Prenatal and Postnatal Nutrition Influence Pancreatic and Intestinal Carbohydrase Activities of Ruminants
Abstract
Simple Summary
Abstract
1. Introduction
2. Carbohydrase Activity in Ruminants
3. Influence of Prenatal Nutrition on Fetal Carbohydrase Activity
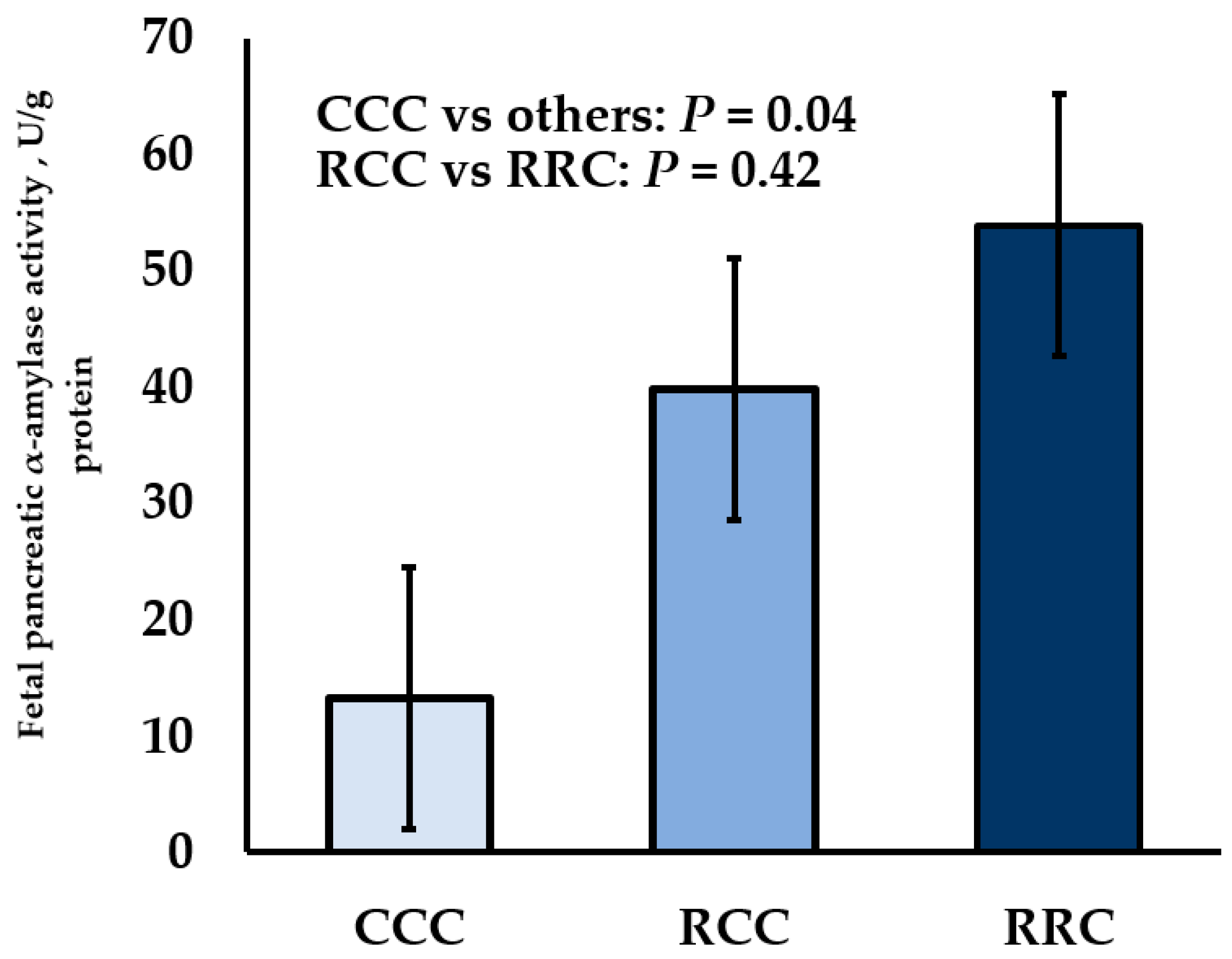
3.1. Maternal Diet Influences on Pancreatic Carbohydrase Activity
3.2. Maternal Diet Influences on Small Intestinal Carbohydrase Activity
4. Influence of Postnatal Nutrition on Neonatal Carbohydrase Activity
4.1. Neonatal Diet Influences on Pancreatic Carbohydrase Activity
4.2. Neonatal Diet Influences on Small Intestinal Carbohydrase Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caton, J.S.; Hess, B.W. Maternal plane of nutrition: Impacts on fetal outcomes and postnatal offspring responses. In Proceedings of the 4th Grazing Livestock Nutrition Conference, Estes Park, CO, USA, 9–10 July 2010; pp. 104–122. [Google Scholar]
- Reed, J.J.; Ward, M.A.; Vonnahme, K.A.; Neville, T.L.; Julius, S.L.; Borowicz, P.P.; Taylor, J.B.; Redmer, D.A.; Grazul-Bilska, A.T.; Reynolds, L.P.; et al. Effects of selenium supply and dietary restriction on maternal and fetal body weight, visceral organ mass, and cellularity estimates, and jejunal vascularity in pregnant ewe lambs. J. Anim. Sci. 2007, 85, 2721–2733. [Google Scholar] [CrossRef]
- Meyer, A.M.; Reed, J.J.; Vonnahme, K.A.; Soto-Navarro, S.A.; Reynolds, L.P.; Ford, S.P.; Hess, B.W.; Caton, J.S. Effects of stage of gestation and nutrient restriction during early to mid-gestation on maternal and fetal visceral organ mass and indices of jejunal growth and vascularity in beef cows. J. Anim. Sci. 2010, 88, 2410–2424. [Google Scholar] [CrossRef]
- Yunusova, R.D.; Neville, T.L.; Vonnahme, K.A.; Hammer, C.J.; Reed, J.J.; Taylor, J.B.; Redmer, D.A.; Reynolds, L.P.; Caton, J.S. Impacts of maternal selenium supply and nutritional plane on visceral tissues and intestinal biology in 180-day-old offspring in sheep. J. Anim. Sci. 2013, 91, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.S.; Bauer, M.L.; Hidari, H. Metabolic components of energy expenditure in growing beef cattle. Asian-Austr. J. Anim. Sci. 2000, 13, 702–710. [Google Scholar] [CrossRef]
- Milligan, L.P.; McBride, B.W. Energy costs of ion pumping by animal tissues. J. Nutr. 1985, 115, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Prezotto, L.D.; Lemley, C.O.; Camacho, L.E.; Doscher, F.E.; Meyer, A.M.; Caton, J.S.; Awda, B.J.; Vonnahme, K.A.; Swanson, K.C. Effects of nutrient restriction and melatonin supplementation on maternal and foetal hepatic and small intestinal energy utilization. J. Anim. Physiol. Anim. Nutr. 2014, 98, 797–807. [Google Scholar] [CrossRef]
- Prezotto, L.D.; Camacho, L.E.; Lemley, C.O.; Keomanivong, F.E.; Caton, J.S.; Vonnahme, K.A.; Swanson, K.C. Nutrient restriction and realimentation in beef cows during early and mid-gestation and maternal and fetal hepatic and small intestinal in vitro oxygen consumption. Animal 2016, 10, 829–837. [Google Scholar] [CrossRef]
