Comparison of Two Different Feather Sampling Methods to Measure Corticosterone in Wild Greater Flamingos (Phoenicopterus roseus) and Wild Mallards (Anas platyrhynchos)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Protocol
2.2. Specimens
2.2.1. Mallard (Anas platyrhynchos)
2.2.2. Greater Flamingo (Phoenicopterus roseus)
2.3. Analysis of CORTf
2.4. Statistical Analysis
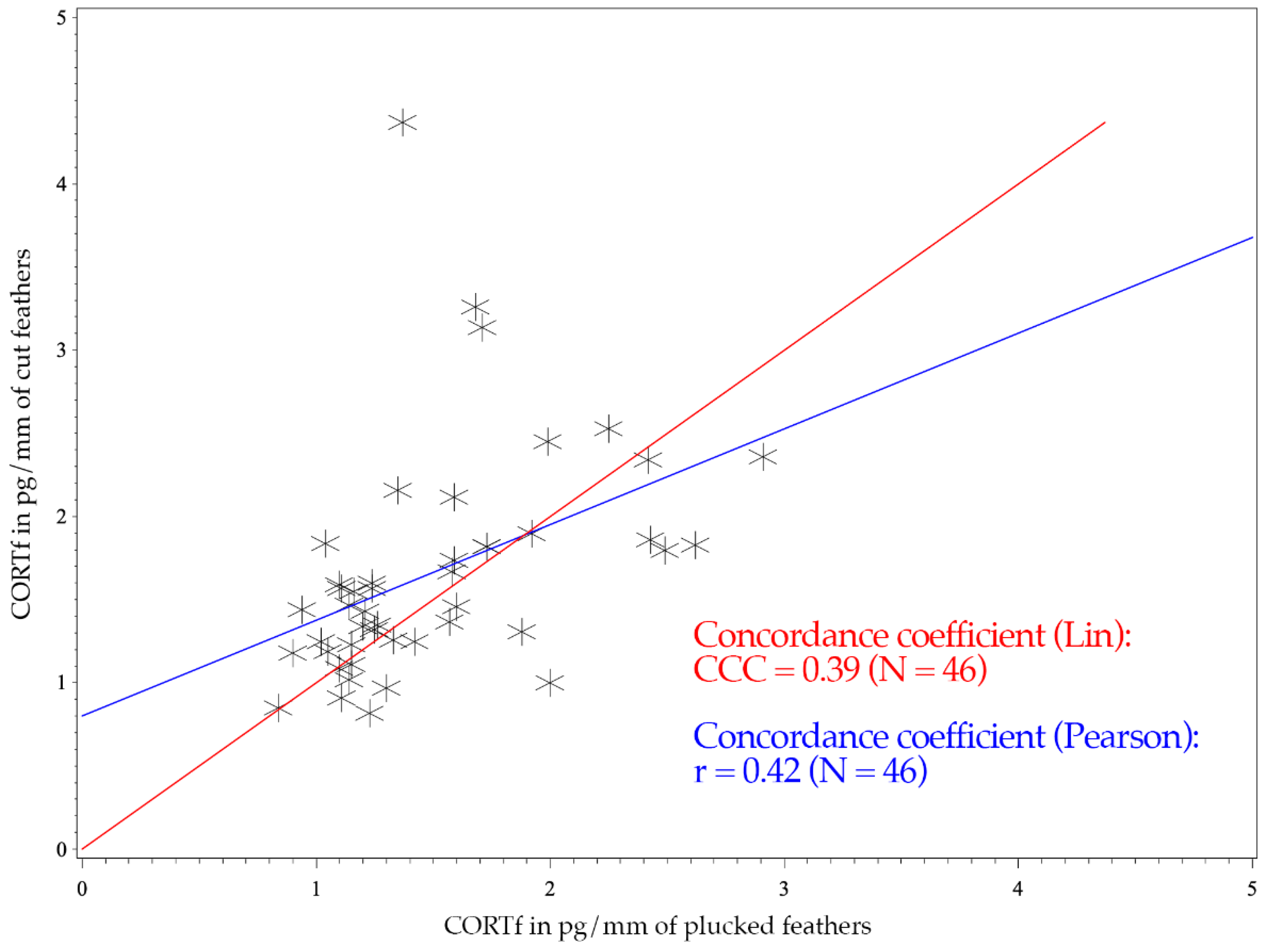
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Dickens, M.J.; Romero, L.M. A consensus endocrine profile for chronically stressed wild animals does not exist. Gen. Comp. Endocrinol. 2013, 191, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Cockrem, J.F. Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Romero, L.M.; Fairhurst, G.D. Measuring corticosterone in feathers: Strengths, limitations, and suggestions for the future. Comp. Biochem. Phys. A 2016, 202, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed] [Green Version]
- Vera, F.; Zenuto, R.; Antenucci, C.D. Expanding the actions of cortisol and corticosterone in wild vertebrates: A necessary step to overcome the emerging challenges. Gen. Comp. Endocrinol. 2017, 246, 337–353. [Google Scholar] [CrossRef]
- Hau, M.; Ricklefs, R.E.; Wikelski, M.; Lee, K.A.; Brawn, J.D. Corticosterone, testosterone and life-history strategies of birds. Proc. Royal Soc. B 2010, 277, 3203–3212. [Google Scholar] [CrossRef]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Sterling, P. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition and Health; John Wiley & Sons: Oxford, UK, 1988; pp. 629–649. [Google Scholar]
- Romero, L.M.; Dickens, M.J.; Cyr, N.E. The reactive scope model—A new model integrating homeostasis, allostasis, and stress. Horm. Behav. 2009, 55, 375–389. [Google Scholar] [CrossRef]
- Kennedy, E.A.; Lattin, C.R.; Romero, L.M.; Dearborn, D.C. Feather coloration in museum specimens is related to feather corticosterone. Behav. Ecol. Sociobiol. 2013, 67, 341–348. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Fairhurst, G.D.; Marchant, T.A.; Soos, C.; Machin, K.L.; Clark, R.G. Experimental relationships between levels of corticosterone in plasma and feathers in a free-living bird. J. Exp. Biol. 2013, 216, 4071–4081. [Google Scholar] [CrossRef] [Green Version]
- Lattin, C.R.; Reed, J.M.; DesRochers, D.W.; Romero, L.M. Elevated corticosterone in feathers correlates with corticosterone-induced decreased feather quality: A validation study. J. Avian Biol. 2011, 42, 247–252. [Google Scholar] [CrossRef]
