Albumen Quality of Fresh and Stored Table Eggs: Hen Genotype as a Further Chance for Consumer Choice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Genotypes and Rearing Conditions of the Hens
2.3. Data Collection
2.4. Statistical Analysis
3. Results and Discussion
3.1. Environmental Conditions and Egg Yield
3.2. Egg Quality
3.3. Albumen Quality of Fresh and Stored Eggs
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leenstra, F.; Ten Napel, J.; Visscher, J.; Van Sambeek, F. Layer breeding programs in changing production environments: A historic perspective. Worlds Poult. Sci. J. 2016, 72, 21–36. [Google Scholar] [CrossRef]
- Zeidler, G. Processing and packaging shell eggs. In Commercial Chicken Meat and Egg Production, 5th ed.; Bell, D.D., Weaver, W.D., Jr., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2002; pp. 1129–1161. [Google Scholar]
- Bessei, W. Impact of animal welfare on worldwide poultry production. Worlds Poult. Sci. J. 2018, 74, 211–224. [Google Scholar] [CrossRef]
- Zeidler, G. Shell egg quality and preservation. In Commercial Chicken Meat and Egg Production, 5th ed.; Bell, D.D., Weaver, W.D., Jr., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2002; pp. 1199–1217. [Google Scholar]
- Damaziak, K.; Marzec, A.; Ridel, J.; Szeliga, J.; Koczywąs, E.; Cisneros, F.; Michalczuk, M.; Lukasiewicz, M.; Gozdowski, D.; Siennicka, A.; et al. Effect of dietary canthaxanthin and iodine on the production performance and egg quality of laying eggs. Poult. Sci. 2018, 97, 4008–4019. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.A.; Silversides, F.G. The effect of storage and strain of hen on egg quality. Poult. Sci. 2000, 78, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Silversides, F.G.; Scott, T.A. Effect of storage and layer are on quality of eggs from two lines of hens. Poult. Sci. 2001, 80, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Hincke, M.T.; Rose-Martel, M.; Hennequet-Antier, C.; Brionne, A.; Cogburn, L.A.; Nys, Y.; Gautron, J. Identifying specific proteins involved in eggshell membrane formation using gene expression analysis and bioinformatics. BMC Genom. 2015, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, E.; Decuypere, E.; Buyse, J.; Everaert, N. Importance of albumen during embryonic development in avian species, with emphasis on domestic chickens. Worlds Poult. Sci. J. 2014, 70, 503–518. [Google Scholar] [CrossRef]
- Nowaczewski, S.; Szablewski, T.; Cegielska-Radziejewska, R.; Kontecka, H. Egg morphometry and eggshell quality in ring-necked pheasant kept in cages. Ann. Anim. Sci. 2013, 13, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Cassandro, M.; Baruchello, M.; Catania, S.; Gobbo, F.; Moronato, M.L.; Baldan, G.; Carnio, D.; Parise, M.; Rizzi, C. Conservazione e Caratterizzazione delle Razze Avicole Venete—Programma Bionet. In Rete Regionale per la Conservazione e Caratterizzazione della Biodiversità di Interesse Agrario; Gruppo di lavoro Avicoli; Veneto Agricoltura: Padova, Italy, 2014. [Google Scholar]
- Sirri, F.; Zampiga, M.; Soglia, F.; Meluzzi, A.; Cavani, C.; Petracci, M. Quality characterization of eggs from Romagnola hens, an Italian local breed. Poult. Sci. 2018, 97, 4131–4136. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Chiofalo, B.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Egg quality from Siciliana and Livorno Italian autochthnous chicken breeds reared in organic system. Animals 2020, 10, 864. [Google Scholar] [CrossRef]
- Rizzi, C. Laying hen biodiversity: A study on the effect of age on the yield performance and quality of eggs produced by two Italian purebred hens. Acta Fythotech. Zootech. 2020, 23, 299–307. [Google Scholar]
- European Commission. Attitudes of European towards Animal Welfare. Special Eurobarometer. 2016. Available online: http://data.europa.eu/euodp/en/data/S2096_84_4_442_ENG (accessed on 18 November 2020).
