Selection for Test-Day Milk Yield and Thermotolerance in Brazilian Holstein Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Climate Data
2.2. Data
2.3. Models
2.4. Analysis of Models
3. Results
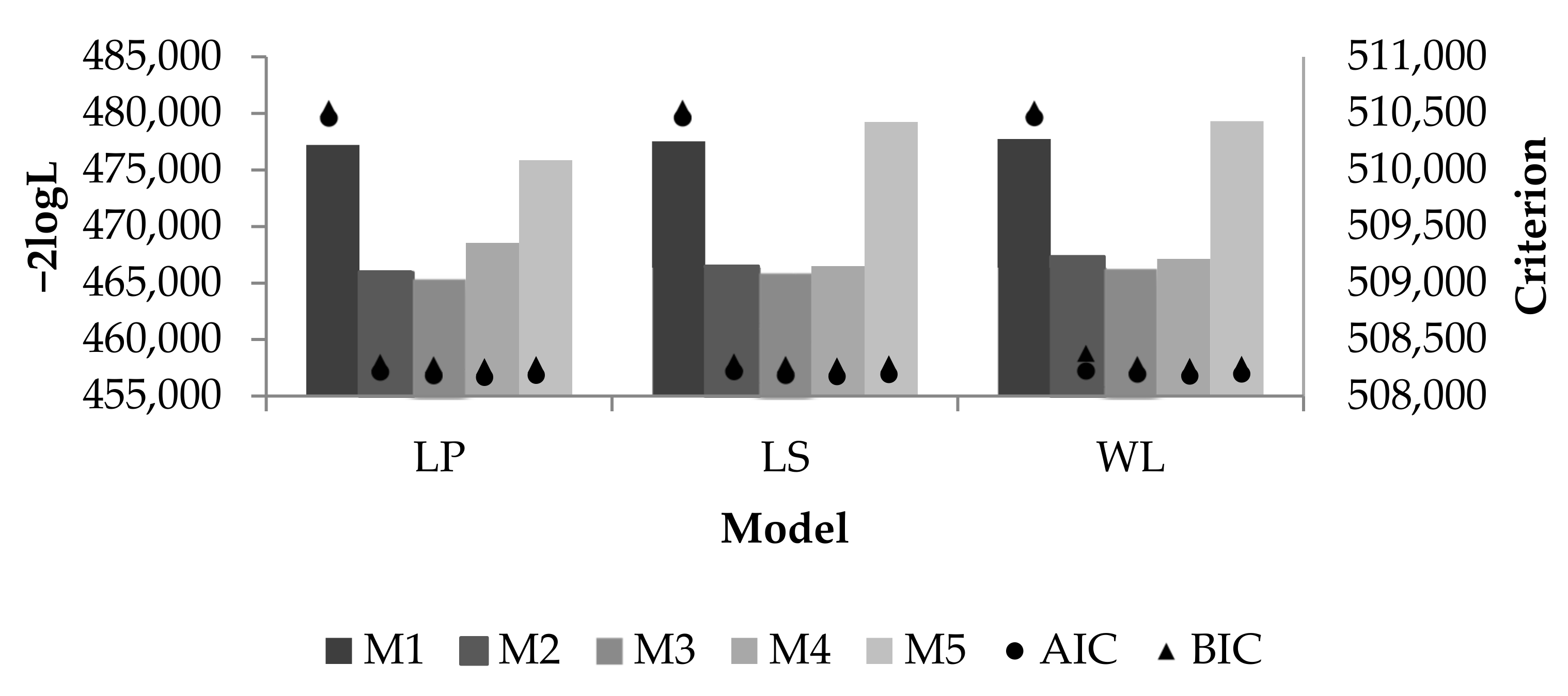
3.1. Loss in Milk Yield and Adjustment of Models
3.2. Heritability
3.3. Genotype-by-Environment Interaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Garcia, A.B.; Angeli, N.; Machado, L.; Cardoso, F.C.; Gonzalez, F. Relationships between heat stress and metabolic and milk parameters in dairy cows in Southern Brazil. Trop. Anim. Health Prod. 2015, 47, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Pegorer, M.F.; Vasconcelos, J.L.; Trinca, L.A.; Hansen, P.J.; Barros, C.M. Influence of sire and sire breed (Gyr versus Holstein) on establishment of pregnancy and embryonic loss in lactating Holstein cows during summer heat stress. Theriogenology 2007, 67, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Rhoads, R.P.; Rhoads, M.L.; Gabler, N.K.; Ross, J.W.; Keating, A.F.; Boddicker, R.L.; Lenka, S.; Sejian, V. Impact of Climate Change on Livestock Production. In Environmental Stress and Amelioration in Livestock Production; Sejian, V., Naqvi, S.M.K., Ezeji, T., Lakritz, J., Lal, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 413–468. [Google Scholar]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Fodor, N.; Foskolos, A.; Topp, C.F.E.; Moorby, J.M.; Pàsztor, L.; Foyer, C.H. Spatially explicit estimation of heat stress-related impacts of climate change on the milk production of dairy cows in the United Kingdom. PLoS ONE 2018, 13, 20197076. [Google Scholar] [CrossRef] [Green Version]
- Key, N.; Sneeringer, S.; Marquardt, D. Climate Change, Heat Stress and U.S. Dairy Production; A Report Summary from the Economic Research Service; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 2014. [Google Scholar]
- Sigdel, A.; Abdollahi-Arpanahi, R.; Aguilar, I.; Peñagaricano, F. Whole genome mapping reveals novel genes and pathways involved in milk production under heat stress in us Holstein cows. Front. Genet. 2019, 10, 928. [Google Scholar] [CrossRef]
- Ansari-Mahyari, S.; Ojali, M.R.; Forutan, M.; Riasi, A.; Brito, L.F. Investigating the genetic architecture of conception and non-return rates in Holstein cattle under heat stress conditions. Trop. Anim. Health Prod. 2019, 51, 1847–1853. [Google Scholar] [CrossRef]
- Wollmann, C.A.; Galvani, E. Zoneamento agroclimático: Linhas de pesquisa e caracterização teórica-conceitual. Soc. Nat. 2013, 25, 179–190. [Google Scholar] [CrossRef] [Green Version]
