Mucosal Hallmarks in the Alimentary Canal of Northern Pike Esox lucius (Linnaeus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
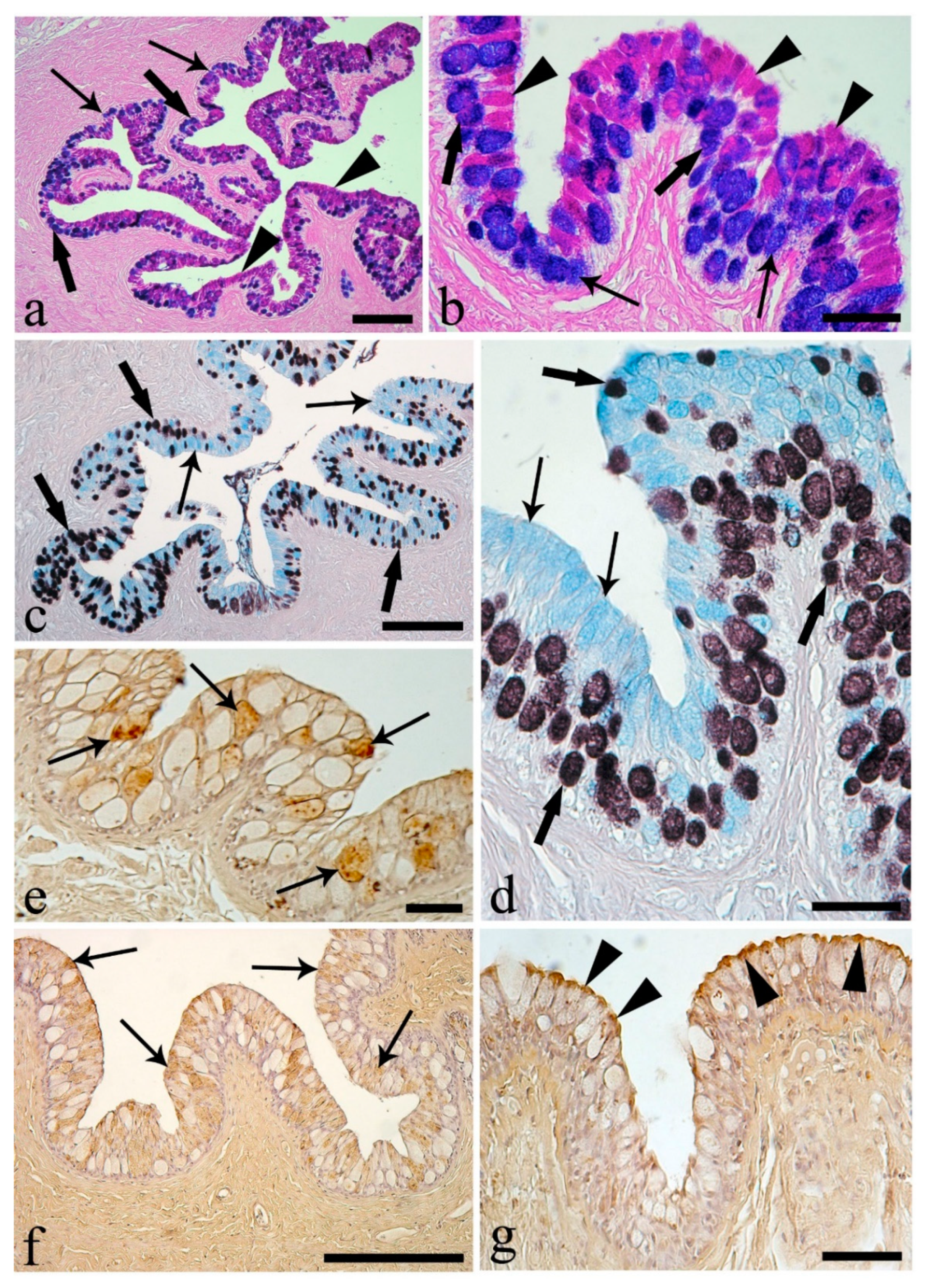
3. Results
4. Discussion
4.1. Esophagus
4.2. Stomach
4.3. Intestine
4.4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, J.M.; Castro, L.F.C. Morphological diversity of the gastrointestinal tract in fishes. In The Multifunctional Gut of Fish; Grosell, M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: New York, NY, USA, 2010; Volume 30, pp. 1–55. [Google Scholar] [CrossRef]
- Bucke, D. The anatomy and histology of the alimentary tract of the carnivorous fish the pike Esox lucius L. J. Fish Biol. 1971, 3, 421–431. [Google Scholar] [CrossRef]
- Petrinec, Z.; Nejedli, S.; Kužir, S.; Opačak, A. Mucosubstances of the digestive tract mucosa in northern pike (Esox lucius L.) and european catfish (Silurus glanis L.). Vet. Arhiv. 2005, 75, 317–327. [Google Scholar]
- Sadeghinezhad, J.; Hooshmand Abbasi, R.; Dehghani Tafti, E.; Boluki, Z. Anatomical, histological and histomorphometric study of the intestine of the northern pike (Esox lucius). Iran. J. Vet. Med. 2015, 9, 207–211. [Google Scholar]
- Díaz, A.O.; García, A.M.; Goldemberg, A.L. Glycoconjugates in the mucosa of the digestive tract of Cynoscion guatucupa: A histochemical study. Acta Histochem. 2008, 110, 76–85. [Google Scholar] [CrossRef]
- Cao, X.J.; Wang, W.M. Histology and mucin histochemistry of the digestive tract of yellow catfish, Pelteobagrus fulvidraco. Anat. Histol. Embryol. 2009, 38, 254–261. [Google Scholar] [CrossRef]
- Leknes, I.L. Histochemical studies on mucin-rich cells in the digestive tract of the Buenos Aires tetra (Hyphessobrycon anisitsi). Acta Histochem. 2011, 113, 353–357. [Google Scholar] [CrossRef]
- Pereira, R.T.; Nebo, C.; de Paula Naves, L.; Fortes-Silva, R.; Cardoso de Oliveira, I.R.; Rosa Paulino, R.; Delarete Drummond, C.; Vieira Rosa, P. Distribution of goblet and endocrine cells in the intestine: A comparative study in Amazonian freshwater Tambaqui and hybrid catfish. J. Morphol. 2020, 281, 55–67. [Google Scholar] [CrossRef]
- Fiertak, A.; Kilarski, W.M. Glycoconjugates of the intestinal goblet cells of four cyprinids. Cell. Mol. Life Sci. 2002, 59, 1724–1733. [Google Scholar] [CrossRef]
- Domeneghini, C.; Arrighi, S.; Radaelli, G.; Bosi, G.; Veggetti, A. Histochemical analysis of glycoconjugate secretion in the alimentary canal of Anguilla anguilla L. Acta Histochem. 2005, 106, 477–487. [Google Scholar] [CrossRef]
- Marchetti, L.; Capacchietti, M.; Sabbieti, M.G.; Accili, D.; Materazzi, G.; Menghi, G. Histology and carbohydrate histochemistry of the alimentary canal in the rainbow trout Oncorhynchus mykiss. J. Fish Biol. 2006, 68, 1808–1821. [Google Scholar] [CrossRef]