- Prezotto, L.D.; Thorson, J.F.; Borowicz, P.P.; Peine, J.L.; Bedenbaugh, M.; Hileman, S.M.; Lents, C.A.; Caton, J.S.; Swanson, K.C. Influences of maternal nutrient restriction and arginine supplementation on visceral metabolism and hypothalamic circuitry of offspring. Domest. Anim. Endocrinol. 2018, 65, 71–79. [Google Scholar] [CrossRef]
- Caton, J.S.; Crouse, M.S.; Reynolds, L.P.; Neville, T.L.; Dahlen, C.R.; Ward, A.K.; Swanson, K.C. Maternal nutrition and programming of offspring energy requirements. Transl. Anim. Sci. 2019, 3, 976–990. [Google Scholar] [CrossRef]
- Elolimy, A.A.; Arroyo, J.M.; Batistel, F.; Iakiviak, M.A.; Loor, J.J. Association of residual feed intake with abundance of ruminal bacteria and biopolymer hydrolyzing enzyme activities during the peripartal period and early lactation in Holstein dairy cows. J. Anim. Sci. Biotechnol. 2018, 9, 43. [Google Scholar] [CrossRef]
- Baldwin, R.L. Modeling Ruminant Digestion and Metabolism; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Harmon, D.L.; Swanson, K.C. Review: Nutritional regulation of intestinal starch and protein assimilation in ruminants. Animal 2020, 14, s17–s28. [Google Scholar] [CrossRef] [PubMed]
- Owens, F.N.; Zinn, R.A.; Kim, Y.K. Limits to starch digestion in the ruminant small intestine. J. Anim. Sci. 1986, 63, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Moharrery, A.; Larsen, M.; Weisbjerg, M.R. Starch digestion in the rumen, small intestine, and hind gut of dairy cows—A meta-analysis. Anim. Feed Sci. Technol. 2014, 192, 1–14. [Google Scholar] [CrossRef]
- Black, J.L. A theoretical consideration of the effect of preventing rumen fermentation on the efficiency of utilization of dietary energy and protein in lambs. Br. J. Nutr. 1971, 25, 31–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harmon, D.L.; McLeod, K.R. Glucose uptake and regulation by intestinal tissues: Implications and whole-body energetics. J. Anim. Sci. 2001, 79, E59–E72. [Google Scholar] [CrossRef]
- Huntington, G.B.; Harmon, D.L.; Richards, C.J. Sites, rates, and limits of starch digestion and glucose metabolism in growing cattle. J. Anim. Sci. 2006, 84, E14–E24. [Google Scholar] [CrossRef]
- Swanson, K.C.; Matthews, J.C.; Woods, C.A.; Harmon, D.L. Postruminal administration of partially hydrolyzed starch and casein influences pancreatic alpha-amylase expression in calves. J. Nutr. 2002, 132, 376–381. [Google Scholar] [CrossRef]
- Richards, C.J.; Swanson, K.C.; Paton, S.J.; Harmon, D.L.; Huntington, G.B. Pancreatic exocrine secretion in steers infused postruminally with casein and cornstarch. J. Anim. Sci. 2003, 81, 1051–1056. [Google Scholar] [CrossRef]
- Trotta, R.J.; Sitorski, L.G.; Acharya, S.; Brake, D.W.; Swanson, K.C. Duodenal infusions of starch with casein or glutamic acid influence pancreatic and small intestinal carbohydrase activities in cattle. J. Nutr. 2020, 150, 784–791. [Google Scholar] [CrossRef]
- Harmon, D.L. Nutritional regulation of postruminal digestive enzymes in ruminants. J. Dairy Sci. 1993, 76, 2102–2111. [Google Scholar] [CrossRef]
