valle, T.; Netto, A.; Rennó, F.; Mingoti, R.; Gandra, J.; Mourão, G.; Fricke, P.; et al. Association of changes among body condition score during the transition period with NEFA and BHBA concentrations, milk production, fertility, and health of Holstein cows. Theriogenology 2017, 104, 30–36. [Google Scholar] [CrossRef]
- Nielsen, H.; Friggens, N.; Løvendahl, P.; Jensen, J.; Ingvartsen, K. Influence of breed, parity, and stage of lactation on lactational performance and relationship between body fatness and live weight. Livest. Prod. Sci. 2003, 79, 119–133. [Google Scholar] [CrossRef]
- Kessel, S.; Stroehl, M.; Meyer, H.H.D.; Hiss, S.; Sauerwein, H.; Schwarz, F.J.; Bruckmaier, R.M. Individual variability in physiological adaptation to metabolic stress during early lactation in dairy cows kept under equal conditions. J. Anim. Sci. 2008, 86, 2903–2912. [Google Scholar] [CrossRef]
- Weber, C.; Hametner, C.; Tuchscherer, A.; Losand, B.; Kanitz, E.; Otten, W.; Singh, S.; Bruckmaier, R.; Becker, F.; Kanitz, W.; et al. Variation in fat mobilization during early lactation differently affects feed intake, body condition, and lipid and glucose metabolism in high-yielding dairy cows. J. Dairy Sci. 2013, 96, 165–180. [Google Scholar] [CrossRef]
- Humer, E.; Khol-Parisini, A.; Gruber, L.; Wittek, T.; Aschenbach, J.R.; Zebeli, Q. Metabolic adaptation and reticuloruminal pH in periparturient dairy cows experiencing different lipolysis early postpartum. Animal 2016, 10, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M.; Moallem, U. Consistent magnitude of postpartum body weight loss within cows across lactations and the relation to reproductive performance. J. Dairy Sci. 2017, 100, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- De Koster, J.; Opsomer, G. Insulin Resistance in Dairy Cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef]
- Kahn, C.R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: A necessary distinction. Metabolism 1978, 27, 1893–1902. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C.; Rodríguez, C.C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2014, 99, 1003–1016. [Google Scholar] [CrossRef]
- Sordillo, L.; Raphael, W. Significance of Metabolic Stress, Lipid Mobilization, and Inflammation on Transition Cow Disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Bewley, J.; Schutz, M. An Interdisciplinary Review of Body Condition Scoring for Dairy Cattle. Prof. Anim. Sci. 2008, 24, 507–529. [Google Scholar] [CrossRef]
- Pires, J.A.; Delavaud, C.; Faulconnier, Y.; Pomiès, D.; Chilliard, Y. Effects of body condition score at calving on indicators of fat and protein mobilization of periparturient Holstein-Friesian cows. J. Dairy Sci. 2013, 96, 6423–6439. [Google Scholar] [CrossRef]
- Gärtner, T.; Gernand, E.; Gottschalk, J.; Donat, K. Relationships between body condition, body condition loss, and serum metabolites during the transition period in primiparous and multiparous cows. J. Dairy Sci. 2019, 102, 9187–9199. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, S.J. Monitoring Metabolic Health of Dairy Cattle in the Transition Period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Contreras, L.; Ryan, C.; Overton, T. Effects of Dry Cow Grouping Strategy and Prepartum Body Condition Score on Performance and Health of Transition Dairy Cows. J. Dairy Sci. 2004, 87, 517–523. [Google Scholar] [CrossRef]