- Bosi, G.; DePasquale, J.A.; Rossetti, E.; Sayyaf Dezfuli, B. Differential mucins secretion by intestinal mucous cells of Chelon ramada in response to an enteric helminth Neoechinorhynchus agilis (Acanthocephala). Acta Histochem. 2020, 122, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Danguy, A.; Afik, F.; Pajak, B.; Gabius, H.J. Contribution of carbohydrate histochemistry to glycobiology. Histol. Histopathol. 1994, 9, 155–171. [Google Scholar] [PubMed]
- Bosi, G.; DePasquale, J.A.; Manera, M.; Castaldelli, G.; Giari, L.; Sayyaf Dezfuli, B. Histochemical and immunohistochemical characterization of rodlet cells in the intestine of two teleosts, Anguilla anguilla and Cyprinus carpio. J. Fish Dis. 2018, 41, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S. Comparative anatomy of the autonomic nervous system. Auton. Neurosci. Basic Clin. 2011, 165, 3–9. [Google Scholar] [CrossRef]
- Takei, Y.; Loretz, C.A. The gastrointestinal tract as an endocrine/neuroendocrine/paracrine organ: Organization, chemical messengers and physiological targets. In The Multifunctional Gut of Fish; Grosell, M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: New York, NY, USA, 2010; Volume 30, pp. 261–317. [Google Scholar] [CrossRef]
- Rønnestad, I.; Gomes, A.S.; Murashita, K.; Angotzi, R.; Jönsson, E.; Volkoff, H. Appetite-controlling endocrine systems in Teleosts. Front. Endocrinol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Bosi, G.; Domeneghini, C.; Arrighi, S.; Giari, L.; Simoni, E.; Sayyaf Dezfuli, B. Response of the gut neuroendocrine system of Leuciscu cephalus (L.) to the presence of Pomphorhynchus laevis Müller, 1776 (Acanthocephala). Histol. Histopathol. 2005, 20, 509–518. [Google Scholar]
- Bosi, G.; Shinn, A.P.; Giari, L.; Sayyaf Dezfuli, B. Enteric neuromodulators and mucus discharge in a fish infected with the intestinal helminth Pomphorhynchus laevis. Parasit. Vectors 2015, 8, 359. [Google Scholar] [CrossRef]
- Sayyaf Dezfuli, B.; Pironi, F.; Giari, L.; Domeneghini, C.; Bosi, G. Effect of Pomphorhynchus laevis (Acanthocephala) on putative neuromodulators in the intestine of naturally infected Salmo trutta. Dis. Aq. Org. 2002, 51, 27–35. [Google Scholar] [CrossRef]
- García-Meilán, I.; Ordóñez-Grande, B.; Machahua, C.; Buenestado, S.; Fontanillas, R.; Gallardo, M.A. Effects of dietary protein-to-lipid ratio on digestive and absorptive processes in sea bass fingerlings. Aquaculture 2016, 463, 163–173. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.F.; Tian, H.Y.; Jiang, G.Z.; Liu, W.B. Feeding rates affect growth, intestinal digestive and absorptive capabilities and endocrine functions of juvenile blunt snout bream Megalobrama amblycephala. Fish Physiol. Biochem. 2016, 42, 689–700. [Google Scholar] [CrossRef]
- Roque Hernández, D.; Barrios, C.E.; Santinón, J.J.; Sánchez, S.; Baldisserotto, B. Effect of fasting and feeding on growth, intestinal morphology and enteroendocrine cell density in Rhamdia quelen juveniles. Aquac. Res. 2018, 49, 1512–1520. [Google Scholar] [CrossRef]