- Pettersson, I.C.; Freire, R.; Nicol, C.J. Factors affecting ranging behavior in commercial free-range hens. Worlds Poult. Sci. J. 2016, 72, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Widowski, T.M.; Hemsworth, P.H.; Barnett, J.L.; Rault, J.-L. Laying hen welfare I. Social environment and space. Worlds Poult. Sci. J. 2016, 72, 333–342. [Google Scholar] [CrossRef]
- Rizzi, C.; Marangon, A. Quality of organic eggs of hybrids and Italian breed hens. Poult. Sci. 2012, 91, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C. Yield performance, laying behaviour traits and egg quality of purebred and hybrid hens reared under outdoor conditions. Animals 2020, 10, 584. [Google Scholar] [CrossRef] [Green Version]
- Commission International de l’Eclairage. CIELab Color System; CIE: Paris, France, 1976. [Google Scholar]
- Coon, C.N. Feeding commercial egg-type layers. In Commercial Chicken Meat and Egg Production, 5th ed.; Bell, D.D., Weaver, W.D., Jr., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2002; pp. 287–328. [Google Scholar]
- Bell, D.D. Management in alternative housing systems. In Commercial Chicken Meat and Egg Production, 5th ed.; Bell, D.D., Weaver, W.D., Jr., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2002; pp. 1041–1057. [Google Scholar]
- Rizzi, C.; Chiericato, G.M. Chemical composition of meat and egg yolk of hybrid and Italian breed hens reared using an organic production system. Poult. Sci. 2010, 89, 1239–1251. [Google Scholar] [CrossRef]
- Hy-Line International. Commercial Layers. Management Guide. Available online: https://www.hyline.com (accessed on 18 November 2020).
- Fernyhough, M.; Nicol, C.J.; Van de Braak, T.; Toscan, M.J.; Tønnessen, M. The ethics of laying hen genetics. J. Agric. Environ. Ethics 2020, 33, 15–36. [Google Scholar] [CrossRef] [Green Version]
- Commission Regulation (EC) No 589/2008 of 23 June 2008 Laying Down Detailed Rules for Implementing Council Regulation (EC) No 1234/2007 as Regards Marketing Standards for Eggs. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R0589 (accessed on 18 November 2020).
- Chen, C.F.; Gourichon, D.; Huang, N.Z.; Lee, Y.P.; Bordas, A.; Tixier-Boichard, M. Performance comparison of dwarf laying hen’s segregating for the naked neck gene in temperate and subtropical environments. Genet. Sel. Evol. 2009, 41, 13. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.; Sah, N.; Wasti, S. Genetic and hormonal regulation of egg formation in the oviduct of laying hens. IntechOpen 2019, 85011. [Google Scholar] [CrossRef] [Green Version]
- Sah, N.; Kuehu, D.L.; Khadka, V.S.; Deng, Y.; Peplowska, K.; Jha, R.; Mishra, B. RNA sequencing-based analysis of the laying hen uterus revealed the novel genes and biological pathways involved in the eggshell biomineralization. Sci. Rep. 2018, 8, 16853. [Google Scholar] [CrossRef] [Green Version]