- Edmonson, A.; Lean, I.; Weaver, L.; Farver, T.; Webster, G. A Body Condition Scoring Chart for Holstein Dairy Cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Sube, A.; Aguirre, C.; Dec, D.; Balocchi, O.; Alonso, M.F. Yield and quality of Lolium perenne L. pastures under irrigation in the Southern Zone of Chile. Agro Sur 2016, 44, 19–27. [Google Scholar]
- Ospina, P.; Nydam, D.; Stokol, T.; Overton, T. Evaluation of nonesterified fatty acids and β-hydroxybutyrate in transition dairy cattle in the northeastern United States: Critical thresholds for prediction of clinical diseases. J. Dairy Sci. 2010, 93, 546–554. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Elson, E.C. Quantitative Determination of Serum Haptoglobin: A Simple and Rapid Method. Am. J. Clin. Pathol. 1974, 62, 655–663. [Google Scholar] [CrossRef]
- Hirvonen, J.; Pyörälä, S.; Jousimies-Somer, H. Acute phase response in heifers with experimentally induced mastitis. J. Dairy Res. 1996, 63, 351–360. [Google Scholar] [CrossRef]
- Millar, H.R.; Simpson, J.G.; Stalker, A.L. An evaluation of the heat precipitation method for plasma fibrinogen estimation. J. Clin. Pathol. 1971, 24, 827–830. [Google Scholar] [CrossRef]
- Kelton, D.F.; Lissemore, K.D.; Martin, R.E. Recommendations for Recording and Calculating the Incidence of Selected Clinical Diseases of Dairy Cattle. J. Dairy Sci. 1998, 81, 2502–2509. [Google Scholar] [CrossRef]
- Sheldon, I.; Owens, S.E. Postpartum uterine infection and endometritis in dairy cattle. Anim. Reprod. 2017, 14, 622–629. [Google Scholar] [CrossRef]
- Huzzey, J.; Veira, D.; Weary, D.; Von Keyserlingk, M.A. Prepartum Behavior and Dry Matter Intake Identify Dairy Cows at Risk for Metritis. J. Dairy Sci. 2007, 90, 3220–3233. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.; Nydam, D.; Stokol, T.; Overton, T. Association between the proportion of sampled transition cows with increased nonesterified fatty acids and β-hydroxybutyrate and disease incidence, pregnancy rate, and milk production at the herd level. J. Dairy Sci. 2010, 93, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; McArt, J.A.; Overton, T.R.; Stokol, T.; Nydam, D.V. Using Nonesterified Fatty Acids and β-Hydroxybutyrate Concentrations During the Transition Period for Herd-Level Monitoring of Increased Risk of Disease and Decreased Reproductive and Milking Performance. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 387–412. [Google Scholar] [CrossRef]
- McCarthy, M.; Mann, S.; Nydam, D.; Overton, T.; McArt, J. Short communication: Concentrations of nonesterified fatty acids and β-hydroxybutyrate in dairy cows are not well correlated during the transition period. J. Dairy Sci. 2015, 98, 6284–6290. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; López-Alonso, M.; Pereira, V.; Benedito, J. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Valverde, I.; Pereira, V.; Sotillo, J.; López-Alonso, M.; Benedito, J.; Rodríguez, C.C. Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res. Vet. Sci. 2006, 80, 133–139. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, N.K.; Singh, O.P.; Pandey, V.; Verma, P.K. Oxidative Stress and Antioxidant Status during Transition Period in Dairy Cows. Asian-Australas. J. Anim. Sci. 2011, 24, 479–484. [Google Scholar] [CrossRef]