- Parillo, F.; Gargiulo, A.M.; Fagioli, O. Complex carbohydrates occurring in the digestive apparatus of Umbrina cirrosa (L.) fry. Vet. Res. Commun. 2004, 28, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Carrassón, M.; Grau, A.; Dopazo, L.R.; Crespo, S. A histological, histochemical and ultrastructural study of the digestive tract of Dentex dentex (Pisces, Sparidae). Histol. Histopathol. 2006, 21, 579–593. [Google Scholar] [PubMed]
- Ogasawara, Y.; Namai, T.; Yoshino, F.; Lee, M.; Ishii, K. Sialic acid is an essential moiety of mucin as a hydroxyl radical scavenger. FEBS Lett. 2007, 581, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Faccioli, C.K.; Alari Chedid, R.; do Amaral, A.C.; Bastos Franceschini Vicentini, I.; Vicentini, C.A. Morphology and histochemistry of the digestive tract in carnivorous freshwater Hemisorubim platyrhynchos (Siluriformes: Pimelodidae). Micron 2014, 64, 10–19. [Google Scholar] [CrossRef]
- Díaz, A.O.; García, A.M.; Devincenti, C.V.; Goldemberg, A.L. Morphological and histochemical characterization of the pharyngeal cavity and oesophagus of mucosa of the digestive tract in Engraulis anchoita (Hubbs and Martini, 1935). Anat. Histol. Embryol. 2003, 32, 341–346. [Google Scholar] [CrossRef]
- Díaz, A.O.; Escalante, A.H.; García, A.M.; Goldemberg, A.L. Histology and histochemistry of the pharyngeal cavity and oesophagus of the silverside Odontesthes bonariensis (Cuvier and Valenciennes). Anat. Histol. Embryol. 2006, 35, 42–46. [Google Scholar] [CrossRef]
- Cardoso, N.D.N.; Firmiano, E.M.D.S.; Gomes, I.D.; Nascimento, A.A.D.; Sales, A.; Araújo, F.G. Histochemical and immunohistochemical study on endocrine cells (5-HT, GAS and SST) of the gastrointestinal tract of a teleost, the characin Astyanax bimaculatus. Acta Histochem. 2015, 117, 595–604. [Google Scholar] [CrossRef]
- Pedini, V.; Dall’Aglio, C.; Parillo, F.; Scocco, P. A lectin histochemical study of the esophagus of shi drum. J. Fish Biol. 2004, 64, 625–631. [Google Scholar] [CrossRef]
- Murray, H.M.; Wright, G.M.; Goff, G.P. A study of the posterior esophagus in winter flounder, Pleuronectes americanus and yellowtail flounder, Pleuronectes ferruginea: Morphological evidence for pregastric digestion? Can. J. Zool. 1994, 72, 1191–1198. [Google Scholar] [CrossRef]
- Domeneghini, C.; Pannelli Straini, R.; Veggetti, A. Gut glycoconjugates in Sparus aurata L. (Pisces, Teleostei). A comparative histochemical study in larval and adult ages. Histol. Histopathol. 1998, 13, 359–372. [Google Scholar] [PubMed]
- Kumari, U.; Mittal, S.; Mittal, A.K. Histological and histochemical investigations of the pharyngeal jaw apparatus of a carp Cirrhinus mrigala. Acta Histochem. 2014, 116, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, I.R. The distribution and function of mucous cells and their secretions in the alimentary tract of Arrhamphus sclerolepis krefftii. J. Fish Biol. 1997, 50, 809–820. [Google Scholar] [CrossRef]
