A Review on Mitigating Fear and Aggression in Dogs and Cats in a Veterinary Setting
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Fear, Anxiety and Stress—Evolutionarily Adaptive
1.2. Stressors in a Veterinary Setting and Individual Responses
1.3. Identifying Stress and Fear in Dogs and Cats
2. Creating a Low-Stress Environment
2.1. Reception Area and Waiting Room
2.2. Examination Room
2.3. Sensory Considerations
3. Low-Stress Handling and Creating Positive Associations
3.1. Treatment Plan
3.2. First Contact with the Animals
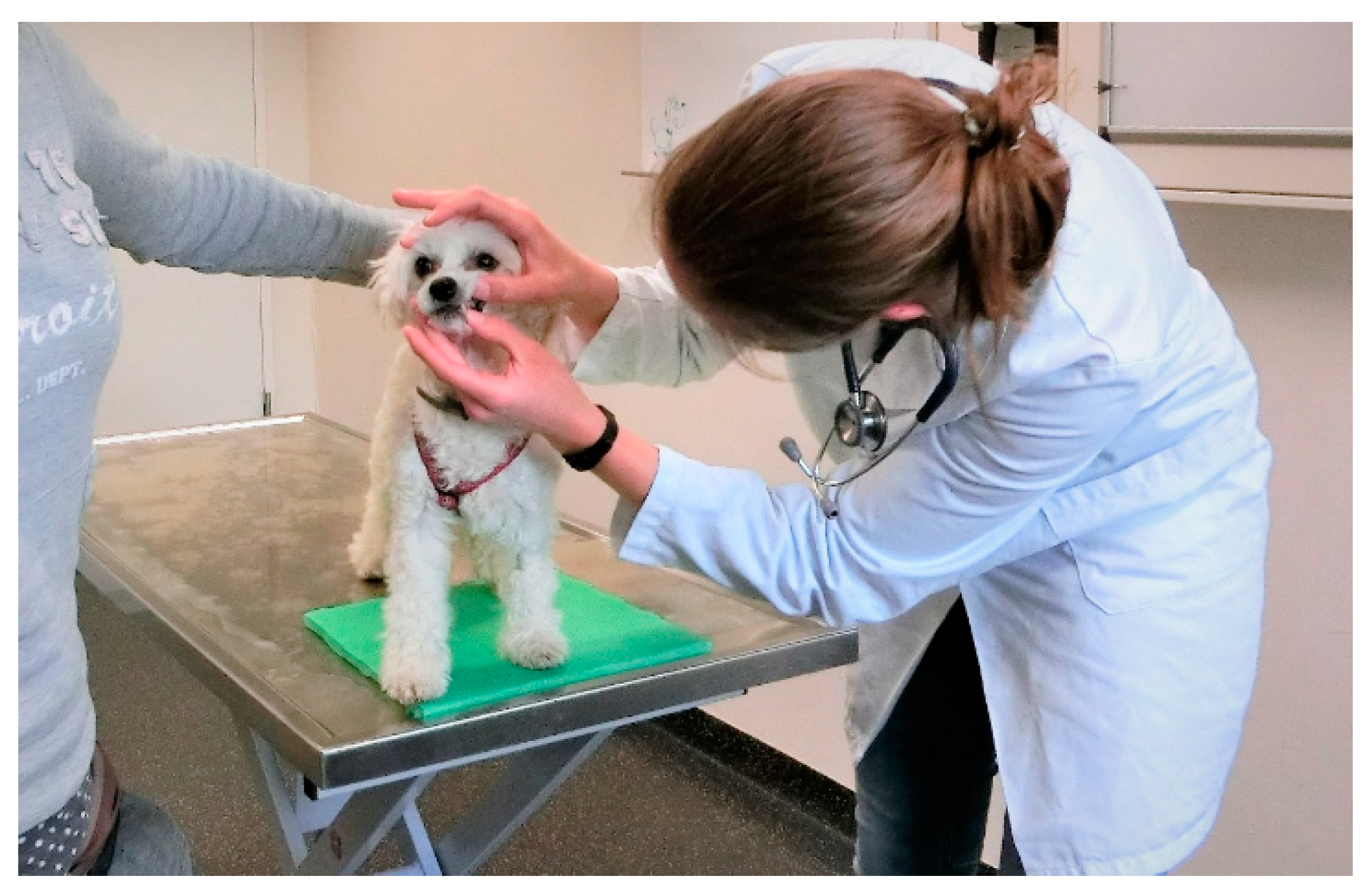
3.3. Considerate Body Language
3.4. Creating Positive Associations
3.5. Balancing Physical and Emotional Health
4. Restraint Methods
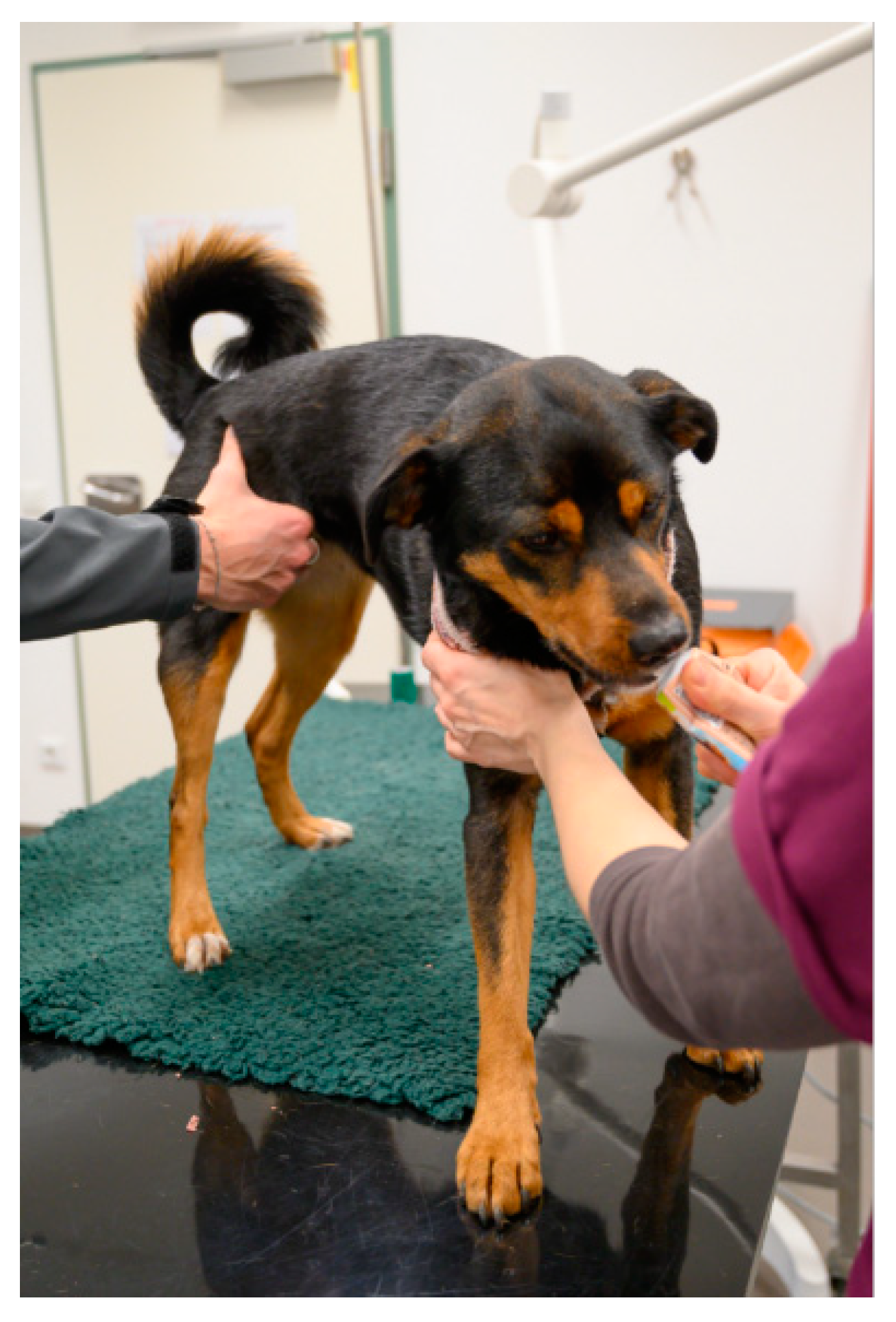
4.1. Tools to Facilitate Restraint and Safety
4.1.1. Towels, Blankets, and Alternatives
4.1.2. Muzzles and Alternatives
4.1.3. Tools That Should Only Be Used When Alternatives Are Not Feasible
5. Reducing the Perception of Pain
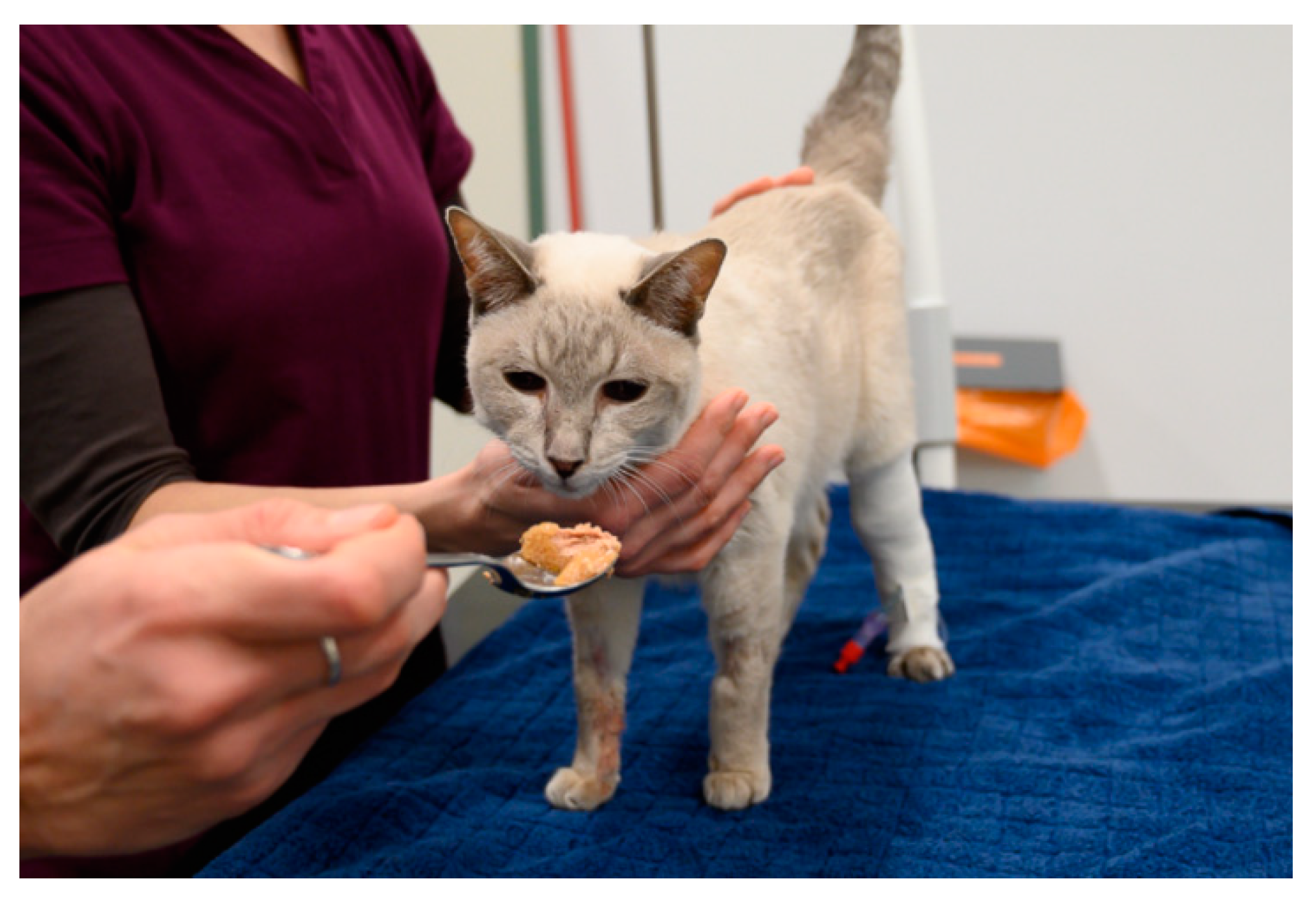
5.1. The Power of Distractions
5.2. Topical Analgesics
5.3. Optimised Use of Needles
6. Minimising Non-Painful Discomfort
7. The Owner’s Influence
8. Inpatients
9. Prevention and Training Measures
9.1. Behaviour Modification Techniques
9.2. Preventing a Resurgence of Fear
9.3. Cooperative Care Training
10. Medication
10.1. Application Options
10.2. Selected Medication Options
10.2.1. Trazodone
10.2.2. Alpha 2 Adrenoreceptor Agonists
10.2.3. Gabapentin
10.2.4. Benzodiazepines
10.2.5. Why Is Acepromazine Not State of the Art Anymore?
11. Pheromone Therapy
12. Conclusions
13. Further information
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mariti, C.; Raspanti, E.; Zilocchi, M.; Carlone, B.; Gazzano, A. The Assessment of Dog Welfare in the Waiting Room of a Veterinary Clinic. Anim. Welf. 2015, 24, 299–305. [Google Scholar] [CrossRef]
- Mariti, C.; Bowen, J.E.; Campa, S.; Grebe, G.; Sighieri, C.; Gazzano, A. Guardians’ Perceptions of Cats’ Welfare and Behavior Regarding Visiting Veterinary Clinics. J. Appl. Anim. Welf. Sci. 2016, 19, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Döring, D.; Roscher, A.; Scheipl, F.; Küchenhoff, H.; Erhard, M.H. Fear-Related Behaviour of Dogs in Veterinary Practice. Vet. J. 2009, 182, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Volk, J.O.; Felsted, K.E.; Thomas, J.G.; Siren, C.W. Executive Summary of the Bayer Veterinary Care Usage Study. J. Am. Vet. Med. Assoc. 2011, 238, 1275–1282. [Google Scholar] [CrossRef]
- Nibblett, B.M.; Ketzis, J.K.; Grigg, E.K. Comparison of Stress Exhibited by Cats Examined in a Clinic versus a Home Setting. Appl. Anim. Behav. Sci. 2015, 173, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Stanford, T.L. Behavior of Dogs Entering a Veterinary Clinic. Appl. Anim. Ethol. 1981, 7, 271–279. [Google Scholar] [CrossRef]
- Pratsch, L.; Arhant, C.; Windschnurer, I.; Affenzeller, N.; Riemer, S. Strategien Zur Angstvermeidung in Der Kleintierpraxis Teil 1–Stressreduzierende Methoden Im Umgang Mit Hund Und Katze. Kleintierpraxis 2020, 65, 548–567. [Google Scholar] [CrossRef]
- Edwards, P.T.; Smith, B.P.; McArthur, M.L.; Hazel, S.J. Fearful Fido: Investigating Dog Experience in the Veterinary Context in an Effort to Reduce Distress. Appl. Anim. Behav. Sci. 2019, 14–25. [Google Scholar] [CrossRef]
- Panksepp, J. Affective Neuroscience: The Foundations of Human and Animal Emotions; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Adolphs, R. The Biology of Fear. Curr. Biol. 2013, 23, R79–R93. [Google Scholar] [CrossRef] [Green Version]