- Picinin, L.C.A.; Bordigon-Luiz, M.T.; Cerqueira, M.M.O.P.; Toaldo, I.M.; Souza, F.N.; Leite, M.O.; Fonseca, L.M.; Lana, A.M.Q. Effect of seasonal conditions and milk management practices on bulk milk quality in Minas Gerais State—Brazil. Arq. Bras. Med. Vet. Zootec. 2019, 71, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Polsky, L.; von Keyserlingk, M.A.G. Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef] [Green Version]
- Ravagnolo, O.; Misztal, I. Genetic component of heat stress in dairy cattle, parameter estimation. J. Dairy Sci. 2000, 83, 2126–2130. [Google Scholar] [CrossRef]
- Bernaducci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The effects of heat stress in Italian Holstein dairy cattle. J. Dairy Sci. 2014, 97, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Sae-Tiao, T.; Koonawootrittriron, S.; Suwanasopee, T.; Elzo, M.A. Trend for diurnal temperature variation and relative humidity and their impact on milk yield of dairy cattle in tropical climates. J Anim. Sci. 2017, 95, 258. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. A Guide to Environmental Research on Animals; National Academy Press: Washington, DC, USA, 1971; 374p. [Google Scholar]
- Negri, R.; Aguilar, I.; Feltes, G.L.; Machado, J.D.; Braccini Neto, J.; Costa-Maia, F.M.; Cobuci, J.A. Inclusion of bioclimatic variables in genetic evaluations of dairy cattle. Anim. Biosci. 2021, 34, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Wilmink, J.B.M. Adjustment of test day milk, fat and protein yields for age, season and stage of lactation. Livest. Prod. Sci. 1987, 16, 335–348. [Google Scholar] [CrossRef]
- Misztal, I. Properties of random regression models using linear splines. J. Anim. Breed. Genet. 2006, 123, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, M.; Hill, W.G.; Thompson, R. Estimating the covariance structure of traits during growth and ageing, illustrated with lactation in dairy cattle. Genet. Res. 1994, 64, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Henderson, C.R., Jr. Analysis of covariance in the mixed model: Higher-level, nonhomogeneous, and random regressions. Biometrics 1982, 3, 623. [Google Scholar] [CrossRef]
- Misztal, I.; Tsuruta, S.; Strabel, T.; Auvray, B.; Druet, T.; Lee, D.H. BLUPF90 and related programs (BGF90). In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002. [Google Scholar]
- Euclides, V.P.B.; Valle, C.B.; Macedo, M.C.M.; Almeida, R.G.; Montagner, D.B.; Barbosa, R.A. Brazilian scientific progress in pasture research during the first decade of XXI century. Rev. Bras. Zootec. 2010, 39, 151–168. [Google Scholar] [CrossRef] [Green Version]
- Léis, C.M.; Cherubini, E.; Ruviaro, C.F.; Silva, V.P.; Lampert, V.N.; Spies, A.; Soares, S.R. Carbon footprint of milk production in Brazil: A comparative case study. Int. J. Life Cycke. Assess. 2015, 20, 46–60. [Google Scholar] [CrossRef] [Green Version]
