Mate Choice in Double-Breeding Female Great Tits (Parus Major): Good Males or Compatible Males
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Area and Data Collection
2.3. Morphological Measurements
2.4. Genetic Analysis
2.5. Acoustic Recordings and Measurements
2.6. Statistical Analyses
3. Results
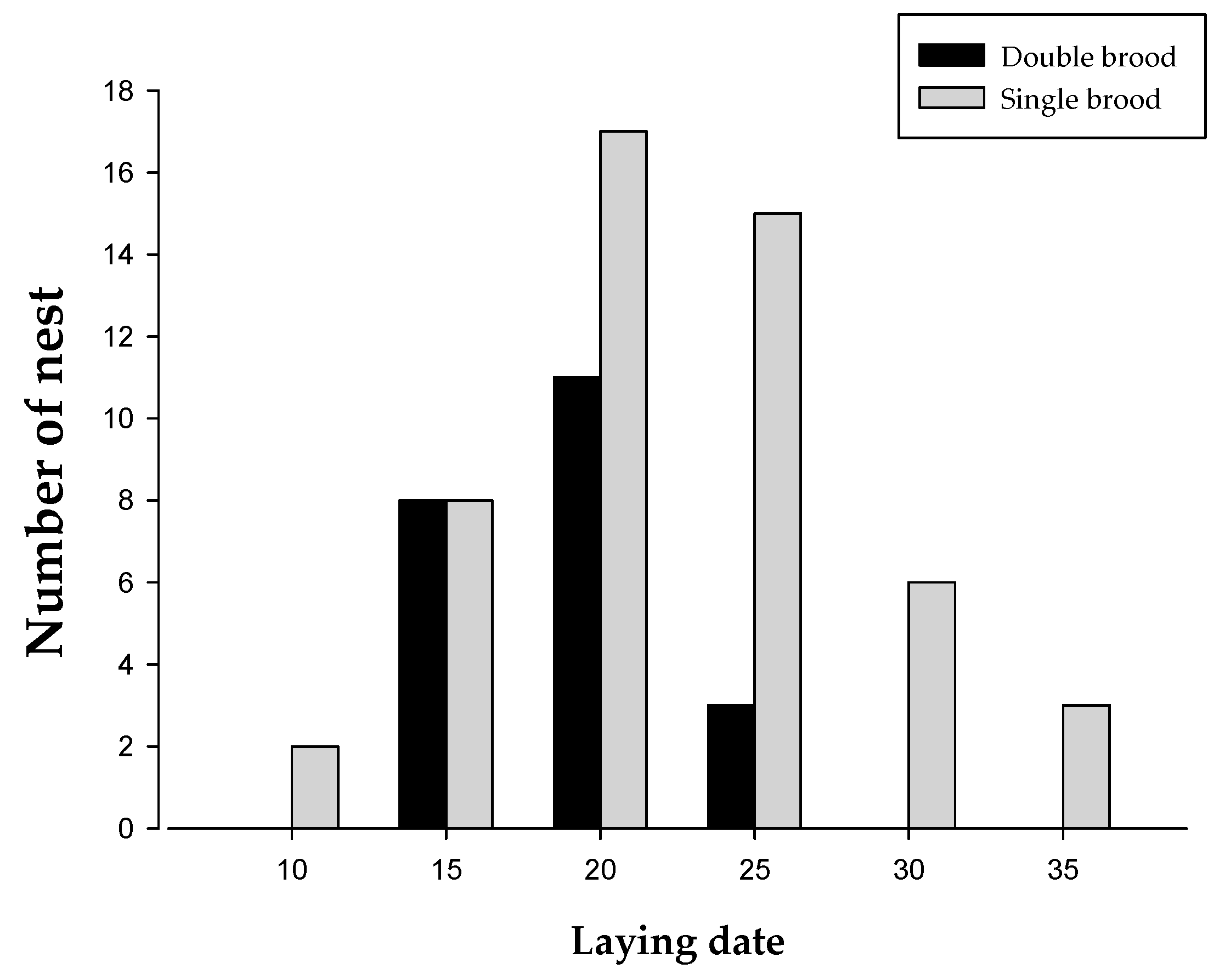
3.1. The Timing of Breeding and Female Quality
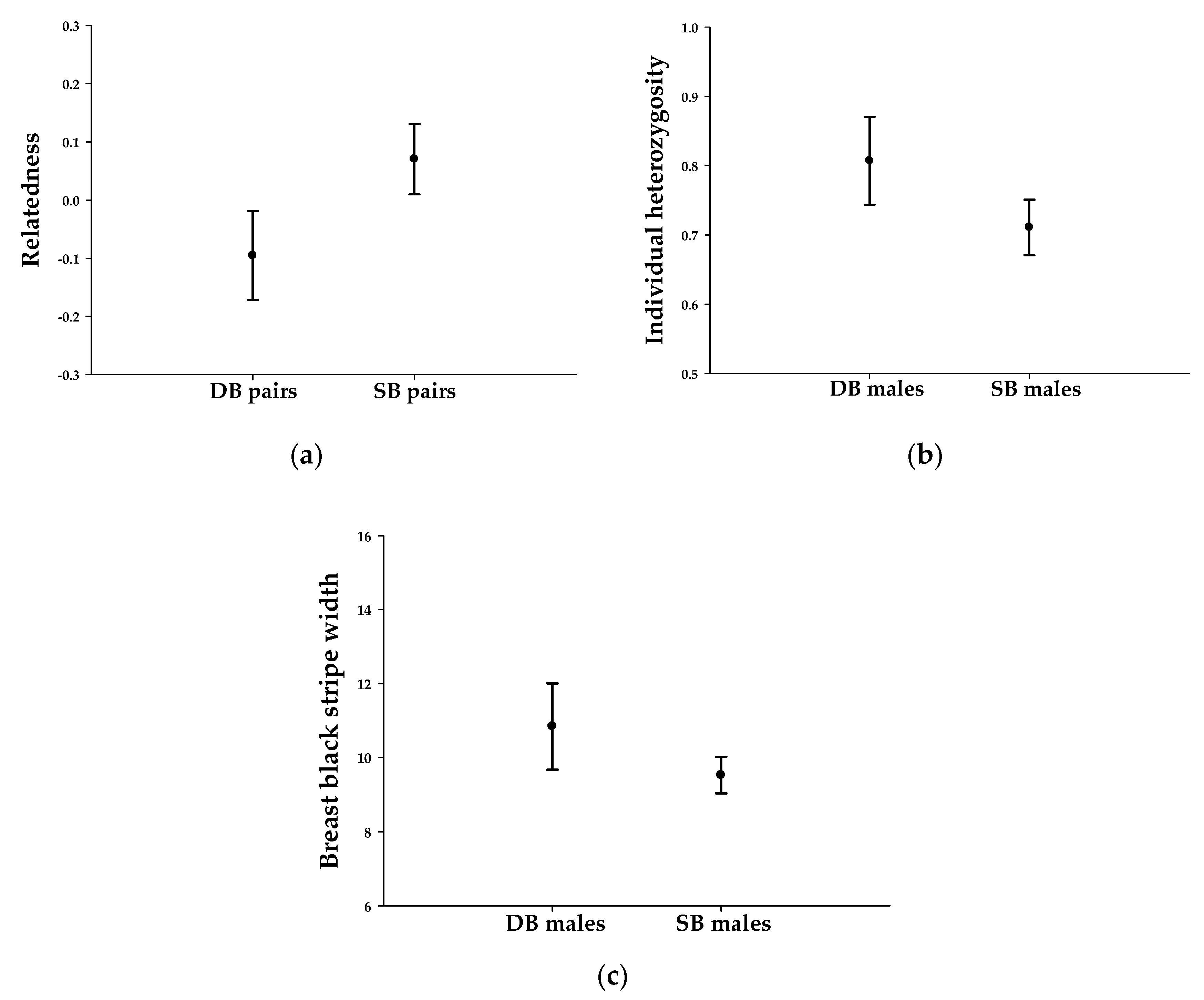
3.2. Good Genes, Compatible Genes, or Both
3.3. Female Trait Preference in Choosing Males for Double Breeding
3.4. Genetic Quality and Number of Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Locus | Sequence | Repeat Type | Expected Allele Size (bp) | Annealing Temperature |
|---|---|---|---|---|
| Pma33 | F-TTCCCCAAGTATCCTGCATC R-AAACCATATCACCCAGTGCC | (GATA)14GAT(GATA)8 | 305 | 55 |
| Pma1 * | F-CTCTTTTCCCAGCCTCCAG R-TATGTTTTGTCTGCTCGGGG | (CA)15(CT)(CA)4 | 118 | 58 |
| Pma30 * | F-GTTTCTGCCCAAATGGTGTG R-TCAGACCTTTCCAATGATGG | (GA)10 | 305 | 58 |
| Pma179 * | F-GGAGGCTTAAACATTCTGTGTG R-GGGCTGAAGGAGTTTGCTAC | (TG)14 | 179~197 | 61 |
| Pmad22 | F-GATCAGAGCTTGCCTCAACAC R-TCTGGGCTGAAATACCTACCC | (CTAT)15(CCAT)12 | 403 | 60 |
| Pma42 * | F-ACTTCCACATGCCAGTTTCC R-TGTTAAGGCAGAGAGGTGGG | (TCCA)15 | 285 | 57 |
| Pma27 * | F-TATAAACCACAGCCACACGC R-CACAACCACAGAGGCATGAG | (CAT)16 | 202 | 55 |
| Pma40 | F-CGTTCCTCCTTTGCTTTCTG R-AATGGCACAACACCTTCTCC | (GA)10 | 416 | 58 |
| Pma71 * | F-TCAGCCTCCAAGGAAAACAG R-GCATAAGCAACACCATGCAG | (TAGG)6(TAGA)11 | 186 | 58 |
| Pma45 * | F-CCCCTGGCTCTTTCATATCC R-GACAGGTGTTGGCACAAGG | (TGA)10 | 307 | 58 |
| PmaC25 * | F-CGTCCTGCTGTTTGTATTTCTG R- CCATGAACCATTTTTAGGGTG | (CAT)11 | 323 | 58 |
| PmaD105 | F- CAAATCACACAGTTGCTGCC R-CCTGGTATAAGACTGGTCAAAACAG | (GTCT)3(ATCT)12 | 404 | 58 |
| PmaGAn28 | F- GTTGGTGCAGCGGTCTACTC R-CATGTTGGGACAGCAGTTTG | (GA)16 | 199 | 58 |
| Pma48m | F- CACTCAGCCTCTCAGATCTG R-CGGGCTGGTACTTATTGGGAG | (TG)11 | 192 | 58 |
References
- Kirkpatrick, M.; Barton, N.H. The strength of indirect selection on female mating preferences. Proc. Natl. Acad. Sci. USA 1997, 94, 1282–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennions, M.D.; Petrie, M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2007, 75, 21–64. [Google Scholar] [CrossRef]
- Consuegra, S.; De Leaniz, C.G. MHC-mediated mate choice increases parasite resistance in salmon. Proc. R. Soc. B Biol. Sci. 2008, 275, 1397–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neff, B.D.; Pitcher, T.E. Genetic quality and sexual selection: An integrated framework for good genes and compatible genes. Mol. Ecol. 2004, 14, 19–38. [Google Scholar] [CrossRef]
- Tregenza, T.; Wedell, N. Genetic compatibility, mate choice and patterns of parentage: Invited Review. Mol. Ecol. 2000, 9, 1013–1027. [Google Scholar] [CrossRef] [Green Version]
- Mays, H.L., Jr.; Hill, G.E. Choosing mates: Good genes versus genes that are a good fit. Trends Ecol. Evol. 2004, 19, 554–559. [Google Scholar] [CrossRef]