- Steimer, T. The Biology of Fear-and Anxiety-Related Behaviors. Dialogues Clin. Neurosci. 2002, 4, 231–249. [Google Scholar]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping Styles in Animals: Current Status in Behavior and Stress-Physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Panksepp, J.; Biven, L. The Archaeology of Mind: Neuroevolutionary Origins of Human Emotions; WW Norton & Company: New York, NY, USA, 2012. [Google Scholar]
- Rhudy, J.L.; Meagher, M.W. Fear and Anxiety: Divergent Effects on Human Pain Thresholds. Pain 2000, 84, 65–75. [Google Scholar] [CrossRef]
- Gähwiler, S.; Bremhorst, A.; Tóth, K.; Riemer, S. Fear Expressions of Dogs during New Year Fireworks: A Video Analysis. Sci. Rep. 2020, 10, 16035. [Google Scholar] [CrossRef] [PubMed]
- Overall, K.L.; Dunham, A.E.; Juarbe-Diaz, S.V. Phenotypic Determination of Noise Reactivity in 3 Breeds of Working Dogs: A Cautionary Tale of Age, Breed, Behavioral Assessment, and Genetics. J. Vet. Behav. Clin. Appl. Res. 2016, 16, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Perusini, J.N.; Fanselow, M.S. Neurobehavioral Perspectives on the Distinction between Fear and Anxiety. Learn. Mem. 2015, 22, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Karatsoreos, I.N.; McEwen, B.S. Psychobiological Allostasis: Resistance, Resilience and Vulnerability. Trends Cogn. Sci. 2011, 15, 576–584. [Google Scholar] [CrossRef]
- Moberg, G.P. Biological Response to Stress: Implications for Animal Welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare; Mobert, G.P., Mench, I.A., Eds.; CABI: Wallingford, UK, 2000; pp. 1–22. [Google Scholar] [CrossRef]
- Quimby, J.M.; Smith, M.L.; Lunn, K.F. Evaluation of the Effects of Hospital Visit Stress on Physiologic Parameters in the Cat. J. Feline Med. Surg. 2011, 13, 733–737. [Google Scholar] [CrossRef]
- Cauvin, A.L.; Witt, A.L.; Groves, E.; Neiger, R.; Martinez, T.; Church, D.B. The Urinary Corticoid:Creatinine Ratio (UCCR) in Healthy Cats Undergoing Hospitalisation. J. Feline Med. Surg. 2003, 5, 329–333. [Google Scholar] [CrossRef]
- Bragg, R.F.; Bennett, J.S.; Cummings, A.; Quimby, J.M. Evaluation of the Effects of Hospital Visit Stress on Physiologic Variables in Dogs. Javma 2015, 246, 212–215. [Google Scholar] [CrossRef]
- Vonderen, I.K.; Kooistra, H.S.; Rijnberk, A. Influence of Veterinary Care on the Urinary Corticoid: Creatinine Ratio in Dogs. J. Vet. Intern. Med. 1998, 12, 431–435. [Google Scholar] [CrossRef]
- Herron, M.E.; Shreyer, T. The Pet-Friendly Veterinary Practice: A Guide for Practitioners. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 451–481. [Google Scholar] [CrossRef] [PubMed]
- John, H.; Muir, W. Handbook of Veterinary Anesthesia; Elsevier Mosby: St. Louis, MO, USA, 2013. [Google Scholar]
- Mills, D.S.; Dube, M.B.; Zulch, H. Stress and Pheromonatherapy in Small Animal Clinical Behaviour; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Overall, K. Manual of Clinical Behavioral Medicine for Dogs and Cats; Elsevier: St Louis, MO, USA, 2013. [Google Scholar]