- Yashpal, M.; Kumari, U.; Mittal, S.; Mittal, A.K. Histochemical characterization of glycoproteins in the buccal epithelium of a catfish Rita rita. Acta Histochem. 2007, 109, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Spicer, S.S.; Schulte, B.A. Diversity of cell glycoconjugates shown histochemically: A perspective. J. Histochem. Cytochem. 1992, 40, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Arellano, J.M.; Dinis, T.; Sarasquete, C. Histomorphological characteristics of the intestine of the Senegal sole, Solea senegalensis. Eur. J. Histochem. 1999, 43, 121–133. [Google Scholar] [PubMed]
- Madrid, J.F.; Ballesta, J.; Castells, M.T.; Marin, J.A.; Pastor, L.M. Characterization of glycoconjugates in the intestinal mucosa of vertebrates by lectin histochemistry. Acta Histochem. Cytochem. 1989, 22, 1–14. [Google Scholar] [CrossRef]
- Ferraris, R.P.; Tan, J.D.; De La Cruz, M.C. Development of the digestive tract of milkfish Chanos chanos (Forskal): Histology and histochemistry. Aquaculture 1987, 61, 241–257. [Google Scholar] [CrossRef]
- Purushothaman, K.; Lau, D.; Saju, J.M.; Musthaq, S.; Lunny, D.P.; Vij, S.; Orbn, L. Morpho-histological characterization of the alimentary canal of an important food fish, Asian seabass (Lates calcarifer). PeerJ 2016. [Google Scholar] [CrossRef]
- Smith, L.S. Digestive functions in teleost fishes. In Fish Nutr.; Halver, J.E., Ed.; Academic Press: New York, NY, USA, 1989; pp. 331–421. [Google Scholar]
- Pedini, V.; Dall’Aglio, C.; Parillo, F.; Scocco, P. Glycoconjugate distribution in gastric fundic mucosa of Umbrina cirrosa L. revealed by lectin histochemistry. J. Fish Biol. 2005, 66, 222–229. [Google Scholar] [CrossRef]
- Wang, Y.X.; Sun, J.F.; Lv, A.J.; Zhang, S.L.; Sung, Y.Y.; Shi, H.Y.; Hu, X.C.; Chen, S.J.; Xing, K.Z. Histochemical distribution of four types of enzymes and mucous cells in the gastrointestinal tract of reared half-smooth tongue sole Cynoglossus semilaevis. J. Fish Biol. 2018, 92, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Bohórquez, D.V.; Liddle, R.A. Gastrointestinal hormones and neurotransmitters. In Sleisenger and Fortran’s Gastrointestinal and Liver Disease, 10th ed.; Feldman, M., Friedman, L.S., Brandt, L.J., Eds.; Saunders/Elsevier: Philadelphia, PA, USA, 2015; pp. 36–54. [Google Scholar]
- Sayyaf Dezfuli, B.; DePasquale, J.A.; Castaldelli, G.; Giari, L.; Bosi, G. A fish model for the study of the relationship between neuroendocrine and immune cells in the intestinal epithelium: Silurus glanis infected with a tapeworm. Fish. Shellfish Immunol. 2017, 64, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Schubert, M.L. Gastric secretion. Curr. Opin. Gastroenterol. 