- Konvičná, J.; Vargová, M.; Paulíková, I.; Kovác, G.; Kostecká, Z. Oxidative stress and antioxidant status in dairy cows during prepartal and postpartal periods. Acta Vet. Brno 2015, 84, 133–140. [Google Scholar] [CrossRef]
- Colakoglu, H.E.; Yazlik, M.O.; Kaya, U.; Çolakoğlu, E.Ç.; Kurt, S.; Öz, B.; Bayramoglu, R.; Vural, M.R.; Küplülü, Ş. MDA and GSH-Px activity in transition dairy cows under seasonal variations and their relationship with reproductive performance. J. Vet. Res. 2017, 61, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Cerón, J.J.; E Silva, F.C.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of Inflammatory Conditions on Liver Activity in Puerperium Period and Consequences for Performance in Dairy Cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef]
- Hiss, S.; Weinkauf, C.; Hachenberg, S.; Sauerwein, H. Short communication: Relationship between metabolic status and the milk concentrations of haptoglobin and lactoferrin in dairy cows during early lactation. J. Dairy Sci. 2009, 92, 4439–4443. [Google Scholar] [CrossRef]
- Rafia, S.; Taghipour-Bazargani, T.; Khaki, Z.; Bokaie, S.; Tabrizi, S.S. Effect of body condition score on dynamics of hemogram in periparturient Holstein cows. Comp. Haematol. Int. 2011, 21, 933–943. [Google Scholar] [CrossRef]
- Roche, J.R.; Meier, S.; Heiser, A.; Mitchell, M.D.; Walker, C.G.; Crookenden, M.A.; Riboni, M.V.; Loor, J.; Kay, J. Effects of precalving body condition score and prepartum feeding level on production, reproduction, and health parameters in pasture-based transition dairy cows. J. Dairy Sci. 2015, 98, 7164–7182. [Google Scholar] [CrossRef]
- Montagner, P.; Krause, A.R.T.; Schwegler, E.; Weschenfelder, M.M.; Maffi, A.S.; Xavier, E.G.; Schneider, A.; Pereira, R.A.; Jacometo, C.B.; Schmitt, E.; et al. Relationship between pre-partum body condition score changes, acute phase proteins and energy metabolism markers during the peripartum period in dairy cows. Ital. J. Anim. Sci. 2017, 16, 329–336. [Google Scholar] [CrossRef]
- Roche, J.R.; Macdonald, K.; Schütz, K.; Matthews, L.; Verkerk, G.; Meier, S.; Loor, J.; Rogers, A.; McGowan, J.; Morgan, S.; et al. Calving body condition score affects indicators of health in grazing dairy cows. J. Dairy Sci. 2013, 96, 5811–5825. [Google Scholar] [CrossRef]
- Schuh, K.; Sadri, H.; Häussler, S.; Webb, L.A.; Urh, C.; Wagner, M.; Koch, C.; Frahm, J.; Dänicke, S.; Dusel, G.; et al. Comparison of performance and metabolism from late pregnancy to early lactation in dairy cows with elevated v. normal body condition at dry-off. Animal 2019, 13, 1478–1488. [Google Scholar] [CrossRef]
- Du, X.; Chen, L.; Huang, D.; Peng, Z.; Zhao, C.; Zhang, Y.; Zhu, Y.; Wang, Z.; Li, X.; Liu, G. Elevated Apoptosis in the Liver of Dairy Cows with Ketosis. Cell. Physiol. Biochem. 2017, 43, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Swartz, T.H. Review: Following the smoke signals: Inflammatory signaling in metabolic homeostasis and homeorhesis in dairy cattle. Animal 2020, 14, s144–s154. [Google Scholar] [CrossRef] [PubMed]
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Raphael, W.; Sordillo, L.M. Dietary Polyunsaturated Fatty Acids and Inflammation: The Role of Phospholipid Biosynthesis. Int. J. Mol. Sci. 2013, 14, 21167–21188. [Google Scholar] [CrossRef] [PubMed]