- Ellis, S.L.H.; Rodan, I.; Carney, H.C.; Heath, S.; Rochlitz, I.; Shearburn, L.D.; Sundahl, E.; Westropp, J.L. AAFP and ISFM Feline Environmental Needs Guidelines. J. Feline Med. Surg. 2013, 15, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Rodan, I.; Sundahl, E.; Carney, H.; Gagnon, A.C.; Heath, S.; Landsberg, G.; Seksel, K.; Yin, S. AAFP and ISFM Feline-Friendly Handling Guidelines. J. Feline Med. Surg. 2011, 13, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.M.; Picketts, V.A.; Mason, G.J.; Dewey, C.E.; Niel, L. Can You Handle It? Validating Negative Responses to Restraint in Cats. Appl. Anim. Behav. Sci. 2018, 204, 94–100. [Google Scholar] [CrossRef]
- Affenzeller, N.; McPeake, K.J.; McClement, J.; Zulch, H. Human-Directed Aggressive Behaviour as the Main Presenting Sign in Dogs Subsequently Diagnosed with Diskospondylitis. Vet. Rec. Case Rep. 2017, 5, e000501. [Google Scholar] [CrossRef]
- Amat, M.; Camps, T.; García-Morato, C.; Manteca Vilanova, X. Handling Aggressive Dogs. Clin. Brief 2016, 18–22. [Google Scholar]
- Barcelos, A.M.; Mills, D.S.; Zulch, H. Clinical Indicators of Occult Musculoskeletal Pain in Aggressive Dogs. Vet. Rec. 2015, 176, 465. [Google Scholar] [CrossRef] [Green Version]
- Mills, D.S.; Demontigny-Bédard, I.; Gruen, M.; Klinck, M.P.; McPeake, K.J.; Barcelos, A.M.; Hewison, L.; Van Haevermaet, H.; Denenberg, S.; Hauser, H.; et al. Pain and Problem Behavior in Cats and Dogs. Animals 2020, 10, 318. [Google Scholar] [CrossRef] [Green Version]
- Berkowitz, L. Pain and Aggression: Some Findings and Implications. Motiv. Emot. 1993, 17, 277–293. [Google Scholar] [CrossRef]
- Moffat, K. Addressing Canine and Feline Aggression in the Veterinary Clinic. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 983–1003. [Google Scholar] [CrossRef]
- Firnkes, A.; Bartels, A.; Bidoli, E.; Erhard, M. Appeasement Signals Used by Dogs during Dog–Human Communication. J. Vet. Behav. 2017, 19, 35–44. [Google Scholar] [CrossRef]
- Hammerle, M.; Horst, C.; Levine, E.; Overall, K.; Radosta, L.; Rafter-Ritchie, M.; Yin, S. 2015 AAHA Canine and Feline Behavior Management Guidelines. J. Am. Anim. Hosp. Assoc. 2015, 51, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Rodan, I. Understanding Feline Behavior and Application for Appropriate Handling and Management. Top. Companion Anim. Med. 2010, 25, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, J.K.F. Minimising Stress for Patients in the Veterinary Hospital: Why It Is Important and What Can Be Done about It. Vet. Sci. 2017, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stellato, A.C.; Hoffman, H.; Gowland, S.; Dewey, C.E.; Widowski, T.M.; Niel, L. Effect of High Levels of Background Noise on Dog Responses to a Routine Physical Examination in a Veterinary Setting. Appl. Anim. Behav. Sci. 2019, 214, 64–71. [Google Scholar] [CrossRef]
- Casey, R.A.; Loftus, B.; Bolster, C.; Richards, G.J.; Blackwell, E.J. Inter-Dog Aggression in a UK Owner Survey: Prevalence, Co-Occurrence in Different Contexts and Risk Factors. Vet. Rec. 2013, 172, 127. [Google Scholar] [CrossRef] [PubMed]
- Moesta, A.; Crowell-Davis, S. Intercat Aggression--General Considerations, Prevention and Treatment. Tierärztl. Prax. Ausg. K Kleintiere Heimtiere 2011, 39, 97–104. [Google Scholar] [PubMed]
- Riccomini, F. How to Minimise Feline Stress in Veterinary Practice. Vet Times 2008, 8, 9–10. [Google Scholar]
- Perego, R.; Proverbio, D.; Spada, E. Increases in Heart Rate and Serum Cortisol Concentrations in Healthy Dogs Are Positively Correlated with an Indoor Waiting-Room Environment. Vet. Clin. Pathol. 2014, 43, 67–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, W.J.; Bain, M. Effect of Different Types of Classical Music Played at a Veterinary Hospital on Dog Behavior and Owner Satisfaction. J. Am. Vet. Med. Assoc. 2017, 251, 195–200. [Google Scholar] [CrossRef]