- Jenni-Eiermann, S.; Helfenstein, F.; Vallat, A.; Glauser, G.; Jenni, L. Corticosterone: Effects on feather quality and deposition into feathers. Methods Ecol. Evol. 2015, 6, 237–246. [Google Scholar] [CrossRef]
- Fairhurst, G.D.; Frey, M.D.; Reichert, J.F.; Szelest, I.; Kelly, D.M.; Bortolotti, G.R. Does environmental enrichment reduce stress? An integrated measure of corticosterone from feathers provides a novel perspective. PLoS ONE 2011, 6, e17663. [Google Scholar] [CrossRef]
- Monclús, L.; Carbajal, A.; Tallo-Parra, O.; Sabés-Alsina, M.; Darwich, L.; Molina-López, R.; Lopez-Bejar, M. Relationship between feather corticosterone and subsequent health status and survival in wild Eurasian Sparrowhawk. J. Ornithol. 2017, 158, 773–783. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.; Blas, J.; Cabezas, S. Tracking stress: Localisation, deposition and stability of corticosterone in feathers. J. Exp. Biol. 2009, 212, 1477–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolotti, G.R. Flaws and pitfalls in the chemical analysis of feathers: Bad news–good news for avian chemoecology and toxicology. Ecol. Appl. 2010, 20, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Monclús, L.; Ballesteros-Cano, R.; De La Puente, J.; Lacorte, S.; Lopez-Bejar, M. Influence of persistent organic pollutants on the endocrine stress response in free-living and captive red kites (Milvus milvus). Environ. Pollut. 2018, 242, 329–337. [Google Scholar] [CrossRef]
- Voit, M.; Merle, R.; Baumgartner, K.; von Fersen, L.; Reese, L.; Ladwig-Wiegard, M.; Will, H.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M. Validation of an Alternative Feather Sampling Method to Measure Corticosterone. Animals 2020, 10, 2054. [Google Scholar] [CrossRef]
- Malik, A.; Valentine, A. Pain in birds: A review for veterinary nurses. Vet. Nurs. J. 2018, 33, 11–25. [Google Scholar] [CrossRef]
- Gentle, M.J.; Waddington, D.; Hunter, L.N.; Jones, R.B. Behavioural evidence for persistent pain following partial beak amputation in chickens. Appl. Anim. Behav. Sci. 1990, 27, 149–157. [Google Scholar] [CrossRef]
- Gentle, M.; Hunter, L. Physiological and behavioural responses associated with feather removal in Gallus gallus var domesticus. Res. Vet. Sci. 1991, 50, 95–101. [Google Scholar] [CrossRef]
- Gentle, M.J. Pain in birds. Anim. Welf. 1992, 1, 235–247. [Google Scholar]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 16 December 2020).