- Nys, Y.; Le Roy, N. Calcium homeostasis and eggshell biomineralization in female chicken. In Vitamin D: Biochemistry, Physiology and Diagnostics, 4th ed.; Feldman, D., Pike, J.W., Bouillon, R., Giovanucci, E., Goltzman, D., Hewison, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 1, pp. 361–382. [Google Scholar]
- Dacke, C.G.; Arkle, S.; Cook, D.J.; Wormstone, I.M.; Jones, S.; Zaidi, M.; Bascal, Z.A. Medullary bone and avian calcium regulation. J. Exp. Biol. 1993, 184, 63–88. [Google Scholar]
- Whitehead, C.C. Overview of bone biology in the egg-laying hens. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Nolte, T.; Jansen, S.; Halle, I.; Scholz, A.M.; Simianer, H.; Sharifi, A.R.; Weigend, S. Egg production and bone stability of local chicken breeds and their crosses fed with faba beans. Animals 2020, 10, 1480. [Google Scholar] [CrossRef]
- Jansen, S.; Baulain, U.; Habig, C.; Weigend, A.; Halle, I.; Scholz, A.M.; Simianer, H.; Sharifi, A.R.; Weigen, S. Relationship between bone stability and egg production in genetically divergent chicken layer lines. Animals 2020, 10, 850. [Google Scholar] [CrossRef]
- Zeidler, G. Shell eggs and their nutritional value. In Commercial Chicken Meat and Egg Production, 5th ed.; Bell, D.D., Weaver, W.D., Jr., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2002; pp. 1109–1128. [Google Scholar]
- Zeidler, G. Further-processing eggs and egg products. In Commercial Chicken Meat and Egg Production, 5th ed.; Bell, D.D., Weaver, W.D., Jr., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2002; pp. 1163–1197. [Google Scholar]
- Marzec, A.; Damaziak, K.; Kowalska, A.; Riedel, J.; Michalczuk, M.; Koczywąs, E.; Cisneros, F.; Lenart, A.; Niemiec, J. Effect of hens age and storage time on functional and physiological properties of eggs. J. Appl. Poult. Res. 2019, 28, 290–300. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Kim, H.O. Structure and chemical composition of eggs. In Egg Bioscience and Biotechnology, 1st ed.; Mine, Y., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008; pp. 1–96. [Google Scholar]
- Stoddard, M.C.; Yong, E.H.; Akkaynak, D.; Sheard, C.; Tobias, J.A.; Mahadevan, L. Avian egg shape: Form, function, and evolution. Science 2017, 356, 1249–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Xu, G.Y.; Liu, Z.Z.; Li, J.Y.; Yang, N. A study on eggshell pigmentation: Biliverdin in blue-shelled chickens. Poult. Sci. 2006, 85, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Scheideler, S.E.; Weber, P.; Monsalve, D. Supplemental vitamin E and Se effects on egg production, egg quality, and egg deposition of α-tocopherol and Se. J. Appl. Poult. Res. 2010, 19, 354–360. [Google Scholar] [CrossRef]