- Hernander, L. Factors Influencing Dogs’ Stress Level in the Waiting Room at a Veterinary Clinic; Student Report 190; Swedish University of Agricultural Sciences, Department of Animal Environment and Health: Skara, Sweden, 2008. [Google Scholar]
- Arhant, C.; Hörschläger, N.; Troxler, J.; Binder, R. Schutz von Hunden Und Katzen in Der Tierärztlichen Kleintierpraxis: Empfehlungen Zur Optimierung Der Ausstattung Und Des Managements Sowie Des Umgangs Mit Patienten Unter Tierschutzaspekten. Wien. Tierarztl. Monatsschr. 2017, 104, 259–276. [Google Scholar]
- Yin, S. Low Stress Handling, Restraint and Behavior Modification of Cats & Dogs; Cattle Dog Publishing: Davis, CA, USA, 2009. [Google Scholar]
- Rodan, I. Understanding the cat and feline-friendly handling. In The Cat: Clinical Medicine and Management; Little, S.E., Ed.; W.B. Saunders: Saint Louis, MO, USA, 2012; pp. 2–19. [Google Scholar]
- Anseeuw, E.; Apker, C.; Ayscue, C.; Barker, L.; Blair, D.; Brennan, J.; Brooks, S.; Case-Pall, D.; Caspersen, H.; Clark, J.; et al. Handling Cats Humanely in the Veterinary Hospital. J. Vet. Behav. Clin. Appl. Res. 2006, 1, 84–88. [Google Scholar] [CrossRef]
- Pratsch, L.; Mohr, N.; Palme, R.; Rost, J.; Troxler, J.; Arhant, C. Carrier Training Cats Reduces Stress on Transport to a Veterinary Practice. Appl. Anim. Behav. Sci. 2018, 206, 64–74. [Google Scholar] [CrossRef]
- Herron, M.E. Low-Stress Handling in Veterinary Practice—The New Norm or Still a Novel Concept? Adv. Small Anim. Med. Surg. 2015, 28, 1–2. [Google Scholar] [CrossRef]
- Mandese, W.W.; Griffin, F.C.; Reynolds, P.S.; Blew, A.C.; Deriberprey, A.S.; Estrada, A.H. Stress in Client-owned Dogs Related to Clinical Exam Location: A Randomised Crossover Trial. J. Small Anim. Pract. 2020. [Google Scholar] [CrossRef]
- Griffin, F.C.; Mandese, W.W.; Reynolds, P.S.; Deriberprey, A.S.; Blew, A.C. Evaluation of Clinical Examination Location on Stress in Cats: A Randomized Crossover Trial. J. Feline Med. Surg. 2020, 1098612X2095904. [Google Scholar] [CrossRef]
- Pageat, P.; Gaultier, E. Current Research in Canine and Feline Pheromones. Vet. Clin. North Am. Small Anim. Pract. 2003, 33, 187–211. [Google Scholar] [CrossRef]
- Stella, J.; Croney, C.; Buffington, T. Environmental Factors That Affect the Behavior and Welfare of Domestic Cats (Felis silvestris catus) Housed in Cages. Appl. Anim. Behav. Sci. 2014, 160, 94–105. [Google Scholar] [CrossRef]
- Hampton, A.; Ford, A.; Cox III, R.E.; Liu, C.; Koh, R. Effects of Music on Behavior and Physiological Stress Response of Domestic Cats in a Veterinary Clinic. J. Feline Med. Surg. 2020, 22, 122–128. [Google Scholar] [CrossRef]
- Lindig, A.M.; McGreevy, P.D.; Crean, A.J. Musical Dogs: A Review of the Influence of Auditory Enrichment on Canine Health and Behavior. Animals 2020, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Howell, A.; Feyrecilde, M. Cooperative Veterinary Care; Wiley Blackwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- Yelland, T.; Whelan, F. An Introduction to Handling Aggressive Patients. Vet. Nurse 2011, 2, 568–576. [Google Scholar] [CrossRef]
- Yin, S. Simple Handling Techniques for Dogs. Compendium 2007, 352–358. Available online: https://s3.amazonaws.com/assets.prod.vetlearn.com/mmah/25/a5874d37a74148afbaf8451147ae0c/filePV_29_06_352.pdf (accessed on 5 January 2021).