- Russell, W.M.S.; Burch, R.L.; Hume, C.W. The Principles of Humane Experimental Technique; Methuen Publishing: London, UK, 1959; Volume 238. [Google Scholar]
- Reese, L.; Baumgartner, K.; Fersen, L.v.; Merle, R.; Ladwig-Wiegard, M.; Will, H.; Haase, G.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M. Feather Corticosterone Measurements of Greater Flamingos Living under Different Forms of Flight Restraint. Animals 2020, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Carbajal, A.; Tallo-Parra, O.; Sabes-Alsina, M.; Mular, I.; Lopez-Bejar, M. Feather corticosterone evaluated by ELISA in broilers: A potential tool to evaluate broiler welfare. Poultr. Sci. 2014, 93, 2884–2886. [Google Scholar] [CrossRef]
- Buchanan, K.L.; Goldsmith, A.R. Noninvasive endocrine data for behavioural studies: The importance of validation. Anim. Behav. 2004, 67, 183–185. [Google Scholar] [CrossRef]
- Rendón-Martos, M.; Garrido, A.; Rendón, M.Á.; Ramírez, J.M. Greater Flamingo Phoenicopterus roseus monitoring and conservation at Fuente de Piedra Lake. Flamingo 2009, 1, 1. [Google Scholar]
- Fundación Unicaja. 2019. Available online: https://www.fundacionunicaja.com/agenda/anillamiento-de-flamencos-en-la-laguna-de-fuente-de-piedra/ (accessed on 16 December 2020).
- Junta de Andalucía. Available online: http://www.juntadeandalucia.es/medioambiente/site/portalweb/menuitem.7e1cf46ddf59bb227a9ebe205510e1ca/?vgnextoid=f9dbb4ca765ba110VgnVCM1000000624e50aRCRD&vgnextchannel=c9984df288927310VgnVCM2000000624e50aRCRD (accessed on 16 December 2020).
- TVT. TVT-Stellungn._Flugunfähigmachen_von_Vögeln__Mai_2015. 2015. Available online: https://www.tierschutz-tvt.de/alle-merkblaetter-und-stellungnahmen/?no_cache=1&download=TVT-Stellungn._Flugunf%C3%A4higmachen_von_V%C3%B6geln__Mai_2015_.pdf&did=175 (accessed on 16 December 2020).