- Walker, D.M. The development of the digestive system of the young animal III. Carbohydrase enzyme development in the young lamb. J. Agric. Sci. 1959, 53, 374–380. [Google Scholar] [CrossRef]
- Hembry, F.G.; Bell, M.C.; Hall, R.F. Intestinal carbohydrase activity and carbohydrate utilization in mature sheep. J. Nutr. 1967, 93, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Siddons, R.C. Carbohydrase activities in the bovine digestive tract. Biochem. J. 1968, 108, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Coombe, N.B.; Siddons, R.C. Carbohydrases of the bovine digestive tract. Br. J. Nutr. 1973, 30, 269–276. [Google Scholar] [CrossRef][Green Version]
- Sir Elkhatim, M.M.; Osman, A.M. The level and distribution of disaccharidases in the camel (C. dromedarius). Biochem. Physiol. 1982, 71, 199–204. [Google Scholar] [CrossRef]
- Majeed, M.A.; Zaidi, I.H.; Ilahi, A. The nature of nasolabial gland secretion (NLGS) in large domestic ruminants. Res. Vet. Sci. 1970, 11, 407–410. [Google Scholar] [CrossRef]
- Huntington, G.B. Starch utilization by ruminants: From basics to the bunk. J. Anim. Sci. 1997, 75, 852–867. [Google Scholar] [CrossRef]
- Brake, D.W.; Swanson, K.C. Ruminant nutrition symposium: Effects of postruminal flows of protein and amino acids on small intestinal starch digestion in beef cattle. J. Anim. Sci. 2018, 96, 739–750. [Google Scholar] [CrossRef]
- Kreikemeier, K.K.; Harmon, D.L.; Peters, J.P.; Gross, K.L.; Armendariz, C.K.; Krehbiel, C.R. Influence of dietary forage and feed intake on carbohydrase activities and small intestinal morphology of calves. J. Anim. Sci. 1990, 68, 2916–2929. [Google Scholar] [CrossRef]
- Lee, B.H.; Rose, D.R.; Lin, A.H.; Quezada-Calvillo, R.; Nichols, B.L.; Hamaker, B.R. Contribution of the individual small intestinal alpha-glucosidases to digestion of unusual alpha-linked glycemic disaccharides. J. Agric. Food Chem. 2016, 64, 6487–6494. [Google Scholar] [CrossRef]
- Galand, G. Brush border membrane sucrase-isomaltase, maltase-glucoamylase and trehalase in mammals. Comparative development, effects of glucocorticoids, molecular mechanisms, and phylogenetic implications. Comp. Biochem. Physiol. B 1989, 94, 1–11. [Google Scholar] [CrossRef]
- Lin, A.H.M.; Nichols, B.L.; Quezada-Calvillo, R.; Avery, S.E.; Sim, L.; Rose, D.R.; Naim, H.Y.; Hamaker, B.R. Unexpected high digestion rate of cooked starch by the Ct-maltase-glucoamylase small intestine mucosal α-glucosidase subunit. PLoS ONE 2012, 7, e35473. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Vonnahme, K.A.; Luther, J.S.; Hammer, C.J.; Maddock Carlin, K.R.; Grazul-Bilska, A.T.; Redmer, D.A. Developmental programming: The concept, large animal models, and the key role of uteroplacental vascular development. J. Anim. Sci. 2010, 88, E61–E72. [Google Scholar] [CrossRef] [PubMed]