- Hennessy, M.B.; T Williams, M.; Miller, D.D.; Douglas, C.W.; Voith, V.L. Influence of Male and Female Petters on Plasma Cortisol and Behaviour: Can Human Interaction Reduce the Stress of Dogs in a Public Animal Shelter? Appl. Anim. Behav. Sci. 1998, 61, 63–77. [Google Scholar] [CrossRef]
- Amat, M.; Camps, T.; Manteca, X. Stress in Owned Cats: Behavioural Changes and Welfare Implications. J. Feline Med. Surg. 2016, 18, 577–586. [Google Scholar] [CrossRef]
- Herron, M.E.; Shofer, F.S.; Reisner, I.R. Survey of the Use and Outcome of Confrontational and Non-Confrontational Training Methods in Client-Owned Dogs Showing Undesired Behaviors. Appl. Anim. Behav. Sci. 2009, 117, 47–54. [Google Scholar] [CrossRef]
- Owczarczak-Garstecka, S.C.; Christley, R.; Watkins, F.; Yang, H.; Bishop, B.; Westgarth, C. Dog Bite Safety at Work: An Injury Prevention Perspective on Reported Occupational Dog Bites in the UK. Saf. Sci. 2019, 118, 595–606. [Google Scholar] [CrossRef]
- de Souza, C.C.F.; Maccariello, C.E.M.; Dias, D.P.M.; dos Santos Almeida, N.A.; de Medeiros, M.A. Autonomic, Endocrine and Behavioural Responses to Thunder in Laboratory and Companion Dogs. Physiol. Behav. 2017, 169, 208–215. [Google Scholar] [CrossRef]
- Arhant, C.; Schmied-Wagner, C.; Aigner, U.; Affenzeller, N. Owner Reports on Use of Muzzles and Their Effects on Dogs; an Online Survey. J. Vet. Behav. 2020. [Google Scholar] [CrossRef]
- Moody, C.M.; Mason, G.J.; Dewey, C.E.; Landsberg, G.M.; Niel, L. Testing Two Behavioural Paradigms for Measuring Post-Handling Cat Aversion Behaviour. Appl. Anim. Behav. Sci. 2019, 210, 73–80. [Google Scholar] [CrossRef]
- Moody, C.M.; Mason, G.J.; Dewey, C.E.; Niel, L. Getting a Grip: Cats Respond Negatively to Scruffing and Clips. Vet. Rec. 2020, 186, 385. [Google Scholar] [CrossRef]
- Pozza, M.E.; Stella, J.L.; Chappuis-Gagnon, A.C.; Wagner, S.O.; Tony Buffington, C.A. Pinch-Induced Behavioral Inhibition (‘clipnosis’) in Domestic Cats. J. Feline Med. Surg. 2008, 10, 82–87. [Google Scholar] [CrossRef]
- Nuti, V.; Cantile, C.; Gazzano, A.; Sighieri, C.; Mariti, C. Pinch-Induced Behavioural Inhibition (Clipthesia) as a Restraint Method for Cats during Veterinary Examinations: Preliminary Results on Cat Susceptibility and Welfare. Anim. Welf. 2016, 25, 115–123. [Google Scholar] [CrossRef]
- DeMore, M.; Cohen, L.L. Distraction for Pediatric Immunization Pain: A Critical Review. J. Clin. Psychol. Med. Settings 2005, 12, 281–291. [Google Scholar] [CrossRef]
- Kakigi, R.; Watanabe, S. Pain Relief by Various Kinds of Interference Stimulation Applied to the Peripheral Skin in Humans: Pain-Related Brain Potentials Following CO2 Laser Stimulation. J. Peripher. Nerv. Syst. JPNS 1996, 1, 189–198. [Google Scholar]
- Shilpapriya, M.; Jayanthi, M.; Reddy, V.N.; Sakthivel, R.; Selvaraju, G.; Vijayakumar, P. Effectiveness of New Vibration Delivery System on Pain Associated with Injection of Local Anesthesia in Children. J. Indian Soc. Pedod. Prev. Dent. 2015, 33, 173–176. [Google Scholar] [PubMed]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Haggard, P.; Iannetti, G.D.; Longo, M.R. Spatial Sensory Organization and Body Representation in Pain Perception. Curr. Biol. 2013, 23, R164–R176. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.J. Handbook of Emotion Regulation; Guilford Publications: New York, NY, USA, 2013. [Google Scholar]
- van Oostrom, H.; Knowles, T.G. The Clinical Efficacy of EMLA Cream for Intravenous Catheter Placement in Client-Owned Dogs. Vet. Anaesth. Analg. 2018, 45, 604–608. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.L.S.; Soares, J.H.N.; Moreira, C.M.R.; Silva, C.P.P.; Carrasco, L.P.S.; Souza, H.J.M. The Effects of Lidocaine--Prilocaine Cream on Responses to Intravenous Catheter Placement in Cats Sedated with Dexmedetomidine and Either Methadone or Nalbuphine. Vet. Anaesth. Analg. 2019, 46, 492–495. [Google Scholar] [CrossRef]
- Wagner, K.A.; Gibbon, K.J.; Strom, T.L.; Kurian, J.R.; Trepanier, L.A. Adverse Effects of EMLA (Lidocaine/Prilocaine) Cream and Efficacy for the Placement of Jugular Catheters in Hospitalized Cats. J. Feline Med. Surg. 2006, 8, 141–144. [Google Scholar] [CrossRef]
- Crisi, P.E.; De Santis, F.; Giordano, M.V.; Cerasoli, I.; Colucci, F.; Di Tommaso, M.; Luciani, A. Evaluation of Eutectic Lidocaine/Prilocaine Cream for Jugular Blood Sampling in Cats. J. Feline Med. Surg. 2020, 1098612X2091730. [Google Scholar] [CrossRef]
- Shenoda, Y.; Ward, M.P.; McKeegan, D.; Fawcett, A. “The Cone of Shame”: Welfare Implications of Elizabethan Collar Use on Dogs and Cats as Reported by Their Owners. Animals 2020, 10, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariti, C.; Ricci, E.; Zilocchi, M.; Gazzano, A. Owners as a Secure Base for Their Dogs. Behaviour 2013, 150, 1275–1294. [Google Scholar] [CrossRef]
- Topál, J.; Miklósi, Á.; Csányi, V.; Dóka, A. Attachment Behavior in Dogs (Canis familiaris): A New Application of Ainsworth’s (1969) Strange Situation Test. J. Comp. Psychol. 1998, 112, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, R.; Custance, D. A Counterbalanced Version of Ainsworth’s Strange Situation Procedure Reveals Secure-Base Effects in Dog--Human Relationships. Appl. Anim. Behav. Sci. 2008, 109, 306–319. [Google Scholar] [CrossRef]
- Gácsi, M.; Maros, K.; Sernkvist, S.; Faragó, T.; Miklósi, Á. Human Analogue Safe Haven Effect of the Owner: Behavioural and Heart Rate Response to Stressful Social Stimuli in Dogs. PLoS ONE 2013, 8, e58475. [Google Scholar] [CrossRef] [Green Version]