- Kempenaers, B.; Verheyen, G.R.; Van Broeckhoven, C.; Burke, T.; Van Broeckhoven, C.; Dhondt, A. Extra-pair paternity results from female preference for high-quality males in the blue tit. Nat. Cell Biol. 1992, 357, 494–496. [Google Scholar] [CrossRef]
- Hasselquist, D.; Bensch, S.; Von Schantz, T. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nat. Cell Biol. 1996, 381, 229–232. [Google Scholar] [CrossRef]
- Foerster, K.; Delhey, K.; Johnsen, A.; Lifjeld, J.T.; Kempenaers, B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nat. Cell Biol. 2003, 425, 714–717. [Google Scholar] [CrossRef]
- Wright, D.J.; Brouwer, L.; Mannarelli, M.-E.; Burke, T.; Komdeur, J.; Richardson, D.S. Social pairing of Seychelles warblers under reduced constraints: MHC, neutral heterozygosity, and age. Behav. Ecol. 2015, 27, 295–303. [Google Scholar] [CrossRef]
- Rowe, L.; Houle, D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. B Biol. Sci. 1996, 263, 1415–1421. [Google Scholar] [CrossRef]
- Trivers, R.L. Parental investment and sexual selection. In Sexual Selection and the Descent of Man; Campbell, B., Ed.; Heinemann Press: London, UK, 1972; pp. 136–179. [Google Scholar]
- Drickamer, L.C. Oestrous female house mice discriminate dominant from subordinate males and sons of dominant from sons of subordinate males by odour cues. Anim. Behav. 1992, 43, 868–870. [Google Scholar] [CrossRef]
- Norris, K. Heritable variation in a plumage indicator of viability in male great tits Parus major. Nat. Cell Biol. 1993, 362, 537–539. [Google Scholar] [CrossRef]
- Hamilton, W.D.; Zuk, M. Heritable true fitness and bright birds: A role for parasites? Science 1982, 218, 384–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, K.M.; Yamaguchi, N.; Kasuya, E.; Yahara, T. Extra-pair mate choice in the female great tit Parus major: Good males or compatible males. J. Ethol. 2008, 27, 349–359. [Google Scholar] [CrossRef]
- Norris, K.J. Female choice and the quality of parental care in the great tit Parus major. Behav. Ecol. Sociobiol. 1990, 27, 275–281. [Google Scholar] [CrossRef]
- Senar, J.C.; Quesada, J. Cross-fostering experiments to compare carotenoid- and melanin-based plumage traits and long-term parental effects in post-moulted great tits. Behaviour 2009, 146, 1235–1251. [Google Scholar] [CrossRef] [Green Version]
- Senar, J.C.; Conroy, M.J.; Quesada, J.; Mateos-Gonzalez, F. Selection based on the size of the black tie of the great tit may be reversed in urban habitats. Ecol. Evol. 2014, 4, 2625–2632. [Google Scholar] [CrossRef]
- Byers, B.E.; Kroodsma, D.E. Female mate choice and songbird song repertoires. Anim. Behav. 2009, 77, 13–22. [Google Scholar] [CrossRef]
- Sung, H.-C.; Handford, P.T. Song characters as reliable indicators of male reproductive quality in the Savannah Sparrow (Passerculus sandwichensis). Can. J. Zool. 2020, 98, 32–38. [Google Scholar] [CrossRef]
- Krebs, J.R.; Kacelnik, A. The Dawn Chorus in the great tit (Parus Major): Proximate and Ultimate Causes. Behaviour 1983, 83, 287–308. [Google Scholar] [CrossRef]