- Tallo-Parra, O.; Manteca, X.; Sabes-Alsina, M.; Carbajal, A.; Lopez-Bejar, M. Hair cortisol detection in dairy cattle by using EIA: Protocol validation and correlation with faecal cortisol metabolites. Animal 2015, 9, 1059–1064. [Google Scholar] [CrossRef] [Green Version]
- Carlitz, E.H.; Kirschbaum, C.; Miller, R.; Rukundo, J.; van Schaik, C.P. Effects of body region and time on hair cortisol concentrations in chimpanzees (Pan troglodytes). Gen. Comp. Endocrinol. 2015, 223, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Terwissen, C.; Mastromonaco, G.; Murray, D. Influence of adrenocorticotrophin hormone challenge and external factors (age, sex, and body region) on hair cortisol concentration in Canada lynx (Lynx canadensis). Gen. Comp. Endocrinol. 2013, 194, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Fairhurst, G.D.; Dawson, R.D.; van Oort, H.; Bortolotti, G.R. Synchronizing feather-based measures of corticosterone and carotenoid-dependent signals: What relationships do we expect? Oecologia 2014, 174, 689–698. [Google Scholar] [CrossRef]
- Grindstaff, J.L.; Lovern, M.B.; Burtka, J.L.; Hallmark-Sharber, A. Structural coloration signals condition, parental investment, and circulating hormone levels in Eastern bluebirds (Sialia sialis). J. Comp. Physiol. A 2012, 198, 625–637. [Google Scholar] [CrossRef]
- Lendvai, Á.; Giraudeau, M.; Németh, J.; Bakó, V.; McGraw, K. Carotenoid-based plumage coloration reflects feather corticosterone levels in male house finches (Haemorhous mexicanus). Behav. Ecol. Sociobiol. 2013, 67, 1817–1824. [Google Scholar] [CrossRef]
- Angelier, F.; Moe, B.; Blanc, S.; Chastel, O. What factors drive prolactin and corticosterone responses to stress in a long-lived bird species (snow petrel Pagodroma nivea)? Physiol. Biochem. Zool. 2009, 82, 590–602. [Google Scholar] [CrossRef] [Green Version]
- Grzimek, B. Grzimeks Tierleben: Enzyklopädie des Tierreiches; DTV, Kindler Verlag: Zurich, Switzerland, 1980. [Google Scholar]
- Blase, R.; Pettinger, F. Die Jägerprüfung in Frage und Antwort: Das Lehr-, Lern-u. Nachschlagewerk für Jäger; Neumann-Neudamm KG: Melsungen, Germany, 1988. [Google Scholar]
- BirdLife International. Anas platyrhynchos (Amended Version of 2017 Assessment). The IUCN Red List of Threatened Species, 2019. Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T22680186A155457360.en (accessed on 16 December 2020).
- Deutscher Jagdverband e.V. Stockente (Anas platyrhynchos). Available online: https://www.jagdverband.de/zahlen-fakten/tiersteckbriefe/stockente-anas-platyrhynchos (accessed on 16 December 2020).
- Miller, E.R.; Fowler, M.E. Fowler’s Zoo and Wild Animal Medicine; Elsevier Health Sciences: St. Louis, MO, USA, 1986; Volume 2. [Google Scholar]
- BirdLife International. Phoenicopterus roseus. The IUCN Red List of Threatened Species, 2018. Available online: https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697360A131878173.en (accessed on 16 December 2020).
- Natural, P. Odiel Marshes: A new breeding site for Greater Flamingos (Phoenicopterus roseus) in Spain. Flamingo 2008, 16, 23–24. [Google Scholar]
- Andreasson, U.; Perret-Liaudet, A.; van Waalwijk van Doorn, L.J.C.; Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; Genc, S.; Kruse, N.; Kuiperij, H.B.; et al. A Practical Guide to Immunoassay Method Validation. Front. Neurol. 2015, 6, 179. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Ghassabian, S. Linearity of Calibration Curves for Analytical Methods: A Review of Criteria for Assessment of Method Reliability. In Calibration and Validation of Analytical Methods: A Sampling of Current Approaches; IntechOpen Limited: London, UK, 2018; Volume 109–127. [Google Scholar]
- Hanneman, S.K.; Cox, C.D.; Green, K.E.; Kang, D.-H. Estimating Intra- and Inter-Assay Variability in Salivary Cortisol. Biol. Res. Nurs. 2011, 13, 243–250. [Google Scholar] [CrossRef]
- Rohwer, S.; Ricklefs, R.E.; Rohwer, V.G.; Copple, M.M. Allometry of the duration of flight feather molt in birds. PLoS Biol. 2009, 7, e1000132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, I.; Lin, K. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar]
