The Effect of Inhibin Immunization in Seminiferous Epithelium of Yangzhou Goose Ganders: A Histological Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Birds and Management
2.3. Immunogen Preparation
2.4. Experimental Design
2.5. Tissue Collection
2.6. Microscopy
- 10 = complete spermatogenesis;
- 9 = spermatozoa present with random appeared spermatogenesis;
- 8 = few spermatozoa;
- 7 = no spermatozoa, but spermatids present;
- 6 = few spermatids present;
- 5 = only spermatocytes present;
- 4 = few spermatocytes present;
- 3 = spermatogonia present;
- 2 = only Sertoli cells present; and
- 1 = almost empty lumen.
2.7. Statistical Analysis
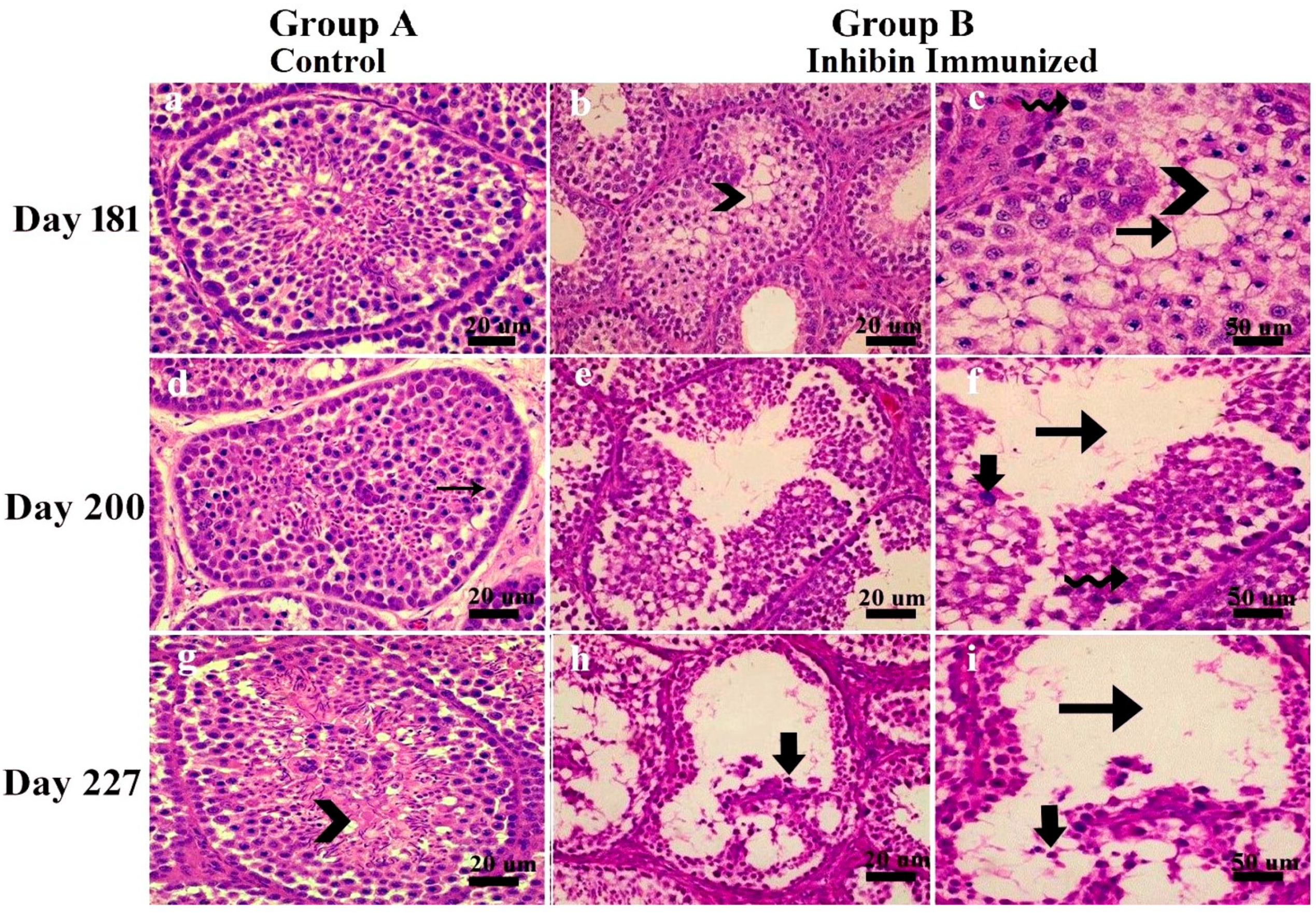
3. Results
3.1. Classification Criteria of Seminiferous Epithelium Apoptosis
- Seminiferous epithelium lumen seemed quite empty, showing degeneration of spermatogonia, spermatocytes, and apoptotic bodies.