- Slagsvold, T.; Sætre, G.-P.; Dale, S. Dawn singing in the Great Tit (Parus Major): Mate attraction, mate guarding, or territorial defence? Behaviour 1994, 131, 115–138. [Google Scholar] [CrossRef]
- Brown, J.L. A theory of mate choice based on heterozygosity. Behav. Ecol. 1997, 8, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Mitton, J.B.; Schuster, W.S.F.; Cothran, E.G.; De Fries, J.C. Correlation between the individual heterozygosity of parents and their offspring. Heredity 1993, 71, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, J.I.; Forcada, J.; Trathan, P.N.; Amos, W. Female fur seals show active choice for males that are heterozygous and unrelated. Nat. Cell Biol. 2007, 445, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Kempenaers, B. Mate choice and genetic quality: A review of the heterozygosity theory. Adv. Study Behav. 2007, 37, 189–278. [Google Scholar] [CrossRef]
- Chapman, J.R.; Akagawa, S.N.; Oltman, D.W.C.; Late, J.S.; Heldon, B.C.S. A quantitative review of heterozygosity-fitness correlations in animal populations. Mol. Ecol. 2009, 18, 2746–2765. [Google Scholar] [CrossRef]
- García-Navas, V.; Ortego, J.; Sanz, J.J. Heterozygosity-based assortative mating in blue tits (Cyanistes caeruleus): Implications for the evolution of mate choice. Proc. R. Soc. B Biol. Sci. 2009, 276, 2931–2940. [Google Scholar] [CrossRef] [Green Version]
- Zeh, J.A.; Zeh, D.W. The Evolution of Polyandry I: Intragenomic Conflict and Genetic Incompatibility; Royal Society of London, Series B; Biological Sciences: London, UK, 1996; Volume 263, pp. 1711–1717. [Google Scholar]
- Charlesworth, A.D. Inbreeding depression and its evolutionary consequences. Ann. Rev. Ecol. Syst. 1987, 18, 237–268. [Google Scholar] [CrossRef]
- Amos, W.; Wilmer, J.W.; Fullard, K.; Burg, T.M.; Croxall, J.P.; Bloch, D.; Coulson, T. The influence of parental relatedness on reproductive success. Proc. R. Soc. B Biol. Sci. 2001, 268, 2021–2027. [Google Scholar] [CrossRef]
- Szulkin, M.; Zelazowski, P.; Nicholson, G.; Sheldon, B.C. Inbreeding avoidance under different null models of random mating in the great tit. J. Anim. Ecol. 2009, 78, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Van De Casteele, T.; Galbusera, P.; Schenck, T.; Matthysen, E. Seasonal and lifetime reproductive consequences of inbreeding in the great tit Parus major. Behav. Ecol. 2003, 14, 165–174. [Google Scholar] [CrossRef]
- Nagy, L.R.; Holmes, R.T. To double-brood or not? Individual variation in the reproductive effort in black-throated blue warblers (Dendroica caerulescens). Auk 2005, 122, 902–914. [Google Scholar] [CrossRef]
- O’Brien, E.L.; Dawson, R.D. Experimental dissociation of individual quality, food and timing of breeding effects on double-brooding in a migratory songbird. Oecologia 2012, 172, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, S.; Tinbergen, J.M.; Daan, S. Multiple breeding in the Great Tit. A trade-off between successive reproductive attempts? Funct. Ecol. 1997, 11, 714–722. [Google Scholar] [CrossRef]