- Juodžente, D.; Karveliene, B.; Riškevičiene, V. The Influence of the Duration of the Preoperative Time Spent in the Veterinary Clinic without the Owner on the Psychogenic and Oxidative Stress in Dogs. J. Vet. Med. Sci. 2018, 70, 1129–1133. [Google Scholar] [CrossRef]
- Höglund, K.; Hanås, S.; Carnabuci, C.; Ljungvall, I.; Tidholm, A.; Häggström, J. Blood Pressure, Heart Rate, and Urinary Catecholamines in Healthy Dogs Subjected to Different Clinical Settings. J. Vet. Intern. Med. 2012, 26, 1300–1308. [Google Scholar] [CrossRef]
- Csoltova, E.; Martineau, M.; Boissy, A.; Gilbert, C. Behavioral and Physiological Reactions in Dogs to a Veterinary Examination: Owner-Dog Interactions Improve Canine Well-Being. Physiol. Behav. 2017, 177, 270–281. [Google Scholar] [CrossRef]
- Merola, I.; Prato-Previde, E.; Marshall-Pescini, S. Social Referencing in Dog-Owner Dyads? Anim. Cogn. 2012, 15, 175–185. [Google Scholar] [CrossRef]
- Merola, I.; Prato-Previde, E.; Marshall-Pescini, S. Dogs’ Social Referencing towards Owners and Strangers. PLoS ONE 2012, 7, e47653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, C.; Heiblum, M.; Tejeda, A.; Galindo, F. Experimental Evaluation of Attachment Behaviors in Owned Cats. J. Vet. Behav. 2007, 2, 119–125. [Google Scholar] [CrossRef]
- Vitale, K.R.; Behnke, A.C.; Udell, M.A.R. Attachment Bonds between Domestic Cats and Humans. Curr. Biol. 2019, 29, R864–R865. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Keeling, L.J.; Rehn, T. Cats and Owners Interact More with Each Other after a Longer Duration of Separation. PLoS ONE 2017, 12, e0185599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hekman, J.P.; Karas, A.Z.; Sharp, C.R. Psychogenic Stress in Hospitalized Dogs: Cross Species Comparisons, Implications for Health Care, and the Challenges of Evaluation. Animals 2014, 4, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Kogan, L.R.; Schoenfeld-Tacher, R.; Simon, A.A. Behavioral Effects of Auditory Stimulation on Kenneled Dogs. J. Vet. Behav. 2012, 7, 268–275. [Google Scholar] [CrossRef]
- Brayley, C.; Montrose, V.T. The Effects of Audiobooks on the Behaviour of Dogs at a Rehoming Kennels. Appl. Anim. Behav. Sci. 2016, 174, 111–115. [Google Scholar] [CrossRef]
- Schipper, L.L.; Vinke, C.M.; Schilder, M.B.H.; Spruijt, B.M. The Effect of Feeding Enrichment Toys on the Behaviour of Kennelled Dogs (Canis familiaris)). Appl. Anim. Behav. Sci. 2008, 114, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Shiverdecker, M.D.; Schiml, P.A.; Hennessy, M.B. Human Interaction Moderates Plasma Cortisol and Behavioral Responses of Dogs to Shelter Housing. Physiol. Behav. 2013, 109, 75–79. [Google Scholar] [CrossRef]
- Coppola, C.L.; Grandin, T.; Enns, R.M. Human Interaction and Cortisol: Can Human Contact Reduce Stress for Shelter Dogs? Physiol. Behav. 2006, 87, 537–541. [Google Scholar] [CrossRef]
- Gourkow, N.; Hamon, S.C.; Phillips, C.J.C. Effect of Gentle Stroking and Vocalization on Behaviour, Mucosal Immunity and Upper Respiratory Disease in Anxious Shelter Cats. Prev. Vet. Med. 2014, 117, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, L.A.; Arrandale, L. The Use of Hides to Reduce Acute Stress in the Newly Hospitalised Domestic Cat (Felis sylvestris catus). Vet. Nurs. J. 2017, 32, 129–132. [Google Scholar] [CrossRef]
- Arrandale, L.; Buckley, L. Towels versus Hides: Which Are Best at Reducing Acute Stress in the Newly Hospitalised Domestic Cat (Felis sylvestris catus)? Vet. Nurs. J. 2017, 32, 285–288. [Google Scholar] [CrossRef]
- Hewson, C. Evidence-Based Approaches to Reducing in-Patient Stress — Part 3: How to Reduce in-Patient Stress. Vet. Nurs. J. 2014, 29, 234–236. [Google Scholar] [CrossRef]
- Reid, P.J. Treatment of Emotional Distress and Disorders -Nonpharmacologic Methods. In Mental Health and Well-being in Animals; McMillan, F.D., Ed.; CABI: Wallingford, UK, 2019; pp. 345–363. [Google Scholar]
- Kamprath, K.; Wotjak, C.T. Nonassociative Learning Processes Determine Expression and Extinction of Conditioned Fear in Mice. Learn. Mem. 2004, 11, 770–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newall, C.; Watson, T.; Grant, K.-A.; Richardson, R. The Relative Effectiveness of Extinction and Counter-Conditioning in Diminishing Children’s Fear. Behav. Res. Ther. 2017, 95, 42–49. [Google Scholar] [CrossRef]
- Affenzeller, N.; Zulch, H.E. Animal Behavior Case of the Month. J. Am. Vet. Med. Assoc. 2017, 251, 1248–1251. [Google Scholar] [CrossRef]
- Riemer, S. Not a One-Way Road–Severity, Progression and Prevention of Firework Fears in Dogs. PLoS ONE 2019, 14, e0218150. [Google Scholar] [CrossRef] [Green Version]
- Raio, C.M.; Brignoni-Perez, E.; Goldman, R.; Phelps, E.A. Acute Stress Impairs the Retrieval of Extinction Memory in Humans. Neurobiol. Learn. Mem. 2014, 112, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Bouton, M.E.; Westbrook, R.F.; Corcoran, K.A.; Maren, S. Contextual and Temporal Modulation of Extinction: Behavioral and Biological Mechanisms. Biol. Psychiatry 2006, 60, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Davis, M. Neural Circuitry of Anxiety and Stress Disorders. In Neuropsychopharmacology: The Fifth Generation of Progress; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2002; pp. 931–951. [Google Scholar]
- Affenzeller, N.; Pratsch, L.; Windschnurer, I.; Arhant, C.; Riemer, S. Strategien Zur Angstvermeidung in Der Kleintierpraxis Teil 2 –Maßnahmen Bei Bestehenden Ängsten, Anxiolytische Medikamente, Prävention. Kleintierpraxis 2020, 66, 24–43. [Google Scholar] [CrossRef]
- Mama, K. Top 5 Short Procedure Sedation Scenarios. Clin. Brief 2020, 3, 26–30. [Google Scholar]