- McBride, G. A Proposal for Strength-of-Agreement Criteria for Lin’s Concordance Correlation Coefficient; NIWA Client Report: HAM2005-062; National Institute of Water & Atmospheric Research Ltd.: Hamilton, New Zealand, 2005. [Google Scholar]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- McKnight, P.E.; Najab, J. Mann-Whitney U Test. In The Corsini Encyclopedia of Psychology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; Volume 1. [Google Scholar]
- Sims, C.G.; Holberton, R.L. Development of the corticosterone stress response in young northern mockingbirds (Mimus polyglottos). Gen. Comp. Endocrinol. 2000, 119, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Dufty, A.M., Jr.; Belthoff, J.R. Corticosterone and the stress response in young western screech-owls: Effects of captivity, gender, and activity period. Physiol. Zool. 1997, 70, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankowich, T.; Blumstein, D.T. Fear in animals: A meta-analysis and review of risk assessment. Proc. Royal Soc. B Biol. Sci. 2005, 272, 2627–2634. [Google Scholar] [CrossRef] [Green Version]
- Webster, M.M.; Rutz, C. How Strange are your Study Animals? Nature 2020, 582, 337–340. [Google Scholar] [CrossRef]





| Sex | Total Length of Feathers (in mm) | Weight of Feathers (in mg) | CORTf (in pg/mm) | CORTf (in pg/mg) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Plucked | Cut | Plucked | Cut | Plucked | Cut | Plucked | Cut | ||
| Female | Mean | 386 | 384 | 81.5 | 84.7 | 1.49 | 1.76 | 7.18 | 8.07 |
| Max | 400 | 400 | 104.3 | 105.2 | 2.62 | 4.37 | 12.12 | 19.21 | |
| Min | 362 | 355 | 57.1 | 57.3 | 0.84 | 0.85 | 3.73 | 3.89 | |
| Male | Mean | 390 | 388 | 84.1 | 89.7 | 1.51 | 1.58 (1.84) | 7.11 | 6.87 (7.96) |
| Max | 412 | 400 | 106.4 | 113.2 | 2.91 | 3.26 (8.10) | 14.31 | 13.35 (34.29) | |
| Min | 375 | 369 | 66.0 | 66.5 | 0.90 | 0.82 | 4.04 | 3.40 | |
| Total mean | 388 | 386 | 82.8 | 87.3 | 1.50 | 1.66 | 7.14 | 7.44 | |
| Sex | Total Length of Feathers (in mm) | Weight of Feathers (in mg) | CORTf (in pg/mm) | CORTf (in pg/mg) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Plucked | Cut | Plucked | Cut | Plucked | Cut | Plucked | Cut | ||
| Female | Mean | 368 | 373 | 63.9 | 70.7 | 2.56 | 2.70 | 14.95 | 14.41 |
| Max | 393 | 395 | 76.8 | 83.4 | 6.29 | 7.22 | 35.28 | 35.61 | |
| Min | 236 | 333 | 39.4 | 56.6 | 1.26 | 1.29 | 6.98 | 5.83 | |
| Male | Mean | 371 | 372 | 77.7 | 83.7 | 2.44 | 2.54 | 11.65 | 11.25 |
| Max | 393 | 396 | 89.5 | 95.6 | 4.11 | 4.21 | 18.46 | 17.71 | |
| Min | 330 | 341 | 63.2 | 74.0 | 1.68 | 1.45 | 8.18 | 6.73 | |
| Total mean | 370 | 372 | 69.9 | 76.4 | 2.51 | 2.63 | 13.52 | 13.04 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voit, M.; Baumgartner, K.; von Fersen, L.; Merle, R.; Reese, L.; Wiegard, M.; Will, H.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M.; et al. Comparison of Two Different Feather Sampling Methods to Measure Corticosterone in Wild Greater Flamingos (Phoenicopterus roseus) and Wild Mallards (Anas platyrhynchos). Animals 2021, 11, 2796. https://doi.org/10.3390/ani11102796
Voit M, Baumgartner K, von Fersen L, Merle R, Reese L, Wiegard M, Will H, Tallo-Parra O, Carbajal A, Lopez-Bejar M, et al. Comparison of Two Different Feather Sampling Methods to Measure Corticosterone in Wild Greater Flamingos (Phoenicopterus roseus) and Wild Mallards (Anas platyrhynchos). Animals. 2021; 11(10):2796. https://doi.org/10.3390/ani11102796
Chicago/Turabian StyleVoit, Marielu, Katrin Baumgartner, Lorenzo von Fersen, Roswitha Merle, Lukas Reese, Mechthild Wiegard, Hermann Will, Oriol Tallo-Parra, Annaïs Carbajal, Manel Lopez-Bejar, and et al. 2021. "Comparison of Two Different Feather Sampling Methods to Measure Corticosterone in Wild Greater Flamingos (Phoenicopterus roseus) and Wild Mallards (Anas platyrhynchos)" Animals 11, no. 10: 2796. https://doi.org/10.3390/ani11102796