- Hoffmann, J.; Postma, E.; Schaub, M. Factors influencing double brooding in Eurasian Hoopoes Upupa epops. Ibis 2014, 157, 17–30. [Google Scholar] [CrossRef]
- Winkel, W.; Winkel, D. Kosten und Nutzen von Zweitbruten bei der Tannenmeise (Parus ater) Costs and benefits of second broods in Coal Tits (Parus ater). J. Ornithol. 1995, 136, 29–36. [Google Scholar] [CrossRef]
- Harrison, T.J.E.; Smith, J.A.; Martin, G.R.; Chamberlain, D.E.; Bearhop, S.; Robb, G.N.; Reynolds, S.J. Does food supplementation really enhance productivity of breeding birds? Oecologia 2010, 164, 311–320. [Google Scholar] [CrossRef]
- Verboven, N.; Verhulst, S. Seasonal variation in the incidence of double broods: The date hypothesis fits better than the quality hypothesis. J. Anim. Ecol. 1996, 65, 264. [Google Scholar] [CrossRef] [Green Version]
- Harvey, P.H.; Greenwood, P.J.; Perrins, C.M.; Martin, A.R. Breeding success of great tits Parus Major in relation to age of male and female parent. Ibis 1979, 121, 216–219. [Google Scholar] [CrossRef]
- Jankowiak, Ł.; Wysocki, D. Do individual breeding experience and parental effort affect breeding season length in blackbirds? Behav. Ecol. 2015, 27, 829–834. [Google Scholar] [CrossRef] [Green Version]
- Whelan, S.; Strickland, D.; Morand-Ferron, J.; Norris, D.R. Male experience buffers female laying date plasticity in a winter-breeding, food-storing passerine. Anim. Behav. 2016, 121, 61–70. [Google Scholar] [CrossRef]
- Husby, A.; Kruuk, L.E.; Visser, M.E. Decline in the Frequency and Benefits of Multiple Brooding in Great Tits as a Consequence of a Changing Environment; Royal Society B; Biological Sciences: London, UK, 2009; Volume 276, pp. 1845–1854. [Google Scholar]
- Wolf, L.; Ketterson, E.D.; Nolan, V., Jr. Female condition and delayed benefits to males that provide parental care: A removal study. Auk 1991, 108, 371–380. [Google Scholar] [CrossRef]
- Monroe, A.P.; Hallinger, K.K.; Brasso, R.L.; Cristol, D.A. Occurrence and implications of double brooding in a southern population of tree swallows. Condor 2008, 110, 382–386. [Google Scholar] [CrossRef]
- Nilsson, J.Å.; Smith, H.G. Incubation feeding as a male tactic for early hatching. Anim. Behav. 1988, 36, 641–647. [Google Scholar] [CrossRef]
- Cantarero, A.; López-Arrabé, J.; Palma, A.; Redondo, A.J.; Moreno, J. Males respond to female begging signals of need: A handicapping experiment in the pied flycatcher, Ficedula hypoleuca. Anim. Behav. 2014, 94, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Verhulst, S.; Hut, R.A. Post-fledging care, multiple breeding and the costs of reproduction in the great tit. Anim. Behav. 1996, 51, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Del Hoyo, J. Handbook of the Birds of the World Vol. 12 Picathartes to Tits and Chickadees; del Hoyo, J., Elliott, A., Christie, D.A., Eds.; Lynx Edicions: Barcelona, Spain, 2007; p. 750. [Google Scholar]