- Seminiferous tubules basal membrane was observed empty. Pyknotic germ and Sertoli cells were also observed.
- Sertoli cells vacuolated, and most tubules had empty lumen depicting impaired spermatogenesis.
- Seminiferous tubules had irregularly shaped and degenerated germ cells.
3.2. Diameter of ST, LD, EH, Number of ST/Field, and Germ Cells
3.3. Johnson’s Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Z.; Sun, A.; Shao, X.; Chen, Z.; Zhu, H. Boosting the economic returns of goose breeding and developing the industry by controlled photoperiod for out-of-season reproduction. Anim. Environ. Welf. 2015, 3, 357. [Google Scholar]
- Rehman, A.; Ahmad, E.; Arshad, U.; Riaz, A.; Akhtar, M.S.; Ahmad, T.; Khan, J.A.; Mohsin, I.; Shi, Z.; Sattar, A. Effects of immunization against inhibin α-subunit on ovarian structures, pregnancy rate, embryonic and fetal losses, and prolificacy rate in goats where estrus was induced during the non-breeding season. Anim. Reprod. Sci. 2021, 224, 106654. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-Y. Inhibins, activins, and follistatins: Gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr. Rev. 1988, 9, 267–293. [Google Scholar] [CrossRef] [PubMed]
- Knight, P. Roles of inhibins, activins, and follistatin in the female reproductive system. Front. Neuroen-Docrinol. 1996, 17, 476–509. [Google Scholar] [CrossRef]
- Cai, K.; Hua, G.; Ahmad, S.; Liang, A.; Han, L.; Wu, C.; Yang, F.; Yang, L. Action mechanism of inhibin α-subunit on the development of Sertoli cells and first wave of spermatogenesis in mice. PLoS ONE 2011, 6, e25585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, F.D.; Hao, S.L.; Yang, W.X. Multiple signaling pathways in Sertoli cells: Recent findings in spermatogenesis. Cell Death Dis. 2019, 10, 541. [Google Scholar] [CrossRef] [Green Version]
- Moreau, J.D.; Satterlee, D.G.; Rejman, J.J.; Cadd, G.G.; Kousoulas, K.G.; Fioretti, W.C. Active immunization of Japanese quail hens with a recombinant chicken inhibin fusion protein enhances production performance. Poult. Sci. 1998, 77, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Avital-Cohen, N.; Heiblum, R.; Argov, N.; Rosenstrauch, A.; Chaiseha, Y.; Mobarkey, N.; Rozenboim, I. The effect of active immunization against vasoactive intestinal peptide (VIP) and inhibin on reproductive performance of aging White Leghorn roosters. Poult. Sci. 2012, 91, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Avital-Cohen, N.; Heiblum, R.; Argov, N.; Rosenstrauch, A.; Chaiseha, Y.; Mobarkey, N.; Rozenboim, I. The effect of active immunization against vasoactive intestinal peptide and inhibin on reproductive performance of young White Leghorn roosters. Poult. Sci. 2011, 90, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.F.; Wei, Q.; Zhu, H.; Chen, Z.; Ahmad, E.; Zhendan, S.; Shi, F. The role of active immunization against inhibin α-subunit on testicular development, testosterone concentration and relevant genes expressions in testis, hypothalamus and pituitary glands in Yangzhou goose ganders. Theriogenology 2019, 128, 122–132. [Google Scholar] [CrossRef]
- Schanbacher, B. Pituitary and testicular responses of beef bulls to active immunization against inhibin alpha. J. Anim. Sci. 1991, 69, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Ahmad, E.; Sattar, A.; Riaz, A.; Khan, J.A.; Naseer, Z.; Akhtar, M.F.; Abbas, M.; Shi, Z. Long term effects of immunization against inhibin on fresh and post-thawed semen quality and sperm kinematics during low and peak breeding seasons in Beetal bucks. Small Rumin. Res. 2021, 201, 106442. [Google Scholar] [CrossRef]
- Mao, D.; Bai, W.; Hui, F.; Yang, L.; Cao, S.; Xu, Y. Effect of inhibin gene immunization on antibody production and reproductive performance in Partridge Shank hens. Theriogenology 2016, 85, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, Z.; Shao, X.; Yu, J.; Wei, C.; Dai, Z.; Shi, Z. Reproductive axis gene regulation during photostimulation and photorefractoriness in Yangzhou goose ganders. Front. Zool. 2017, 14, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, M.F.; Ahmad, E.; Mustafa, S.; Chen, Z.; Shi, Z.; Shi, F. Spermiogenesis, stages of seminiferous epithelium and variations in seminiferous tubules during active states of spermatogenesis in Yangzhou goose ganders. Animals 2020, 10, 570. [Google Scholar] [CrossRef] [Green Version]