2013, 29, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.T.; Costa, L.S.; Oliveira, I.R.C.; Araújo, J.C.; Aerts, M.; Vigliano, F.A.; Rosa, P.V. Relative distribution of gastrin, CCK-8, NPY and CGRP-immunoreactive cells in the digestive tract of dorado (Salminus brasiliensis). Tissue Cell 2015, 47, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.S.; Fang, Z.P.; Huang, F.J. Identification, localization and morphology of APUD cells in gastroenteropancreatic system of stomach-containing teleosts. World J. Gastroenterol. 2000, 6, 842–847. [Google Scholar] [CrossRef]
- Larsson, L.-I. Developmental biology of gastrin and somatostatin cells in the antropyloric mucosa of the stomach. Microsc. Res. Tech. 2000, 48, 272–281. [Google Scholar] [CrossRef]
- Lin, X.; Wang, P.; Ou, Y.; Li, J.; Wen, J. An immunohistochemical study on endocrine cells in the neuroendocrine system of the digestive tract of milkfish Chanos chanos (Forsskal, 1775). Aquac. Res. 2017, 48, 1439–1449. [Google Scholar] [CrossRef]
- Groff, K.E.; Youson, J.H. An immunohistochemical study of the endocrine cells within the pancreas, intestine and stomach of the gar (Lepisosteus osseus L.). Gen. Comp. Endocrinol. 1997, 106, 1–16. [Google Scholar] [CrossRef]
- Vieira-Coelho, M.A.; Soares-da-Silva, P. Dopamine formation, from its immediate precursor 3,4-dihydroxyphenylalanine, along the rat digestive tract. Fundam. Clin. Pharmacol. 1993, 7, 235–243. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Aneman, Å.; Friberg, P.; Hooper, D.; Fåndriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocr. Metab. 1997, 82, 3864–3871. [Google Scholar] [CrossRef]
- Schultz, E. Catechol-O-methyltransferase and aromatic L-amino acid decarboxylase activities in human gastrointestinal tissues. Life Sci. 1991, 49, 721–725. [Google Scholar] [CrossRef]
- Eldrup, E.; Richter, E.A. DOPA, dopamine, and DOPAC concentrations in the rat gastrointestinal tract decrease during fasting. Am. J. Physiol.-Endocrinol. Metab. 2000, 279, E815–E822. [Google Scholar] [CrossRef] [PubMed]
- Mandic, S.; Volkoff, H. The effects of fasting and appetite regulators on catecholamine and serotonin synthesis pathways in goldfish (Carassius auratus). Comp. Biochem. Physiol. A.-Mol. Integr. Physiol. 2018, 223, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Day, R.; Salzet, M. The neuroendocrine phenotype, cellular plasticity, and the search for genetic switches: Redefining the diffuse neuroendocrine system. Neuro Endocrinol. Lett. 2002, 23, 447–451. [Google Scholar] [PubMed]
- Beorlegui, C.; Martínez, A.; Sesma, P. Endocrine cells and nerves in the pyloric ceca and the intestine of Oncorhynchus mykiss (Teleostei): An immunocytochemical study. Gen. Comp. Endocrinol. 1992, 86, 483–495. [Google Scholar] [CrossRef]
- Elbal, M.T.; Agulleiro, B. An immunocytochemical and ultrastructural study of endocrine cells in the gut of a teleost fish, Sparus auratus. Gen. Comp. Endocrinol. 1986, 64, 339–354. [Google Scholar] [CrossRef]