- Nomi, D.; Yuta, T.; Koizumi, I. Male feeding contribution facilitates multiple brooding in a biparental songbird. Ibis 2017, 160, 293–300. [Google Scholar] [CrossRef]
- Sun, H.; Gao, W.; Gong, L.; Yang, Y.L.; Wang, H.T. The study of composition and diversity of birds in Zuojia nature preserved area, Jilin Province. J. Northeast. Norm. Univ. (Nat. Sci. Ed.) 2008, 1, 21. [Google Scholar]
- Senar, J.C.; Pascual, J. Keel and tarsus length may provide a good predictor of avian body size. Ardea 1997, 85, 269–274. [Google Scholar]
- Lemel, J.; Wallin, K. Status signalling, motivational condition and dominance: An experimental study in the great tit, Parus major L. Anim. Behav. 1993, 45, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Järvi, T.; Bakken, M. The function of the variation in the breast stripe of the great tit (Parus major). Anim. Behav. 1984, 32, 590–596. [Google Scholar] [CrossRef]
- Poeysae, H. Feeding consequences of the dominance status in great tit Parus major groups. Ornis Fenn. 1988, 65, 69–75. [Google Scholar]
- Figuerola, J.; Senar, J.C. Measurement of plumage badges: An evaluation of methods used in the Great Tit Parus major. Ibis 2008, 142, 482–484. [Google Scholar] [CrossRef]
- Quesada, J.; Senar, J.C. The role of melanin- and carotenoid-based plumage coloration in nest defence in the Great Tit. Ethology 2007, 113, 640–647. [Google Scholar] [CrossRef]
- Harper, D.G. Some comments on the repeatability of measurements. Ringing Migr. 1994, 15, 84–90. [Google Scholar] [CrossRef]
- Saladin, V.; Bonfils, D.; Binz, T.; Richner, H. Isolation and characterization of 16 microsatellite loci in the Great Tit Parus major. Mol. Ecol. Notes 2003, 3, 520–522. [Google Scholar] [CrossRef] [Green Version]
- Kawano, K.M. Isolation of polymorphic microsatellite markers in the great tit (Parus major minor). Mol. Ecol. Notes 2003, 3, 314–315. [Google Scholar] [CrossRef]
- Marshall, T.C.; Slate, J.; Kruuk, L.E.B.; Pemberton, J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998, 7, 639–655. [Google Scholar] [CrossRef] [Green Version]
- Goudet, J. FSTAT (Version 1.2): A computer program to calculate F-statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Dawson, D.A.; Horsburgh, G.J.; Küpper, C.; Stewart, I.R.K.; Ball, A.D.; Durrant, K.L.; Hansson, B.; Bacon, I.; Bird, S.; Klein, Á.; et al. New methods to identify conserved microsatellite loci and develop primer sets of high cross-species utility–as demonstrated for birds. Mol. Ecol. Resour. 2010, 10, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Gompert, Z. Coancestry: A program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol. Ecol. Resour. 2011, 11, 141–145. [Google Scholar] [CrossRef]
- Ritland, K. Estimators for pairwise relatedness and individual inbreeding coefficients. Genet. Res. 1996, 67, 175–185. [Google Scholar] [CrossRef]