- Palladino, R.A.; O’Donovan, M.; Kennedy, E.; Murphy, J.J.; Boland, T.M.; Kenny, D.A. Fatty acid composition and nutritive value of twelve cultivars of perennial ryegrass. Grass Forage Sci. 2009, 64, 219–226. [Google Scholar] [CrossRef]
- Sepúlveda-Varas, P.; Weary, D.M.; Von Keyserlingk, M.A. Lying behavior and postpartum health status in grazing dairy cows. J. Dairy Sci. 2014, 97, 6334–6343. [Google Scholar] [CrossRef]
- Kaufman, E.; Leblanc, S.J.; McBride, B.; Duffield, T.; Devries, T.J. Short communication: Association of lying behavior and subclinical ketosis in transition dairy cows. J. Dairy Sci. 2016, 99, 7473–7480. [Google Scholar] [CrossRef]
- Barragan, A.; Piñeiro, J.; Schuenemann, G.; Rajala-Schultz, P.J.; Sanders, D.; Lakritz, J.; Bas, S. Assessment of daily activity patterns and biomarkers of pain, inflammation, and stress in lactating dairy cows diagnosed with clinical metritis. J. Dairy Sci. 2018, 101, 8248–8258. [Google Scholar] [CrossRef]
- Piñeiro, J.; Menichetti, B.; Barragan, A.; Relling, A.; Weiss, W.; Bas, S.; Schuenemann, G. Associations of pre- and postpartum lying time with metabolic, inflammation, and health status of lactating dairy cows. J. Dairy Sci. 2019, 102, 3348–3361. [Google Scholar] [CrossRef]
- Adewuyi, A.; Roelofs, J.; Gruys, E.; Toussaint, M.; Van Eerdenburg, F. Relationship of Plasma Nonesterified Fatty Acids and Walking Activity in Postpartum Dairy Cows. J. Dairy Sci. 2006, 89, 2977–2979. [Google Scholar] [CrossRef]
- Munksgaard, L.; Jensen, M.B.; Pedersen, L.J.; Hansen, S.W.; Matthews, L. Quantifying behavioural priorities—Effects of time constraints on behaviour of dairy cows, Bos taurus. Appl. Anim. Behav. Sci. 2005, 92, 3–14. [Google Scholar] [CrossRef]
- Farney, J.; Mamedova, L.; Coetzee, J.F.; Minton, J.; Hollis, L.; Bradford, B.J. Sodium salicylate treatment in early lactation increases whole-lactation milk and milk fat yield in mature dairy cows. J. Dairy Sci. 2013, 96, 7709–7718. [Google Scholar] [CrossRef]
- Carpenter, A.; Ylioja, C.; Vargas, C.; Mamedova, L.; Mendonça, L.; Coetzee, J.F.; Hollis, L.; Gehring, R.; Bradford, B.J. Hot topic: Early postpartum treatment of commercial dairy cows with nonsteroidal antiinflammatory drugs increases whole-lactation milk yield. J. Dairy Sci. 2016, 99, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kvidera, S.; Horst, E.; Abuajamieh, M.; Mayorga, E.; Fernandez, M.V.S.; Baumgard, L.H. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 2017, 100, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Ballou, M.A. Growth and development symposium: Inflammation: Role in the etiology and pathophysiology of clinical mastitis in dairy cows1. J. Anim. Sci. 2012, 90, 1466–1478. [Google Scholar] [CrossRef]
| Items | Diets | |
|---|---|---|
| Close-Up Dry Cows | Lactating Cows | |
| Ingredients, % of DM | ||
| Wheat Straw | 21.78 | 3.59 |
| High-moisture corn | - | 6.93 |
| Soybean meal | 7.26 | 5.02 |
| Steam-flaked corn | - | 6.31 |
| Canola meal | 14.36 | 3.90 |
| Triticale grain ground | - | 9.93 |
| Corn gluten meal | 5.57 | - |
| Corn silage | 44.55 | 12.23 |
| Pasture 1 | - | 47.62 |
| Molasses beet | - | 2.14 |
| Mycotoxin binder 2 | 0.08 | 0.04 |
| Magnesium sulphate | 0.57 | - |
| Magnesium oxide | - | 0.32 |
| Sodium bicarbonate | - | 0.80 |