- Sheikh, H.; Pasha, I.; Katiya, A.G. Factors affecting whipping ability of fresh and stale eggs. Pak. J. Food Sci. 2009, 19, 1–6. [Google Scholar]
- Gajcevic, Z.; Gordana, K.; Has-Schon, E.; Pavic, V. Effects of organic Se supplement to layer diet on table egg freshness and Se content. Ital. J. Anim. Sci. 2009, 8, 189–199. [Google Scholar] [CrossRef]
- Kirunda, D.F.K.; McKee, F.R. Relating Quality characteristics of aged eggs and fresh eggs to vitelline membrane strengths as determined by a texture analyzer. Poult. Sci. 2000, 79, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Lomakina, K.; Míková, K.A. Study of the factors affecting the foaming properties of egg-white—A review. Czech. J. Food Sci. 2006, 24, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Carey, C. Incubation in extreme environment. In Avian Incubation: Behaviour, Environment and Evolution, 1st ed.; Deeming, D.C., Ed.; Oxford University Press: Oxford, UK, 2002; pp. 238–252. [Google Scholar]
- Deeming, D.C. Functional characteristics of eggs. In Avian Incubation: Behaviour, Environment and Evolution, 1st ed.; Deeming, D.C., Ed.; Oxford University Press: Oxford, UK, 2002; pp. 28–41. [Google Scholar]
- Turner, J.S. Maintenance of egg temperature. In Avian Incubation: Behaviour, Environment and Evolution, 1st ed.; Deeming, D.C., Ed.; Oxford University Press: Oxford, UK, 2002; pp. 119–142. [Google Scholar]
- Sah, N.; Mishra, B. Regulation of egg formation in the oviduct of laying hens. Worlds Poult. Sci. J. 2018, 74, 509–522. [Google Scholar] [CrossRef]
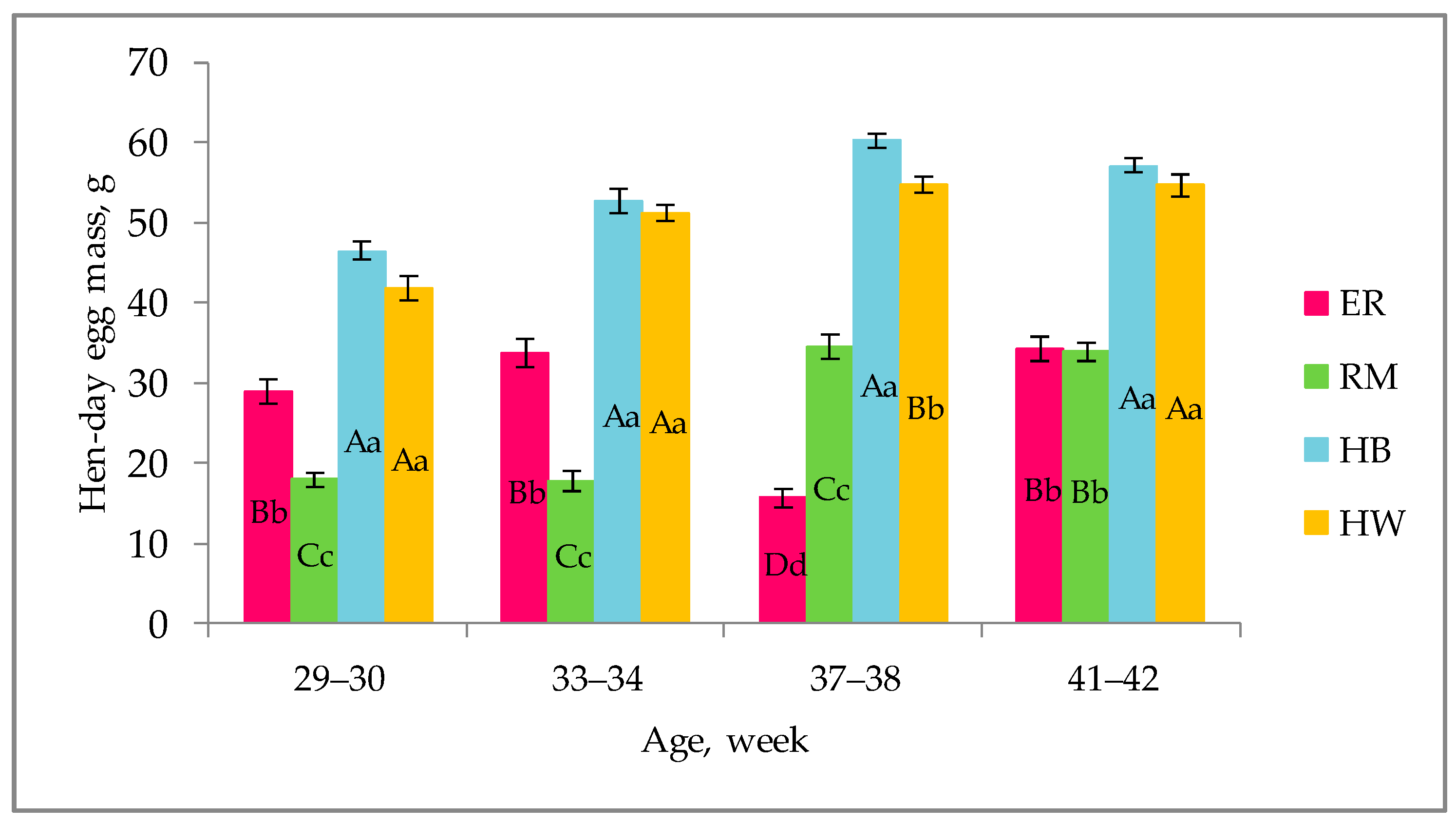




| Items | Environmental Conditions | Genotypes 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature, °C | RH, % | ER | RM | HB | HW | |||
| Laying Period | Average | Min | Max | Average | Cumulative egg mass, kg | |||
| 26–30 weeks | 28 | 18 | 32 | 63 | 0.92 | 0.27 | 1.31 | 1.47 |
| 31–34 weeks | 21 | 10 | 24 | 64 | 1.81 | 0.95 | 2.52 | 2.79 |
| 35–38 weeks | 19 | 11 | 24 | 83 | 2.65 | 1.72 | 4.12 | 4.25 |
| 39–42 weeks | 10 | 5 | 12 | 69 | 3.42 | 2.75 | 5.80 | 5.78 |
| Hen-day egg production, % | ||||||||
| 25 weeks | 47.8 | 4.4 | 81.2 | 78.9 | ||||
| 25–44 weeks | 56.3 | 45.6 | 85.2 | 85.8 | ||||
| Egg mass/body gain, g/g | ||||||||
| 25–44 weeks | 9.6 | 4.4 | 17.6 | 34.3 | ||||
| Items | Genotypes 1 | |||
|---|---|---|---|---|
| ER | RM | HB | HW | |
| Eggshell thickness 2, μm | ||||
| 31 weeks | 338 a | 372 Aa | 376 | 342 AaBb |
| 35 weeks | 339 a | 342 Bb | 368 | 329 Bb |
| 39 weeks | 315 b | 368 Aa | 366 | 332 ABb |
| 43 weeks | 320 a | 383 Aa | 364 | 349 Aa |