- García-Navas, V.; Ferrer, E.S.; Cáliz-Campal, C.; Bueno-Enciso, J.; Barrientos, R.; Sanz, J.J.; Ortego, J. Spatiotemporal and genetic contingency of extrapair behaviour in a songbird. Anim. Behav. 2015, 106, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Diehl, J.; Van Oers, K.; Snijders, L. Repeatability of signalling traits in the avian dawn chorus. Front. Zool. 2019, 16, 1–11. [Google Scholar] [CrossRef]
- Bircher, N.; Van Oers, K.; Hinde, C.A.; Naguib, M. Extraterritorial forays by great tits are associated with dawn song in unexpected ways. Behav. Ecol. 2020, 31, 873–883. [Google Scholar] [CrossRef]
- McGregor, P.K.; Krebs, J.R.; Perrins, C.M. Song repertoires and lifetime reproductive success in the great tit (Parus major). Am. Nat. 1981, 118, 149–159. [Google Scholar] [CrossRef]
- Crawley, M.J. Statistical Computing: An Introduction to Data Analysis Using S-Plus; Wiley: Chichester, England, 2002. [Google Scholar]
- Jørgensen, S. Model selection and multimodel inference. Ecol. Model. 2004, 172, 96–97. [Google Scholar] [CrossRef]
- Crick, H.Q.P.; Gibbons, D.W.; Magrath, R.D. Seasonal changes in clutch size in British birds. J. Anim. Ecol. 1993, 62, 263. [Google Scholar] [CrossRef]
- Visser, M.; Van Noordwijk, A.J.; Tinbergen, J.M.; Lessells, C.M. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. B Biol. Sci. 1998, 265, 1867–1870. [Google Scholar] [CrossRef] [Green Version]
- Thorley, J.B.; Lord, A.M. Laying date is a plastic and repeatable trait in a population of blue tits Cyanistes caeruleus. Ardea 2015, 103, 69–78. [Google Scholar] [CrossRef]
- Virkkala, R. Ecology of the siberian Tit Parus cinctus in relation to habitat euality: Effects of forest management. Ornis Scand. 1990, 21, 139. [Google Scholar] [CrossRef]
- White, F.N.; Kinney, J.L. Avian incubation. Science 1974, 186, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Stodola, K.W.; Buehler, D.A.; Kim, D.H.; Franzreb, K.E.; Linder, E.T. Biotic and abiotic factors governing nestling-period length in the ovenbird (Seiurus Aurocapilla). Auk 2010, 127, 204–211. [Google Scholar] [CrossRef]
- Ortego, J.; Calabuig, G.; Bonal, R.; Muñoz, A.; Aparicio, J.M.; Cordero, P.J. Temporal variation of heterozygosity-based assortative mating and related benefits in a lesser kestrel population. J. Evol. Biol. 2009, 22, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Whitehouse, K.; Spraker, T.R.; Lyons, E.; Melin, S.R.; Gulland, F.; Delong, R.L.; Amos, W. Contrasting effects of heterozygosity on survival and hookworm resistance in California sea lion pups. Mol. Ecol. 2006, 15, 1973–1982. [Google Scholar] [CrossRef]
- Olano-Marin, J.; Mueller, J.C.; Kempenaers, B. Heterozygosity and survival in blue tits (Cyanistes caeruleus): Contrasting effects of presumably functional and neutral loci. Mol. Ecol. 2011, 20, 4028–4041. [Google Scholar] [CrossRef]
- Gil, D.; Graves, J.; Hazon, N.; Wells, A. Male attractiveness and differential testosterone investment in Zebra Finch eggs. Science 1999, 286, 126–128. [Google Scholar] [CrossRef]
- Petrie, M.; Williams, A. Peahens Lay More Eggs for Peacocks with Larger Trains. Biol. Sci. 1993, 251, 127–131. [Google Scholar]