- Pederzoli, A.; Bertacchi, I.; Gambarelli, A.; Mola, L. Immunolocalization of vasoactive intestinal peptide and substance P in the developing gut of Dicentrarchis labrax (L.). Eur. J. Histochem. 2004, 48, 179–184. [Google Scholar] [CrossRef]
- Zizza, S.; Desantis, S. Morphology and lectin-binding sites of pyloric caeca epithelium in normal and GnRH-treated Atlantic bluefin tuna (Thunnus thynnus, Linnaeus 1758). Microsc. Res. Tech. 2011, 74, 863–873. [Google Scholar] [CrossRef]
- Shi, G.; Wang, J.X.; Liu, X.Z.; Wang, R.X. Study on histology and histochemistry of digestive tract in Sebastiscus marmoratus. Chin. J. Fish. 2007, 31, 293–302. [Google Scholar]
- Scocco, P.; Menghi, G.; Ceccarelli, P. Histochemical differentiation of glycoconjugates occurring in the tilapine intestine. J. Fish Biol. 1997, 51, 848–857. [Google Scholar] [CrossRef]
- Hernández, D.R.; Vigliano, F.A.; Sánchez, S.; Bermúdez, R.; Domitrovic, H.A.; Quiroga, M.I. Neuroendocrine system of the digestive tract in Rhamdia quelen juvenile: An immunohistochemical study. Tissue. Cell 2012, 44, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Nardocci, G.; Navarro, C.; Cortés, P.P.; Imarai, M.; Montoya, M.; Valenzuela, B.; Jara, P.; Acuña-Castillo, C.; Fernández, R. Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish. Shellfish Immunol. 2014, 40, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, S.; Trompette, A.; Claustre, J.; El Homsi, M.; Garzón, J.; Jourdan, G.; Scoazec, J.-Y.; Plaisancié, P. Beta-Casomorphin-7 regulates the secretion and expression of gastrointestinal mucins through a mu-opioid pathway. Am. J. Physiol. 2006, 290, G1105–G1113. [Google Scholar]
- Anderson, T.A. Histological and cytological structure of the gastrointestinal tract of the luderick, Girella tricuspidata (Pisces, Kyphosidae), in relation to diet. J. Morphol. 1986, 190, 109–119. [Google Scholar] [CrossRef] [PubMed]








| Acronym | Vector Laboratories Code | Lectin | Species Source: Latin Name (Common Name) | Major Carbohydrate Specificity |
|---|---|---|---|---|
| ConA | B-1005 | Concanavalin A | Canavalia ensiformis (Jack bean) | α-Mannose, α-Glucose |
| DBA | B-1035 | Dolichos biflorus agglutinin | Dolichos biflorus (horse gram) | α-GalNAc |
| PNA | B-1075 | Peanut agglutinin | Arachis hypogaea (peanut) | Gal β 1-3GalNAc |
| DSL | B-1185 | Datura stramonius lectin | Datura stramonium (thorn apple) | (GlcNAc)n, Gal β 1-4GlcNAc |
| WGA | B-1025 | Wheat germ agglutinin | Triticum vulgare (wheat germ) | (GlcNAc)n, Sia |
| UEA I | B-1065 | Ulex europaeus agglutinin I | Ulex europaeus (gorse seed) | α-Fucose |
| Antibody Anti- | Clonality | Host | Source, Code | Dilution and Incubation at Room Temperature |
|---|---|---|---|---|
| Somatostatin-14 | Polyclonal | Rabbit | Genosys Biotechnologies Inc., Cambridge, UK, CA-08-325 | 1:200; 24 h |
| Monoclonal | Mouse | Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, sc-74556 | 1:50; 24 h | |