| p-value | 0.0135 | <0.0001 | 0.2395 | 0.0065 |
| RMSE | 33.14 | 25.67 | 23.02 | 23.85 |
| component | linear p = 0.007 | quadratic p = 0.0001 | ns | quadratic p = 0.0009 |
| Eggshell weight 3, g | ||||
| 31 weeks | 5.15 b | 5.67 b | 6.48 b | 5.72 b |
| 35 weeks | 5.30 ab | 5.92 b | 6.79 a | 6.00 b |
| 39 weeks | 5.65 a | 6.73 a | 6.77 a | 6.66 a |
| 43 weeks | 5.60 a | 6.80 a | 6.91 a | 6.60 a |
| p-value | 0.0084 | <0.0001 | 0.0226 | <0.0001 |
| RMSE | 0.62 | 0.56 | 0.55 | 0.55 |
| Component | linear p = 0.0016 | linear p < 0.0001 | linear p = 0.0056 | linear p < 0.0001 |
| Albumen weight 4, g | ||||
| 31 weeks | 31.9 Bb | 32.3 Bb | 37.1 Bb | 36.2 Bc |
| 35 weeks | 32.7 AaBb | 33.7 AaBb | 41.4 Aa | 38.7 ABb |
| 39 weeks | 32.0 Bb | 34.9 Aa | 41.5 Aa | 38.6 ABb |
| 43 weeks | 34.6 Aa | 35.5 Aa | 42.5 Aa | 40.0 Aa |
| p-value | 0.0020 | < 0.0001 | < 0.0001 | 0.0001 |
| RMSE | 2.91 | 2.27 | 3.26 | 3.11 |
| Component | linear p = 0.0030 | linear p < 0.0001 | linear p < 0.0001 | linear p < 0.0001 |
| Items | Genotypes 1 | |||
|---|---|---|---|---|
| ER | RM | HB | HW | |
| Albumen height 2, mm | ||||
| 31 weeks | 6.93 AaBb | 7.10 | 7.99 AaBb | 8.08 ab |
| 35 weeks | 6.57 BbCc | 7.20 | 8.19 AaB | 8.36 a |
| 39 weeks | 7.53 Aa | 7.27 | 8.63 Aa | 8.15 ab |
| 43 weeks | 6.09 Cc | 6.47 | 7.45 Bb | 7.38 b |
| p-value | <0.0001 | 0.0797 | 0.0006 | 0.0308 |
| RMSE | 1.02 | 1.16 | 1.02 | 1.34 |
| Component | cubic p < 0.0001 | ns | quadratic p = 0.0005 | linear p = 0.0345 |
| Haugh Units 3 | ||||
| 31 weeks | 85.3 AaBb | 85.4 | 90.0 Aa | 90.7 Aa |
| 35 weeks | 81.6 BbCc | 86.0 | 89.9 Aa | 91.1 Aa |
| 39 weeks | 88.4 Aa | 84.2 | 91.2 Aa | 89.2 AaBb |
| 43 weeks | 77.5 Cc | 79.8 | 84.1 Bb | 84.4 Bb |
| p-value | <0.0001 | 0.0690 | <0.0001 | 0.0025 |
| RMSE | 6.67 | 8.66 | 5.53 | 7.48 |
| component | cubic p < 0.0001 | ns | linear p = 0.0004 | linear p = 0.0010 |
| Items | Genotypes 1 | |||
|---|---|---|---|---|
| ER | RM | HB | HW | |
| Albumen height 2, μm | ||||
| 31 weeks | 5.18 | 5.07 | 5.99 a | 5.52 |
| 35 weeks | 5.05 | 5.44 | 6.10 a | 5.63 |
| 39 weeks | 5.17 | 5.22 | 5.95 a | 5.69 |
| 43 weeks | 4.65 | 5.49 | 5.39 b | 5.60 |
| p-value | 0.2456 | 0.3616 | 0.0493 | 0.9449 |
| RMSE | 1.02 | 0.86 | 0.97 | 1.00 |
| component | ns | ns | linear p = 0.0258 | ns |
| Haugh Units 3 | ||||
| 31 weeks | 73.9 Aa | 73.1 | 78.2 Aa | 75.6 |
| 35 weeks | 70.9 AaCc | 73.5 | 76.5 Aa | 73.2 |
| 39 weeks | 73.2 AaCc | 71.2 | 74.4 AaB | 73.7 |
| 43 weeks | 64.7 BbC | 73.0 | 68.5 Bb | 71.6 |
| p-value | 0.0045 | 0.7144 | 0.0002 | 0.3596 |
| RMSE | 9.44 | 6.84 | 7.84 | 7.83 |
| component | linear p = 0.0040 | ns | linear p < 0.0001 | ns |
| Items | Genotypes 1 | |||
|---|---|---|---|---|