- Keomanivong, F.E.; Camacho, L.E.; Lemley, C.O.; Kuemper, E.A.; Yunusova, R.D.; Borowicz, P.P.; Kirsch, J.D.; Vonnahme, K.A.; Caton, J.S.; Swanson, K.C. Effects of realimentation after nutrient restriction during mid- to late gestation on pancreatic digestive enzymes, serum insulin and glucose levels, and insulin-containing cell cluster morphology. J. Anim. Physiol. Anim. Nutr. 2017, 101, 589–604. [Google Scholar] [CrossRef]
- Keomanivong, F.E.; Lemley, C.O.; Camacho, L.E.; Yunusova, R.; Borowicz, P.P.; Caton, J.S.; Meyer, A.M.; Vonnahme, K.A.; Swanson, K.C. Influence of nutrient restriction and melatonin supplementation of pregnant ewes on maternal and fetal pancreatic digestive enzymes and insulin-containing clusters. Animal 2016, 10, 440–448. [Google Scholar] [CrossRef]
- Trotta, R.J.; Vasquez-Hidalgo, M.A.; Vonnahme, K.A.; Swanson, K.C. Effects of nutrient restriction during midgestation to late gestation on maternal and fetal postruminal carbohydrase activities in sheep. J. Anim. Sci. 2020, 98, skz393. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Beef Cattle, 7th revised ed.; The National Academies Press: Washington DC, USA, 2000. [Google Scholar]
- Trotta, R.J.; Keomanivong, F.E.; Peine, J.L.; Caton, J.S.; Swanson, K.C. Influence of maternal nutrient restriction and rumen-protected arginine supplementation on post-ruminal digestive enzyme activity of lamb offspring. Livest. Sci. 2020, 241, 104246. [Google Scholar] [CrossRef]
- da Cruz, W.F.G.; Schoonmaker, J.P.; de Resende, F.D.; Siqueira, G.R.; Rodrigues, L.M.; Zamudio, G.D.R.; Ladeira, M.M. Effects of maternal protein supplementation and inclusion of rumen-protected fat in the finishing diet on nutrient digestibility and expression of intestinal genes in Nellore steers. Anim. Sci. J. 2019, 90, 1200–1211. [Google Scholar] [CrossRef]
- Greene, M.A.; Britt, J.L.; Powell, R.R.; Feltus, F.A.; Bridges, W.C.; Bruce, T.; Klotz, J.L.; Miller, M.F.; Duckett, S.K. Ergot alkaloid exposure during gestation alters: 3. Fetal growth, muscle fiber development and miRNA transcriptome. J. Anim. Sci. 2019, 97, 3153–3168. [Google Scholar] [CrossRef]
- Greene, M.A.; Britt, J.L.; Bertrand, J.K.; Klotz, J.L.; Bridges, W., Jr.; Andrae, J.G.; Duckett, S.K. Feeding tall fescue seed during mid and late gestation influences subsequent postnatal growth, puberty, and carcass quality of offspring. Animals 2020, 10, 1859. [Google Scholar] [CrossRef]
- Trotta, R.J.; Klotz, J.L.; Matthews, J.C.; Swanson, K.C. PSIX-7 Grazing toxic endophyte-infected tall fescue does not influence pancreatic or small intestinal digestive enzyme activities in beef steers. J. Anim. Sci. 2020, 98 (Suppl. 4), 410–411. [Google Scholar] [CrossRef]
- Klotz, J.L.; Britt, J.L.; Miller, M.F.; Snider, M.A.; Aiken, G.E.; Long, N.M.; Pratt, S.L.; Andrae, J.G.; Duckett, S.K. Ergot alkaloid exposure during gestation alters: II. Uterine and umbilical artery vasoactivity. J. Anim. Sci. 2019, 97, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.; Gerkowitch, V.P.; Russel, R.I. Pre- and post-weaning disaccharidases patterns in isografts of fetal mouse intestine. Gastroenterology 1973, 64, 292–297. [Google Scholar] [CrossRef]