| Mineral mix podological | - | 1.20 |
| Anionic salts 3 | 5.02 | - |
| Rumen buffer 4 | 0.81 | - |
| Chemical analysis | ||
| NEL, Mcal/kg of DM | 1.35 | 1.84 |
| Crude protein, % DM | 17.82 | 20.33 |
| Crude fat, % DM | 2.93 | 3.72 |
| aNDFom, % DM | 40.38 | 30.93 |
| ADF, % DM | 27.18 | 16.49 |
| Calcium, % DM | 0.76 | 0.54 |
| Phosphorus, % DM | 0.37 | 0.45 |
| Magnesium, % DM | 0.53 | 0.47 |
| Item 1 | Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| HFM | LFM | SEM | Group | Time | G × T | |
| BHB, mmol/L | 0.67 | 0.65 | 0.05 | 0.70 | 0.45 | >0.15 |
| MDA, nmol/mL | 13.92 | 12.11 | 0.72 | 0.01 | <0.01 | 0.14 |
| Haptoglobin, mg/mL | 0.07 | 0.02 | 0.02 | 0.03 | <0.01 | 0.01 |
| Fibrinogen, mg/mL | 3.19 | 3.53 | 0.28 | 0.22 | <0.01 | 0.10 |
| Total proteins, mg/mL | 76.26 | 75.33 | 1.17 | 0.43 | <0.01 | >0.15 |
| Items 1 | Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| HFM | LFM | SEM | Groups | Time | G × T | |
| Activity (no. steps/h) | 283.9 | 327.8 | 14.06 | <0.01 | <0.01 | <0.01 |
| Lying time (min/d) | 516.9 | 481.3 | 16.50 | 0.03 | <0.01 | >0.15 |
| Lying bouts (no./d) | 9.8 | 10.0 | 0.44 | 0.67 | <0.01 | <0.01 |
| Lying bout duration (min) | 57.9 | 52.8 | 3.14 | 0.11 | <0.01 | <0.01 |
| Item 1 | Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| HFM | LFM | SEM | Group | Time | G × T | |
| Milk yield (kg/d) | 27.96 | 29.61 | 1.04 | 0.11 | <0.01 | >0.15 |
| Protein (%) | 3.51 | 3.49 | 0.06 | 0.83 | <0.01 | 0.12 |
| Fat (%) | 4.11 | 3.98 | 0.14 | 0.35 | <0.01 | >0.15 |
| Protein yield (kg/d) | 0.92 | 0.99 | 0.03 | 0.03 | <0.01 | 0.08 |
| Fat yield (kg/d) | 1.07 | 1.13 | 0.04 | 0.14 | <0.01 | 0.04 |
| Milk urea (mg/dL) | 37.07 | 38.01 | 1.25 | 0.45 | <0.01 | 0.14 |
| Somatic cell linear score 2 | 2.22 | 2.02 | 0.35 | 0.59 | <0.01 | 0.09 |
| Variables 1 | Groups | p-Value | |
|---|---|---|---|
| HFM | LFM | ||
| Conception rate at first service, % | 52 (10) | 50 (8.6) | 0.88 |
| Pregnancy rate, % | 88.1 (5.4) | 88 (5.5) | 0.66 |
| Calving to first service interval, d | 72 (2.3) | 72.6 (1.9) | 0.84 |
| Calving to conception interval, d | 98.2 (7.9) | 104.9 (7.9) | 0.55 |
| Services per conception, no. | 1.8 (0.2) | 2.2 (0.2) | 0.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, A.; Mellado, R.; Bustamante, H. Prepartum Fat Mobilization in Dairy Cows with Equal Body Condition and Its Impact on Health, Behavior, Milk Production and Fertility during Lactation. Animals 2020, 10, 1478. https://doi.org/10.3390/ani10091478
Rodríguez A, Mellado R, Bustamante H. Prepartum Fat Mobilization in Dairy Cows with Equal Body Condition and Its Impact on Health, Behavior, Milk Production and Fertility during Lactation. Animals. 2020; 10(9):1478. https://doi.org/10.3390/ani10091478
Chicago/Turabian StyleRodríguez, Alfredo, Ricardo Mellado, and Hedie Bustamante. 2020. "Prepartum Fat Mobilization in Dairy Cows with Equal Body Condition and Its Impact on Health, Behavior, Milk Production and Fertility during Lactation" Animals 10, no. 9: 1478. https://doi.org/10.3390/ani10091478
APA StyleRodríguez, A., Mellado, R., & Bustamante, H. (2020). Prepartum Fat Mobilization in Dairy Cows with Equal Body Condition and Its Impact on Health, Behavior, Milk Production and Fertility during Lactation. Animals, 10(9), 1478. https://doi.org/10.3390/ani10091478