| ER | RM | HB | HW | |
| Albumen height 2, μm | ||||
| 31 weeks | 4.97 Aa | 4.63 | 4.94 | 5.35 Aa |
| 35 weeks | 4.12 Bb | 5.08 | 5.22 | 4.57 Bb |
| 39 weeks | 4.05 Bb | 4.81 | 4.55 | 4.69 ABb |
| 43 weeks | 3.82 Bb | 4.87 | 4.62 | 4.56 Bb |
| p-value | 0.0007 | 0.5395 | 0.0571 | 0.0025 |
| RMSE | 0.68 | 1.00 | 0.88 | 0.71 |
| component | linear p = 0.0001 | ns | ns | linear p = 0.0026 |
| Haugh Units 3 | ||||
| 31 weeks | 72.8 Aa | 69.5 | 70.6 Aa | 74.2 Aa |
| 35 weeks | 63.2 Bb | 71.1 | 69.5 Aab | 65.5 Bb |
| 39 weeks | 61.2 Bbc | 67.8 | 61.4 Bc | 65.0 Bb |
| 43 weeks | 57.1 Bc | 69.1 | 62.7 ABbc | 61.5 Bb |
| p-value | <0.0001 | 0.7579 | 0.0009 | <0.0001 |
| RMSE | 6.64 | 8.24 | 8.52 | 6.54 |
| component | linear p < 0.001 | ns | linear p = 0.0005 | linear p < 0.0001 |
| Items | Genotypes 1 | |||
|---|---|---|---|---|
| ER | RM | HB | HW | |
| Albumen height 2, μm | ||||
| 31 weeks | 4.38 Aa | 4.26 | 4.25 ab | 4.24 Aa |
| 35 weeks | 3.34 Bb | 4.44 | 3.79 b | 3.51 Bb |
| 39 weeks | 3.74 AaBb | 4.25 | 3.92 ab | 3.87 AaBb |
| 43 weeks | 3.75 AaBb | 4.82 | 4.43 a | 4.19 Aa |
| p-value | 0.0003 | 0.1013 | 0.0148 | 0.0006 |
| RMSE | 0.85 | 0.81 | 0.78 | 0.67 |
| component | quadratic p = 0.0052 | ns | quadratic p = 0.0019 | quadratic p = 0.0002 |
| Haugh Units 3 | ||||
| 31 weeks | 68.0 Aa | 66.1 | 64.1 Aa | 64.8 Aa |
| 35 weeks | 54.1 Bb | 65.9 | 54.5 Bb | 52.7 Cc |
| 39 weeks | 59.2 ABb | 63.0 | 54.7 Bb | 56.3 BbCc |
| 43 weeks | 56.6 Bb | 67.1 | 60.3 AaBb | 59.4 AaBb |
| p-value | <0.0001 | 0.3439 | 0.0003 | <0.0001 |
| RMSE | 9.86 | 7.46 | 9.33 | 7.40 |
| component | linear p = 0.0018 | ns | quadratic p < 0.0001 | quadratic p < 0.0001 |
| Items | Genotypes 1 | |||||||
|---|---|---|---|---|---|---|---|---|
| ER | RM | HB | HW | |||||
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| 1 d egg | −0.19 | 0.04 | −0.38 | 0.0008 | −0.29 | 0.002 | −0.41 | <0.0001 |
| 7 d egg | 0.03 | 0.75 | −0.14 | 0.22 | −0.05 | 0.59 | −0.27 | 0.006 |
| 14 d egg | −0.51 | <0.0001 | −0.08 | 0.54 | −0.14 | 0.22 | −0.46 | <0.0001 |
| 21 d egg | −0.45 | <0.0001 | 0.06 | 0.64 | −0.12 | 0.23 | −0.28 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzi, C. Albumen Quality of Fresh and Stored Table Eggs: Hen Genotype as a Further Chance for Consumer Choice. Animals 2021, 11, 135. https://doi.org/10.3390/ani11010135
Rizzi C. Albumen Quality of Fresh and Stored Table Eggs: Hen Genotype as a Further Chance for Consumer Choice. Animals. 2021; 11(1):135. https://doi.org/10.3390/ani11010135
Chicago/Turabian StyleRizzi, Chiara. 2021. "Albumen Quality of Fresh and Stored Table Eggs: Hen Genotype as a Further Chance for Consumer Choice" Animals 11, no. 1: 135. https://doi.org/10.3390/ani11010135