- Colegrave, N.; Kotiaho, J.S.; Tomkins, J.L.; Sakare, J. Mate choice or polyandry: Reconciling genetic compatibility and good genes sexual selection. Evol. Ecol. Res. 2002, 4, 911–917. [Google Scholar]
- Wilson, A.J.; Nussey, D.H. What is individual quality? An evolutionary perspective. Trends Ecol. Evol. 2010, 25, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Botero-Delgadillo, E.; Gilsenan, C.; Mueller, J.C.; Kempenaers, B. Negative effects of individual heterozygosity on reproductive success in a wild bird population. Mol. Ecol. 2020, 29, 3196–3216. [Google Scholar] [CrossRef] [PubMed]
- Coltman, D.W.; Slate, J. Microsatellite measures of inbreeding: A meta-analysis. Evolution 2003, 57, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Velando, A.; Barros, Á.; Morán, P. Heterozygosity-fitness correlations in a declining seabird population. Mol. Ecol. 2015, 24, 1007–1018. [Google Scholar] [CrossRef]
- Bichet, C.; Vedder, O.; Sauer-Gürth, H.; Becker, P.H.; Wink, M.; Bouwhuis, S. Contrasting heterozygosity-fitness correlations across life in a long-lived seabird. Mol. Ecol. 2019, 28, 671–685. [Google Scholar] [CrossRef]
- Seddon, N.; Amos, W.; Mulder, R.A.; Tobias, J.A. Male heterozygosity predicts territory size, song structure and reproductive success in a cooperatively breeding bird. Proc. R. Soc. B Biol. Sci. 2004, 271, 1823–1829. [Google Scholar] [CrossRef] [Green Version]
- Ryder, T.B.; Tori, W.; Blake, J.; Loiselle, B.A.; Parker, P. Mate choice for genetic quality: A test of the heterozygosity and compatibility hypotheses in a lek-breeding bird. Behav. Ecol. 2009, 21, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.P.; Badyaev, A.V. Adaptive genetic complementarity in mate choice coexists with selection for elaborate sexual traits. Proc. R. Soc. B Biol. Sci. 2006, 273, 1913–1919. [Google Scholar] [CrossRef] [Green Version]
- Arct, A.; Drobniak, S.M.; Mellinger, S.; Gustafsson, L.; Cichoń, M. Parental genetic similarity and offspring performance in blue tits in relation to brood size manipulation. Ecol. Evol. 2019, 9, 10085–10091. [Google Scholar] [CrossRef] [Green Version]
- Apanius, V.; Penn, D.; Slev, P.R.; Ruff, L.R.; Potts, W.K. The nature of selection on the major histocompatibility complex. Crit. Rev. Immunol. 1997, 17, 179–224. [Google Scholar] [CrossRef]
- Kokko, H.; Brooks, R.; McNamara, J.M.; Houston, A.I. The sexual selection continuum. Proc. R. Soc. B Biol. Sci. 2002, 269, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R. Can older males deliver the good genes? Trends Ecol. Evol. 2001, 16, 308–313. [Google Scholar] [CrossRef]
| Male Characteristics | Single Breeding | Double Breeding | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
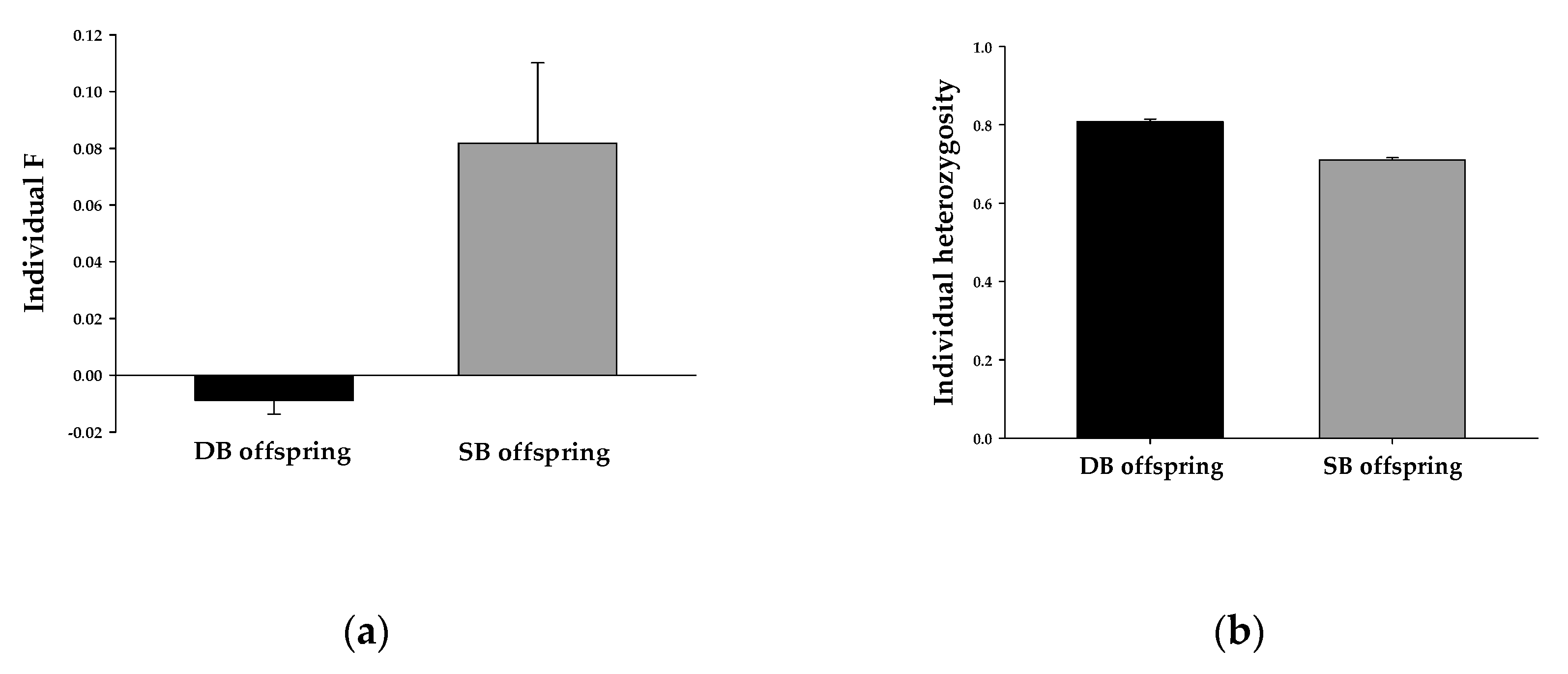
| Individual F | 0.012 ± 0.009 (n = 51) | 0.037 ± 0.017 (n = 51) | 0.002 ± 0.018 (n = 22) | 0.004 ± 0.024 (n = 22) |