- Van Beers, E.H.; Büller, H.A.; Grand, R.J.; Einerhand, A.W.C.; Dekker, J. Intestinal brush border glycohydrolases: Structure, function, and development. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 197–262. [Google Scholar] [CrossRef] [PubMed]
- Trotta, R.J.; Lemley, C.O.; Vonnahme, K.A.; Swanson, K.C. Effects of nutrient restriction and melatonin supplementation from mid- to late-gestation on maternal and fetal small intestinal carbohydrase activities in sheep. Domest. Anim. Endocrinol. 2021, 74, 106555. [Google Scholar] [CrossRef]
- Lemley, C.O.; Meyer, A.M.; Camacho, L.E.; Neville, T.L.; Newman, D.J.; Caton, J.S.; Vonnahme, K.A. Melatonin supplementation alters uteroplacental hemodynamics and fetal development in an ovine model of intrauterine growth restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R454–R467. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Sheep, 6th ed.; The National Academies Press: Washington DC, USA, 1985. [Google Scholar]
- Li, Z.; Wang, B.; Li, H.; Jian, L.; Luo, H.; Wang, B.; Zhang, C.; Zhao, X.; Xue, Y.; Peng, S.; et al. Maternal folic acid supplementation differently affects the small intestinal phenotype and gene expression of newborn lambs from differing litter sizes. Animals 2020, 10, 2138. [Google Scholar] [CrossRef]
- Shirazi-Beechey, S.P.; Smith, M.W.; Wang, Y.; James, P.S. Postnatal development of lamb intestinal digestive enzymes is not regulated by diet. J. Physiol. 1991, 437, 691–698. [Google Scholar] [CrossRef]
- Gilbert, M.S.; Pantophlet, A.J.; Berends, H.; Pluschke, A.M.; van den Borne, J.J.G.C.; Hendriks, W.H.; Schols, H.A.; Gerrits, W.J.J. Fermentation in the small intestine contributes substantially to intestinal starch disappearance in calves. J. Nutr. 2015, 145, 1147–1155. [Google Scholar] [CrossRef]
- Brannon, P.M. Adaptation of the exocrine pancreas to diet. Ann. Rev. Nutr. 1990, 10, 85–105. [Google Scholar] [CrossRef]
- Swanson, K.C.; Matthews, J.C.; Woods, C.A.; Harmon, D.L. Influence of substrate and/or neurohormonal mimic on in vitro pancreatic enzyme release from calves postruminally infused with partially hydrolyzed starch and/or casein. J. Anim. Sci. 2003, 81, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.C.; Benson, J.A.; Matthews, J.C.; Harmon, D.L. Pancreatic exocrine secretion and plasma concentration of some gastrointestinal hormones in response to abomasal infusion of starch hydrolyzate and/or casein. J. Anim. Sci. 2004, 82, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Britt, D.G.; Huber, J.T. Effect of adding sugars to a carbohydrate-free diet on intestinal disaccharidase activities in the young calf. J. Dairy Sci. 1974, 57, 420–426. [Google Scholar] [CrossRef]