- Whittaker, M.A.; Laule, G. The Use of Positive Reinforcement Techniques in the Medical Management of Captive Animals. In Proceedings of the American Association of Zoo Veterinarians Annual Conference Proceedings, Omaha, NE, USA, 17–22 October 1998. [Google Scholar]
- Ramirez, K. Marine Mammal Training: The History of Training Animals for Medical Behaviors and Keys to Their Success. Vet. Clin. Exot. Anim. Pract. 2012, 15, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, J.; Wilson, K.; Lanman, C. The Effects of Operant Training on Blood Collection for Domestic Cats. Appl. Anim. Behav. Sci. 2013, 143, 128–134. [Google Scholar] [CrossRef]
- Stellato, A.; Jajou, S.; Dewey, C.E.; Widowski, T.M.; Niel, L. Effect of a Standardized Four-Week Desensitization and Counter-Conditioning Training Program on Pre-Existing Veterinary Fear in Companion Dogs. Animals 2019, 9, 767. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.; Fisher, J.; Neville, P. The Treatment of Phobias in the Dog. Appl. Anim. Behav. Sci. 1997, 52, 275–289. [Google Scholar] [CrossRef]
- Gruen, M.E.; Roe, S.C.; Griffith, E.; Hamilton, A.; Sherman, B.L. Use of Trazodone to Facilitate Postsurgical Confinement in Dogs. J. Am. Vet. Med. Assoc. 2014, 245, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Crowell-Davis, S.L.; Murray, T.F.; de Souza Dantas, L.M. Veterinary Psychopharmacology; Blackwell: Ames, IA, USA, 2019. [Google Scholar]
- Gilbert-Gregory, S.E.; Stull, J.W.; Rice, M.R.; Herron, M.E. Effects of Trazodone on Behavioral Signs of Stress in Hospitalized Dogs. J. Am. Vet. Med. Assoc. 2016, 249, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Stevens, B.; Frantz, E.; Orlando, J.; Griffith, E.; Harden, L.; Gruen, M.; Sherman, B. Efficacy of a single dose of trazodone hydrochloride given to cats prior to veterinary visits to reduce signs of transport-and examination-related anxiety. J. Am. Vet. Med. Assoc. 2016, 249, 202–207. [Google Scholar] [CrossRef]
- Korpivaara, M.; Laapas, K.; Huhtinen, M.; Schöning, B.; Overall, K. Dexmedetomidine Oromucosal Gel for Noise-Associated Acute Anxiety and Fear in Dogs—A Randomised, Double-Blind, Placebo-Controlled Clinical Study. Vet. Rec. 2017, 180, 356. [Google Scholar] [CrossRef] [Green Version]
- Korpivaara, M.; Aspegrén, J.; Huhtinen, M.; Overall, K. Oromucosal Dexmedetomidine Gel for Alleviation of Fear and Anxiety in Dogs during Minor Veterinary or Husbandry Procedures. In Proceedings of the BSAVA Congress Proceedings 2017; BSAVA Library: Birmingham, UK, 2017; p. 503. [Google Scholar]
- Hauser, H.; Campbell, S.; Korpivaara, M.; Stefanovski, D.; Quinlan, M.; Siracusa, C. In-Hospital Administration of Dexmedetomidine Oromucosal Gel for Stress Reduction in Dogs During Veterinary Visits: A Randomized, Double-Blinded, Placebo-Controlled Study. J. Vet. Behav. 2020, 39, 77–85. [Google Scholar] [CrossRef]
- Amat, M.; Le Brech, S.; Garcíamorato, C.; Temple, D.; Salichs, M.; Prades, B.; Camps, T.; Manteca, X. Preventing Travel Anxiety Using Dexmedetomidine Hydrochloride Oromucosal Gel. In Proceedings of the 11th International Veterinary Behaviour Meeting: Samorin, Slovakia, 14–16 September 2017; CABI: Wallingford, UK, 2017; Volume 45, p. 20. [Google Scholar]
- Nagore, L.; Soler, C.; Gil, L.; Serra, I.; Soler, G.; Redondo, J. Sedative Effects of Dexmedetomidine, Dexmedetomidine–Pethidine and Dexmedetomidine–Butorphanol in Cats. J. Vet. Pharmacol. Ther. 2013, 36, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ansah, O.; Raekallio, M.; Vainio, O. Correlation between Serum Concentrations Following Continuous Intravenous Infusion of Dexmedetomidine or Medetomidine in Cats and Their Sedative and Analgesic Effects. J. Vet. Pharmacol. Ther. 2000, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ansah, O.; Raekallio, M.; Vainio, O. Comparison of Three Doses of Dexmedetomidine with Medetomidine in Cats Following Intramuscular Administration. J. Vet. Pharmacol. Ther. 1998, 21, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Granholm, M.; McKusick, B.C.; Westerholm, F.C.; Aspegrén, J.C. Evaluation of the Clinical Efficacy and Safety of Dexmedetomidine or Medetomidine in Cats and Their Reversal with Atipamezole. Vet. Anaesth. Analg. 2006, 33, 214–223. [Google Scholar] [CrossRef]
- Santos, L.C.P.; Ludders, J.W.; Erb, H.N.; Martin-Flores, M.; Basher, K.L.; Kirch, P. A Randomized, Blinded, Controlled Trial of the Antiemetic Effect of Ondansetron on Dexmedetomidine-Induced Emesis in Cats. Vet. Anaesth. Analg. 2011, 38, 320–327. [Google Scholar] [CrossRef]
- Thawley, V.J.; Drobatz, K.J. Assessment of Dexmedetomidine and Other Agents for Emesis Induction in Cats: 43 Cases (2009–2014). J. Am. Vet. Med. Assoc. 2015, 247, 1415–1418. [Google Scholar] [CrossRef]
- Ogata, N.; Dodman, N.H. The Use of Clonidine in the Treatment of Fear-Based Behavior Problems in Dogs: An Open Trial. J. Vet. Behav. Clin. Appl. Res. 2011, 6, 130–137. [Google Scholar] [CrossRef]
- Stahl, S.M. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- van Haaften, K.A.; Eichstadt Forsythe, L.R.; Stelow, E.A.; Bain, M.J. Effects of a Single Preappointment Dose of Gabapentin on Signs of Stress in Cats during Transportation and Veterinary Examination. J. Am. Vet. Med. Assoc. 2017, 251, 1175–1181. [Google Scholar] [CrossRef]
- Shafford, H.L. Serenity Now: Practical Sedation Options for Cats. Available online: https://vetanesthesiaspecialists.com/wp-content/uploads/2015/11/SerenityNowSedationOptions_Feline_ABVP2015_HeidiLShafford.pdf (accessed on 14 March 2020).