- Harfuch, L.; Nassar, A.M.; Zambianco, W.M.; Gurgel, A.C. Modelling Beef and Dairy Sectors Productivities and their Effects on Land Use Change in Brazil. Rev. Econ. Sociol. Rural. 2016, 54, 281–304. [Google Scholar] [CrossRef] [Green Version]
- Intergovernmental Panel on Climate Change. Climate Change 2007: The Physical Science Basis; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Thornton, P.K.; Van de Steeg, J.; Notenbaert, A.M.; Herrero, M. The impacts of climate change on livestock and livestock systems in developing countries: A review of what we know and what we do not know. Agric. Syst. 2009, 101, 113–127. [Google Scholar] [CrossRef]
- Malhi, Y.; Wright, J. Spatial patterns and recent trends in the climate of tropical rainforest regions. Phil. Trans. R. Soc. 2004, 359, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Do, C.; Choy, Y.; Dang, C.; Mahboob, A.; Cho, K. Estimation of the genetic milk yield parameters of Holstein cattle under heat stress in South Korea. Asian Australas. J. Anim. Sci. 2019, 32, 334–340. [Google Scholar] [CrossRef]
- Hammami, H.; Vandenplas, J.; Vanrobays, M.L.; Rekik, B.; Bastin, C.; Gengler, N. Genetic analysis of heat stress effects on yield traits, udder health, and fatty acids of Walloon Holstein cows. J. Dairy Sci. 2015, 98, 4956–4968. [Google Scholar] [CrossRef] [Green Version]
- Santana, M.L.J.; Bignardi, A.B.; Pereira, R.J.; Menéndez-Buxadera, A.; El Faro, L. Random regression models to account for the effect of genotype by environment interaction due to heat stress on milk yield of Holstein cows under tropical conditions. J. Appl. Genet. 2016, 57, 119–127. [Google Scholar] [CrossRef]
- Santana, M.L.J.; Bignardi, A.B.; Pereira, R.J.; Stefani, G.; El Faro, L. Genetics of heat tolerance for milk yield and quality in Holsteins. Animal 2017, 11, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Carabaño, M.J.; Ramón, M.; Díaz, C.; Molina, A.; Pérez-Guzmán, M.D.; Serradilha, J.M. Breeding for resilience to heat stress effects in dairy ruminants. A comprehensive review. J. Anim. Sci. 2017, 95, 1813–1826. [Google Scholar]
- Kolmodin, R.; Strandberg, E.; Madsen, P.; Jense, J.; Jorjani, H. Genotype by Environment Interaction in Nordic Dairy Cattle Studied Using Reaction Norms. Acta Agric. Scand. 2002, 52, 11–24. [Google Scholar] [CrossRef]
- Aguilar, I.; Misztal, I.; Tsuruta, S. Genetic components of heat stress for dairy cattle with multiple lactations. J. Dairy Sci. 2009, 92, 5702–5711. [Google Scholar] [CrossRef] [Green Version]