- Trotta, R.J.; Ward, A.K.; Swanson, K.C. Influence of dietary fructose supplementation on visceral organ mass, carbohydrase activity, and mRNA expression of genes involved in small intestinal carbohydrate assimilation in neonatal calves. J. Dairy Sci. 2020, 103, 10060–10073. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Yang, X.J.; Guo, L.; Zheng, C.; Wang, D.D.; Cai, C.J.; Liu, S.M.; Yao, Y.H. Effects of dietary leucine and phenylalanine on pancreas development, enzyme activity, and relative gene expression in milk-fed Holstein calves. J. Dairy Sci. 2018, 101, 4235–4244. [Google Scholar] [CrossRef]
- Reiners, J.; Steele, M.; Carlin, K.; Swanson, K. Effects of incremental amounts of supplemental leucine to milk-fed neonatal Holstein bull calves on pancreatic and intestinal enzyme activity. In Proceedings of the XIIIth International Symposium on Ruminant Physiology, Leipzig, Germany, 3–6 September 2019; p. 605. [Google Scholar]
- Guo, L.; Tian, H.; Shen, J.; Zheng, C.; Liu, S.; Cao, Y.; Cai, C.; Yao, J. Phenylalanine regulates initiation of digestive enzyme mRNA translation in pancreatic acinar cells and tissue segments in dairy calves. Biosci. Rep. 2018, 38, BSR20171189. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Zheng, C.; Liu, B.; Yin, Q.; Cao, Y.; Yao, J. Leucine affects α-amylase synthesis through PI3K/Akt-mTOR signaling pathways in pancreatic acinar cells of dairy calves. J. Agric. Food Chem. 2018, 66, 5149–5156. [Google Scholar] [CrossRef]
- Guo, L.; Yao, J.H.; Zheng, C.; Tian, H.B.; Liu, Y.L.; Liu, S.M.; Gai, C.J.; Xu, X.R.; Cao, Y.C. Leucine regulates α-amylase and trypsin synthesis in dairy calf pancreatic tissue in vitro via the mammalian target of rapamycin signalling pathway. Animal 2019, 13, 1899–1906. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, K.; Liu, S.; Guo, L.; Yao, Y.; Cai, C. Leucine regulates the exocrine function in pancreatic tissue of dairy goats in vitro. Biomed. Res. Int. 2019, 2019, 7521715. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, K.; Liu, S.; Guo, L.; Cai, C.; Yao, J. Isoleucine regulates the synthesis of pancreatic enzymes via the activation of mRNA expression and phosphorylation in the mammalian target of rapamycin signalling pathways in pancreatic tissues. Biomed. Res. Int. 2019, 2019, 6302950. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tian, H.; Yao, J.; Ren, H.; Yin, Q.; Cao, Y. Leucine improves α-amylase secretion through the general secretory signaling pathway in pancreatic acinar cells of dairy calves. Am. J. Physiol. Cell Physiol. 2020, 318, C1284–C1293. [Google Scholar] [CrossRef] [PubMed]
- Harada, E.; Kato, S. Effect of short-chain fatty acids on the secretory response of the ovine exocrine pancreas. Am. J. Physiol. 1983, 244, G284–G290. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Tsuda, T. Effects of acetylcholine and short-chain fatty acids on acinar cells of the exocrine pancreas in sheep. J. Physiol. 1984, 356, 479–489. [Google Scholar] [CrossRef]
- Swanson, K.C.; Matthews, J.C.; Matthews, A.D.; Howell, J.A.; Richards, C.J.; Harmon, D.L. Dietary carbohydrate source and energy intake influence the expression of pancreatic alpha-amylase in lambs. J. Nutr. 2000, 130, 2157–2165. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Guo, G.; Huo, W.J.; Yang, W.Z. Effects of isovalerate supplementation on morphology and functional gene expression of small intestine mucosa in pre- and post-weaned dairy calves. J. Agric. Sci. 2018, 156, 272–281. [Google Scholar] [CrossRef]