- Lewis-Johnes, D. A modified Johnsen’s count for evaluation of spermatogenesis in the rat. IRCS Med. Sci. 1985, 13, 510–511. [Google Scholar]
- Voge, J.; Wheaton, J. Effects of immunization against two inhibin antigens on daily sperm production and hormone concentrations in ram lambs. J. Anim. Sci. 2007, 85, 3249–3255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchand, C.; Gomot, L.; de Reviers, M. Autoradiography and tritiated thymidine labeling during spermato-genesis of the Barbary duck (Cairina moschata L.). Comptes Rendus Des Seances De La Soc. De Biol. Et De Ses Fil. 1977, 171, 927–931. [Google Scholar]
- Van Dissel-Emiliani, F.M.; Grootenhuis, A.J.; Jong, F.H.D.; Rooij, D.G.D. Inhibin reduces spermatogonial numbers in testes of adult mice and Chinese hamsters. Endocrinology 1989, 125, 1898–1903. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Chaturvedi, C.M. Apoptotic mechanism behind the testicular atrophy in photorefractory and photosensitive quail: Involvement of GnIH induced p-53 dependent Bax-Caspase-3 mediated pathway. J. Photochem. Photobiol. B Biol. 2017, 176, 124–135. [Google Scholar] [CrossRef]
- Islam, M.N.; Tsukahara, N.; Sugita, S. Apoptosis-mediated seasonal testicular regression in the Japanese Jungle crow (Corvus macrorhynchos). Theriogenology 2012, 77, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, L.K.; Ross, W.L.; Young, K.A. Increases in apoptosis and declines in Bcl-XL protein characterize testic-ular regression in American crows (Corvus brachyrhynchos). Reprod. Fertil. Dev. 2007, 19, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Young, K.A.; Ball, G.F.; Nelson, R.J. Photoperiod-induced testicular apoptosis in European starlings (Sturnus vulgaris). Biol. Reprod. 2001, 64, 706–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittami, J.; Goldsmith, A.; Follett, B. Seasonal changes in follicle-stimulating hormone in a breeding population of bar-headed geese, Anser indicus. Gen. Comp. Endocrinol. 1985, 57, 195–197. [Google Scholar] [CrossRef]
- Péczely, P.; Czifra, G.; Sepr, A.; Teplán, I. Effect of low light intensity on testicular function in photorefractory domestic ganders. Gen. Comp. Endocrinol. 1985, 57, 293–300. [Google Scholar] [CrossRef]
- Russell, L.D.; Alger, L.E.; Nequin, L.G. Hormonal control of pubertal spermatogenesis. Endocrinology 1987, 120, 1615–1632. [Google Scholar] [CrossRef]
- Shi, Z.; Tian, Y.; Wu, W.; Wang, Z. Controlling reproductive seasonality in the geese: A review. World’s Poult. Sci. J. 2008, 64, 343–355. [Google Scholar] [CrossRef]
- Meachem, S.; Nieschlag, E.; Simoni, M. Inhibin B in male reproduction: Pathophysiology and clinical relevance. Eur. J. Endocrinol. 2001, 145, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Dai, R.; Lei, L.; Lin, P.; Lu, X.; Wang, X.; Tang, K.; Wang, A.; Jin, Y. Establishment and evaluation of a stable steroidogenic goat Leydig cell line. Anim. Sci. J. 2016, 87, 492–502. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell. Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Møller, A.P. Testes size, ejaculate quality and sperm competition in birds. Biol. J. Linn. Soc. 1988, 33, 273–283. [Google Scholar] [CrossRef]
- Jamieson, B.G. Reproductive Biology and Phylogeny of Birds, Part a: Phylogeny, Morphology, Hormones and Fertilization; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, M.F.; Ahmad, E.; Ali, I.; Shafiq, M.; Chen, Z. The Effect of Inhibin Immunization in Seminiferous Epithelium of Yangzhou Goose Ganders: A Histological Study. Animals 2021, 11, 2801. https://doi.org/10.3390/ani11102801
Akhtar MF, Ahmad E, Ali I, Shafiq M, Chen Z. The Effect of Inhibin Immunization in Seminiferous Epithelium of Yangzhou Goose Ganders: A Histological Study. Animals. 2021; 11(10):2801. https://doi.org/10.3390/ani11102801
Chicago/Turabian StyleAkhtar, Muhammad Faheem, Ejaz Ahmad, Ilyas Ali, Muhammad Shafiq, and Zhe Chen. 2021. "The Effect of Inhibin Immunization in Seminiferous Epithelium of Yangzhou Goose Ganders: A Histological Study" Animals 11, no. 10: 2801. https://doi.org/10.3390/ani11102801