| Substance P | Polyclonal | Rabbit | Peninsula Labs. Int., Belmont, CA, USA, T-4170 | 1:200; 24 h |
| Monoclonal | Mouse | Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, sc-14184 | 1:50; 24 h | |
| Leu-enkephalin | Polyclonal | Rabbit | Peninsula Labs. Int., Belmont, CA, USA, IHC 8601 | 1:500; 24 h |
| Monoclonal | Mouse | Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, sc-47705 | 1:200; 24 h | |
| Tyrosine hydroxylase | Polyclonal | Rabbit | Millipore, Burlington, MA, USA, AB152 | 1:250; 24 h |
| Monoclonal | Mouse | Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, sc-25269 | 1:50; 24 h | |
| Anti-biotinylated secondary antibodies | ||||
| Anti-rabbit IgG | Goat | Vector Labs, Burlingame, CA, USA, BA-1000 | 1:1000; 2 h | |
| Anti-mouse IgG | Goat | Vector Labs, Burlingame, CA, USA, BA-9200 | 1:1000; 2 h | |
| Morphometric Parameters | Esophagus | Stomach | Intestine | ||
|---|---|---|---|---|---|
| Proximal | Medial | Distal | |||
| MFsH | 198.5 ± 4.2 | 163.2 ± 3.8 | 480.2 ± 15.0 | 536.1 ± 15.0 | 575.7 ± 11.6 |
| MFsW | 167.3 ± 4.1 | 83.1 ± 2.7 | 123.3 ± 1.8 | 75.0 ± 1.3 | 123.1 ± 2.9 |
| EpH | 66.3 ± 1.5 | 39.3 ± 1.1 | 54.7 ± 1.5 | 33.7 ± 0.8 | 44.1 ± 1.00 |
| Mucous Cells | Esophagus | Stomach | Intestine | ||
|---|---|---|---|---|---|
| Proximal | Medial | Distal | |||
| AB | 97.6 ± 2.8 | - | 97.7 ± 2.5 | 49.6 ± 1.0 | 46.3 ± 1.7 |
| PAS | 199.2 ± 5.3 | 230.0 ± 7.1 | 6.3 ± 0.4 | 12.4 ± 0.4 | 24.8 ± 1.2 |
| AB/PAS | 99.4 ± 2.8 | 504.2 ± 10.0 | 61.9 ± 2.3 | 33.6 ± 0.7 | 40.1 ± 1.1 |
| Total | 377.6 ± 6.8 | 731.2 ± 13.6 | 167.7 ± 2.7 | 92.9 ± 1.1 | 109.3 ± 1.7 |
| Mucous Cells | Esophagus | Stomach | Intestine | ||
|---|---|---|---|---|---|
| Proximal | Medial | Distal | |||
| AB | 233.8 ± 6.3 | 710.7 ± 10.1 | 140.5 ± 3.7 | 78.4 ± 2.5 | 95.7 ± 1.4 |
| HID | 172.3 ± 4.9 | - | 29.8 ± 1.4 | 0.9 ± 0.1 | - |
| Total | 405.9 ± 8.2 | 710.7 ± 10.1 | 170.3 ± 4.1 | 79.3 ± 2.5 | 95.7 ± 1.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosi, G.; Lorenzoni, M.; Carosi, A.; Sayyaf Dezfuli, B. Mucosal Hallmarks in the Alimentary Canal of Northern Pike Esox lucius (Linnaeus). Animals 2020, 10, 1479. https://doi.org/10.3390/ani10091479
Bosi G, Lorenzoni M, Carosi A, Sayyaf Dezfuli B. Mucosal Hallmarks in the Alimentary Canal of Northern Pike Esox lucius (Linnaeus). Animals. 2020; 10(9):1479. https://doi.org/10.3390/ani10091479
Chicago/Turabian StyleBosi, Giampaolo, Massimo Lorenzoni, Antonella Carosi, and Bahram Sayyaf Dezfuli. 2020. "Mucosal Hallmarks in the Alimentary Canal of Northern Pike Esox lucius (Linnaeus)" Animals 10, no. 9: 1479. https://doi.org/10.3390/ani10091479
APA StyleBosi, G., Lorenzoni, M., Carosi, A., & Sayyaf Dezfuli, B. (2020). Mucosal Hallmarks in the Alimentary Canal of Northern Pike Esox lucius (Linnaeus). Animals, 10(9), 1479. https://doi.org/10.3390/ani10091479