- Guilloteau, P.; Zabielski, R.; David, J.C.; Blum, J.W.; Morisset, J.A.; Biernat, M.; Wolinksi, J.; Laubitz, D.; Hamon, Y. Sodium butyrate as a growth promoter in milk replacer formula for young calves. J. Dairy Sci. 2009, 92, 1038–1049. [Google Scholar] [CrossRef]
- Krzeminski, R.; Mikolajczyk, M.; Kulasek, G. The effect of intraduodenal infusion of 0.1 N HCl on the volume and composition of pancreatic juice and bile in wethers. J. Anim. Physiol. Anim. Nutr. 1990, 64, 139–142. [Google Scholar] [CrossRef]
- Koch, C.; Gerbert, C.; Frieten, D.; Dusel, G.; Eder, K.; Zitnan, R.; Hammon, H.M. Effects of ad libitum milk replacer feeding and butyrate supplementation on the epithelial growth and development of the gastrointestinal tract in Holstein calves. J. Dairy Sci. 2019, 102, 8513–8526. [Google Scholar] [CrossRef]
- Reiners, J.; Hoffman, T.; Anderson, S.E.; Swanson, K. Effects of supplemental dietary leucine fed to lambs on pancreatic and intestinal starch digesting enzymes. In 2020 North Dakota Beef and Sheep Report; Swanson, K., Ed.; North Dakota State University Agricultural Experiment Station: Fargo, ND, USA, 2020; pp. 14–16. [Google Scholar]
- Gorka, P.; Pietrzak, P.; Kotunia, A.; Zabielski, R.; Kowalski, Z.M. Effect of method of delivery of sodium butyrate on maturation of the small intestine in newborn calves. J. Dairy Sci. 2014, 97, 1026–1035. [Google Scholar] [CrossRef]
- Richards, C.J.; Branco, A.F.; Bohnert, D.W.; Huntington, G.B.; Macari, M.; Harmon, D.L. Intestinal starch disappearance increased in steers abomasally infused with starch and protein. J. Anim. Sci. 2002, 80, 3361–3368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brake, D.W.; Titgemeyer, E.C.; Bailey, E.A.; Anderson, D.E. Small intestinal digestion of raw cornstarch in cattle consuming a soybean hull-based diet is improved by duodenal casein infusion. J. Anim. Sci. 2014, 92, 4047–4056. [Google Scholar] [CrossRef] [PubMed]
- Brake, D.W.; Titgemeyer, E.C.; Anderson, D.E. Duodenal supply of glutamate and casein both improve intestinal starch digestion in cattle but by apparently different mechanisms. J. Anim. Sci. 2014, 92, 4057–4067. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Salgado, P.; Toullec, R.; Lalles, J.P. Enzymes of the small intestine of the calf: Effect of dietary protein source on the activities of some enzymes in the small intestinal mucosa and digesta. J. Sci. Food Agric. 2002, 82, 1772–1779. [Google Scholar] [CrossRef]
- Schonhusen, U.; Junghans, P.; Floter, A.; Steinhoff-Wagner, A.; Gors, S.; Schneider, F.; Metges, C.C.; Hammon, H.M. First-pass uptake and oxidation of glucose by splanchnic tissue in young goats fed soy protein-based milk diets with or without amino acid supplementation. J. Dairy Sci. 2013, 96, 2400–2412. [Google Scholar] [CrossRef]