| Individual heterozygosity (Hs) | 0.725 ± 0.018 (n = 51) | 0.712 ± 0.020 (n = 51) | 0.790 ± 0.039 (n = 22) | 0.807 ± 0.030 (n = 22) |
| Breast black stripe width | - | 9.525 ± 0.256 (n = 51) | - | 10.838 ± 0.561 (n = 22) |
| Tarsus length | 21.298 ± 0.139 (n = 51) | 21.690 ± 0.188 (n = 51) | 21.553 ± 0.262 (n = 22) | 21.216 ± 0.264 (n = 22) |
| Repertoire size | - | 3.55 ± 0.174 (n = 42) | - | 3.95 ± 0.381 (n = 21) |
| Relatedness | - | 0.070 ± 0.030 (n = 51) | - | −0.096 ± 0.037 (n = 22) |
| Variable | Estimate | se | df | Z | p |
|---|---|---|---|---|---|
| Intercept | −12.829 | 6.948 | 1 | −1.846 | 0.065 |
| Individual F | 8.995 | 8.109 | 1 | 1.265 | 0.206 |
| Individual heterozygosity (Hs) | 7.019 | 3.650 | 1 | 1.923 | 0.054 |
| Tarsus length | 0.303 | 0.268 | 1 | 1.131 | 0.258 |
| Model | AIC | |
|---|---|---|
| Good | Individual F + Individual heterozygosity + Breast black stripe width + Tarsus length + Repertoire size | 84.7 |
| Compatible | Relatedness | 87.2 |
| Good and Compatible | Individual F + Individual heterozygosity + Breast black stripe width + Tarsus length + Repertoire size + Relatedness | 76.4 |
| NO. | Model | np | Δ AICC | Deviance | ωi |
|---|---|---|---|---|---|
| 1 | Individual heterozygosity + Relatedness+ Breast black stripe width | 6 | 0.00 | 65.8 | 0.34 |
| 2 | Tarsus length + Individual heterozygosity + Relatedness + Breast black stripe width | 7 | 0.98 | 64.3 | 0.21 |
| 3 | Individual heterozygosity + Relatedness + Breast black stripe width + Individual F | 7 | 2.41 | 65.8 | 0.10 |
| 4 | Tarsus length + Heterozygosity + Relatedness + Breast black stripe width + Individual F | 8 | 3.33 | 64.2 | 0.07 |
| 5 | Tarsus length + Relatedness + Breast black stripe width | 6 | 3.75 | 66.4 | 0.05 |
| Variable | Estimate | se | df | Z | p |
|---|---|---|---|---|---|
| Intercept | −6.347 | 5.860 | 1 | 1.072 | 0.284 |
| Individual F | −0.891 | 2.872 | 1 | 0.305 | 0.761 |
| Individual heterozygosity (Hs) | 5.866 | 2.653 | 1 | 2.172 | 0.030 |
| Breast black stripe width | 0.384 | 0.161 | 1 | 2.343 | 0.019 |
| Tarsus length | −0.295 | 0.234 | 1 | 1.239 | 0.215 |
| Relatedness | −5.124 | 1.842 | 1 | 2.731 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.; E, M.; Wei, Y.; Sun, W.; Wang, H. Mate Choice in Double-Breeding Female Great Tits (Parus Major): Good Males or Compatible Males. Animals 2021, 11, 140. https://doi.org/10.3390/ani11010140
Fan Q, E M, Wei Y, Sun W, Wang H. Mate Choice in Double-Breeding Female Great Tits (Parus Major): Good Males or Compatible Males. Animals. 2021; 11(1):140. https://doi.org/10.3390/ani11010140
Chicago/Turabian StyleFan, Qianxi, Mingju E, Yusheng Wei, Wei Sun, and Haitao Wang. 2021. "Mate Choice in Double-Breeding Female Great Tits (Parus Major): Good Males or Compatible Males" Animals 11, no. 1: 140. https://doi.org/10.3390/ani11010140
APA StyleFan, Q., E, M., Wei, Y., Sun, W., & Wang, H. (2021). Mate Choice in Double-Breeding Female Great Tits (Parus Major): Good Males or Compatible Males. Animals, 11(1), 140. https://doi.org/10.3390/ani11010140