- Brügemann, K.; Gernand, E.; Von Borstel, U.; Koenig, S. Genetic analyses of protein yield in dairy cows applying random regression models with time-dependent and temperature x humidity-dependent covariates. J. Dairy Sci. 2011, 94, 4129–4139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Werf, J.H.J.; Goddard, M.E.; Meyer, K. The Use of Covariance Functions and Random Regressions for Genetic Evaluation of Milk Production Based on Test Day Records. J. Dairy Sci. 1998, 81, 3300–3308. [Google Scholar] [CrossRef]







| Item | Statistics |
|---|---|
| Number of test-day records | 94,549 |
| Number of animals with records | 11,294 |
| Number of animals in pedigree file | 32,409 |
| Number of dams in pedigree file | 8639 |
| Number of sires in pedigree file | 641 |
| Number of contemporary groups | 5257 |
| Number of herds | 129 |
| Mean test-day milk (kg) | 25.81 (7.21) |
| Mean records/animal | 8.37 |
| Models | Fixed Effects | Regressor | ||||||
|---|---|---|---|---|---|---|---|---|
| Contemporary Group | Milking Frequency | Variable t | DIM | DTV | DIM | THI | DTV | |
| M1 | * | * | * | * | - | - | ○○○○ | - |
| * | * | * | * | - | - | + + + + | - | |
| * | * | * | * | - | - | ◊ ◊ ◊ | - | |
| M2 | * | * | * | - | - | ○○○○ | ○○ | - |
| * | * | * | - | - | + + + + | + + + | - | |
| * | * | * | - | - | ◊ ◊ ◊ | ◊ ◊ ◊ | - | |
| M3 | * | * | * | - | - | ○○○○ | ○○ | ○○ |
| * | * | * | - | - | + + + + | + + + | + + + | |
| * | * | * | - | - | ◊ ◊ ◊ | ◊ ◊ ◊ | ◊ ◊ ◊ | |
| M4 | * | * | * | - | * | ○○○○ | ○○ | - |
| * | * | * | - | * | + + + + | + + + | - | |
| * | * | * | - | * | ◊ ◊ ◊ | ◊ ◊ ◊ | - | |
| M5 | * | * | * | * | - | - | ○○ | ○○ |
| * | * | * | * | - | - | + + + | + + + | |
| * | * | * | * | - | - | ◊ ◊ ◊ | ◊ ◊ ◊ | |
| N Daughters | Sires | EBV_TDMY | |
|---|---|---|---|
| THI 74 | THI 84 | ||
| >101 | 13 | 100% | 91% |
| 51 to 100 | 43 | 100% | 84% |
| 41 to 50 | 12 | 100% | 84% |
| 31 to 40 | 23 | 100% | 75% |
| 21 to 30 | 59 | 100% | 74% |
| 11 to 20 | 127 | 100% | 73% |
| <10 | 364 | 100% | 71% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negri, R.; Aguilar, I.; Feltes, G.L.; Cobuci, J.A. Selection for Test-Day Milk Yield and Thermotolerance in Brazilian Holstein Cattle. Animals 2021, 11, 128. https://doi.org/10.3390/ani11010128
Negri R, Aguilar I, Feltes GL, Cobuci JA. Selection for Test-Day Milk Yield and Thermotolerance in Brazilian Holstein Cattle. Animals. 2021; 11(1):128. https://doi.org/10.3390/ani11010128
Chicago/Turabian StyleNegri, Renata, Ignacio Aguilar, Giovani Luis Feltes, and Jaime Araújo Cobuci. 2021. "Selection for Test-Day Milk Yield and Thermotolerance in Brazilian Holstein Cattle" Animals 11, no. 1: 128. https://doi.org/10.3390/ani11010128
APA StyleNegri, R., Aguilar, I., Feltes, G. L., & Cobuci, J. A. (2021). Selection for Test-Day Milk Yield and Thermotolerance in Brazilian Holstein Cattle. Animals, 11(1), 128. https://doi.org/10.3390/ani11010128