- Burakowska, K.; Gorka, P.; Kent-Dennis, C.; Kowalski, Z.M.; Laarveld, B.; Penner, G.B. Effect of heat-treated canola meal and glycerol inclusion on performance and gastrointestinal development of Holstein calves. J. Dairy Sci. 2020, 103, 7998–8019. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, S.; Yang, X.; Guo, L.; Cai, C.; Yao, J. Effects of dietary leucine and phenylalanine on gastrointestinal development and small intestinal enzyme activities in milk-fed holstein dairy calves. Biosci. Rep. 2019, 39, BSR20181733. [Google Scholar] [CrossRef]
- Dollar, A.M.; Porter, J.W.G. Utilization of carbohydrates by the young calf. Nature 1957, 179, 1299–1300. [Google Scholar] [CrossRef]
- Huber, J.T.; Jacobson, N.L.; Allen, R.S.; Hartman, P.A. Digestive enzyme activities in the young calf. J. Dairy Sci. 1961, 44, 1494–1501. [Google Scholar] [CrossRef]
- Shirazi-Beechey, S.P.; Kemp, R.B.; Dyer, J.; Beechey, R.B. Changes in the functions of the intestinal brush border membrane during the development of the ruminant habit in lambs. Comp. Biochem. Physiol. 1989, 94B, 801–806. [Google Scholar] [CrossRef]
- Rosensweig, N.S.; Herman, R.H. Control of jejunal sucrase and maltase activity by dietary sucrose or fructose in man. A model for the study of enzyme regulation in man. J. Clin. Invest. 1968, 47, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Greene, H.L.; Stifel, F.B.; Herman, R.H. Dietary stimulation of sucrase in a patient with sucrase-isomaltase deficiency. Biochem. Med. 1972, 6, 409–418. [Google Scholar] [CrossRef]
| Item | Study | |||
|---|---|---|---|---|
| Trotta et al. [58] | Reiners et al. [60] | Koch et al. [73] | Reiners et al. [74] | |
| Species | Calf | Calf | Calf | Lamb |
| Age at slaughter | 42 d | 39 d | 80 d | 163 d |
| Feeding length | 28 d | 28 d | 52 d | 42 d |
| Diet | Milk-replacer | Milk-replacer | Milk-replacer | Milk-replacer |
| Supplement | Fructose | Leucine | Butyrate | Leucine |
| Amount | 2.2 g/kg BW | 0.2, 0.4, 0.8 g/kg BW | 0.24% of DM | 2.9% of DM |
| Maltase | → | linear ↓ | → | ↓ |
| Isomaltase | → | linear ↓ | - | ↓ |
| Glucoamylase | ↑ | → | - | → |
| Lactase | → | quadratic 2 | → | → |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trotta, R.J.; Swanson, K.C. Prenatal and Postnatal Nutrition Influence Pancreatic and Intestinal Carbohydrase Activities of Ruminants. Animals 2021, 11, 171. https://doi.org/10.3390/ani11010171
Trotta RJ, Swanson KC. Prenatal and Postnatal Nutrition Influence Pancreatic and Intestinal Carbohydrase Activities of Ruminants. Animals. 2021; 11(1):171. https://doi.org/10.3390/ani11010171
Chicago/Turabian StyleTrotta, Ronald J., and Kendall C. Swanson. 2021. "Prenatal and Postnatal Nutrition Influence Pancreatic and Intestinal Carbohydrase Activities of Ruminants" Animals 11, no. 1: 171. https://doi.org/10.3390/ani11010171
APA StyleTrotta, R. J., & Swanson, K. C. (2021). Prenatal and Postnatal Nutrition Influence Pancreatic and Intestinal Carbohydrase Activities of Ruminants. Animals, 11(1), 171. https://doi.org/10.3390/ani11010171