- Crowell-Davis, S.L.; Seibert, L.M.; Sung, W.; Parthasarathy, V.; Curtis, T.M. Use of Clomipramine, Alprazolam, and Behavior Modification for Treatment of Storm Phobia in Dogs. J. Am. Vet. Med. Assoc. 2003, 222, 744–748. [Google Scholar] [CrossRef]
- Riemer, S. Effectiveness of Treatments for Firework Fears in Dogs. J. Vet. Behav. Clin. Appl. Res. 2020, 37, 61–70. [Google Scholar] [CrossRef]
- Bergeron, R.; Scott, S.L.; Émond, J.P.; Mercier, F.; Cook, N.J.; Schaefer, A.L. Physiology and Behavior of Dogs during Air Transport. Can. J. Vet. Res. 2002, 66, 211–216. [Google Scholar] [PubMed]
- Väisänen, M.; Raekallio, M.; Kuusela, E.; Huttunen, P.; Leppäluoto, J.; Kirves, P.; Vainio, O. Evaluation of the Perioperative Stress Response in Dogs Administered Medetomidine or Acepromazine as Part of the Preanesthetic Medication. Am. J. Vet. Res. 2002, 63, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.S.; Ramos, D.; Estelles, M.G.; Hargrave, C. A Triple Blind Placebo-Controlled Investigation into the Assessment of the Effect of Dog Appeasing Pheromone (DAP) on Anxiety Related Behaviour of Problem Dogs in the Veterinary Clinic. Appl. Anim. Behav. Sci. 2006, 98, 114–126. [Google Scholar] [CrossRef]
- Kronen, P.W.; Ludders, J.W.; Erb, H.N.; Moon, P.F.; Gleed, R.D.; Koski, S. A Synthetic Fraction of Feline Facial Pheromones Calms but Does Not Reduce Struggling in Cats before Venous Catheterization. Vet. Anaesth. Analg. 2006, 33, 258–265. [Google Scholar] [CrossRef]
- Pereira, J.S.; Fragoso, S.; Beck, A.; Lavigne, S.; Varejão, A.S.; da Graça Pereira, G. Improving the Feline Veterinary Consultation: The Usefulness of Feliway Spray in Reducing Cats’ Stress. J. Feline Med. Surg. 2016, 18, 959–964. [Google Scholar] [CrossRef]
- Conti, L.M.C.; Champion, T.; Guberman, Ú.C.; Mathias, C.H.T.; Fernandes, S.L.; Silva, E.G.M.; Lázaro, M.A.; Lopes, A.D.C.G.; Fortunato, V.R. Evaluation of Environment and a Feline Facial Pheromone Analogue on Physiologic and Behavioral Measures in Cats. J. Feline Med. Surg. 2017, 19, 165–170. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, J.-K.; Abd El-aty, A.M.; Hwang, S.-H.; Lee, J.-H.; Lee, S.-M. Efficacy of Dog-Appeasing Pheromone (DAP) for Ameliorating Separation-Related Behavioral Signs in Hospitalized Dogs. Can. Vet. J. 2010, 51, 380–384. [Google Scholar]
- Siracusa, C.; Manteca, X.; Cuenca, R.; del Mar Alcalá, M.; Alba, A.; Lavín, S.; Pastor, J. Effect of a Synthetic Appeasing Pheromone on Behavioral, Neuroendocrine, Immune, and Acute-Phase Perioperative Stress Responses in Dogs. J. Am. Vet. Med. Assoc. 2010, 237, 673–681. [Google Scholar] [CrossRef]
- Amaya, V.; Paterson, M.; Descovich, K.; Phillips, C.J.C. Effects of Olfactory and Auditory Enrichment on Heart Rate Variability in Shelter Dogs. Animals 2020, 10, 1385. [Google Scholar] [CrossRef]
- Hermiston, C.; Montrose, V.T.; Taylor, S. The Effects of Dog-Appeasing Pheromone Spray upon Canine Vocalizations and Stress-Related Behaviors in a Rescue Shelter. J. Vet. Behav. 2018, 26, 11–16. [Google Scholar] [CrossRef]
- Taylor, S.; Webb, L.; Montrose, V.T.; Williams, J. The Behavioral and Physiological Effects of Dog Appeasing Pheromone upon Canine Behavior during Separation from Owner. J. Vet. Behav. 2020, 40, 36–42. [Google Scholar] [CrossRef]
- Frank, D.; Beauchamp, G.; Palestrini, C. Systematic Review of the Use of Pheromones for Treatment of Undesirable Behavior in Cats and Dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1308–1316. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riemer, S.; Heritier, C.; Windschnurer, I.; Pratsch, L.; Arhant, C.; Affenzeller, N. A Review on Mitigating Fear and Aggression in Dogs and Cats in a Veterinary Setting. Animals 2021, 11, 158. https://doi.org/10.3390/ani11010158
Riemer S, Heritier C, Windschnurer I, Pratsch L, Arhant C, Affenzeller N. A Review on Mitigating Fear and Aggression in Dogs and Cats in a Veterinary Setting. Animals. 2021; 11(1):158. https://doi.org/10.3390/ani11010158
Chicago/Turabian StyleRiemer, Stefanie, Carmen Heritier, Ines Windschnurer, Lydia Pratsch, Christine Arhant, and Nadja Affenzeller. 2021. "A Review on Mitigating Fear and Aggression in Dogs and Cats in a Veterinary Setting" Animals 11, no. 1: 158. https://doi.org/10.3390/ani11010158
APA StyleRiemer, S., Heritier, C., Windschnurer, I., Pratsch, L., Arhant, C., & Affenzeller, N. (2021). A Review on Mitigating Fear and Aggression in Dogs and Cats in a Veterinary Setting. Animals, 11(1), 158. https://doi.org/10.3390/ani11010158




