Cellular Modifications in Spermatogenesis during Seasonal Testicular Regression: An Update Review in Mammals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Proliferation and Apoptosis in Seminiferous Epithelium
2.2. Proliferation and Apoptosis in Germinal Cells during the Regression of Seminiferous Epithelium in Seasonally Breeding Mammals
2.3. Proliferation and Apoptosis of Sertoli Cells in the Regression of the Seminiferous Epithelium of Mammals Related to Seasonal Reproduction
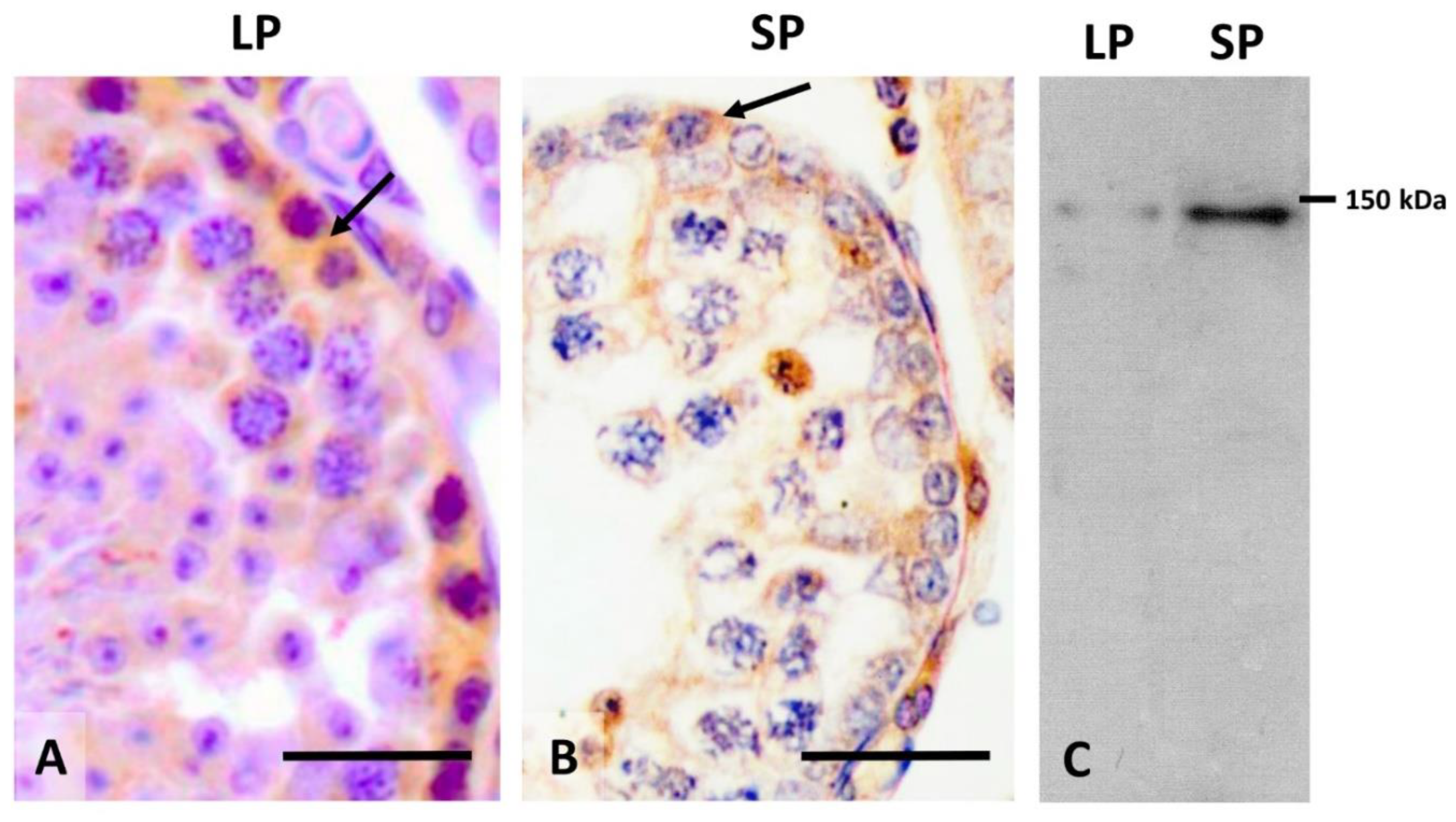
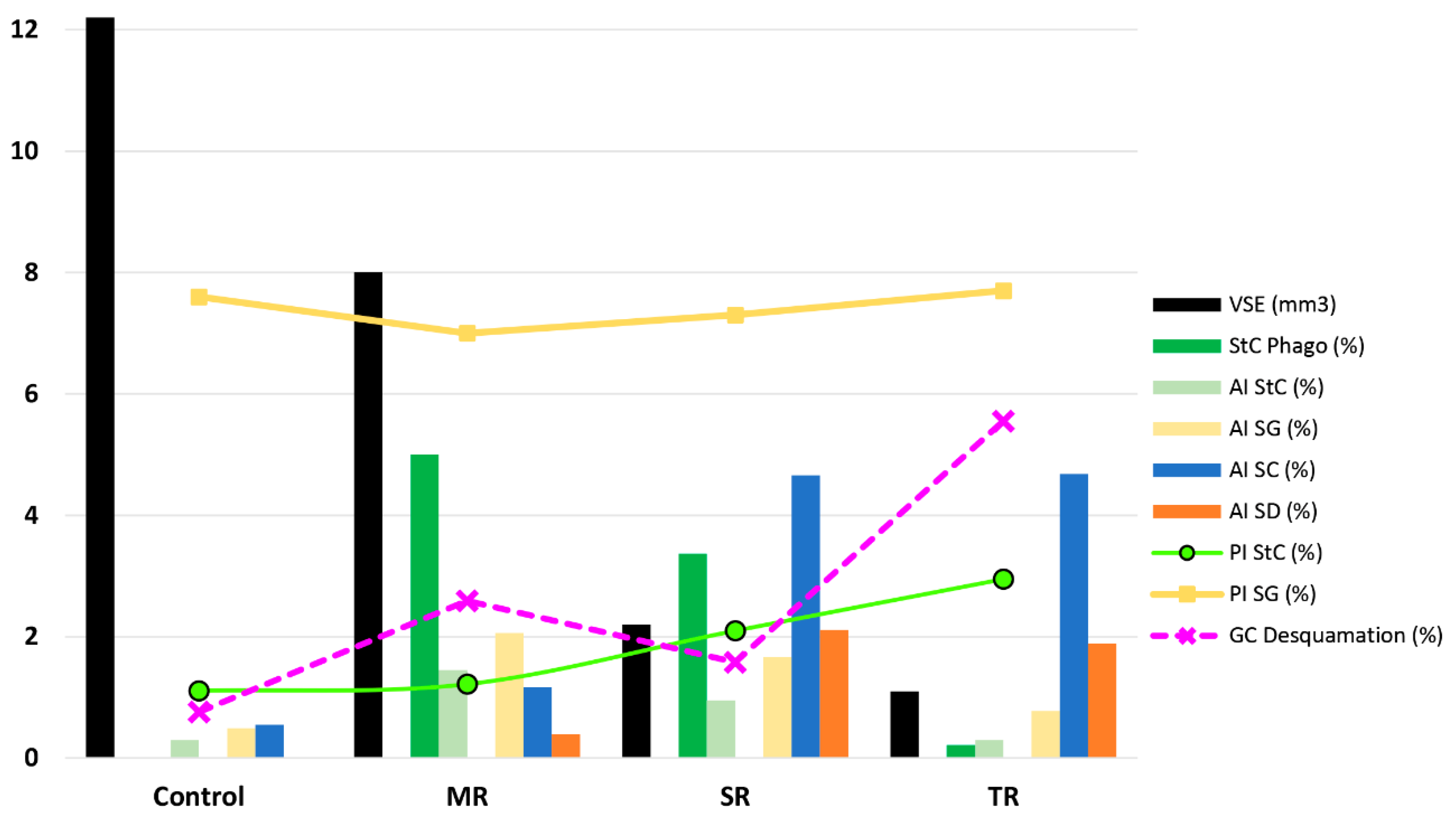
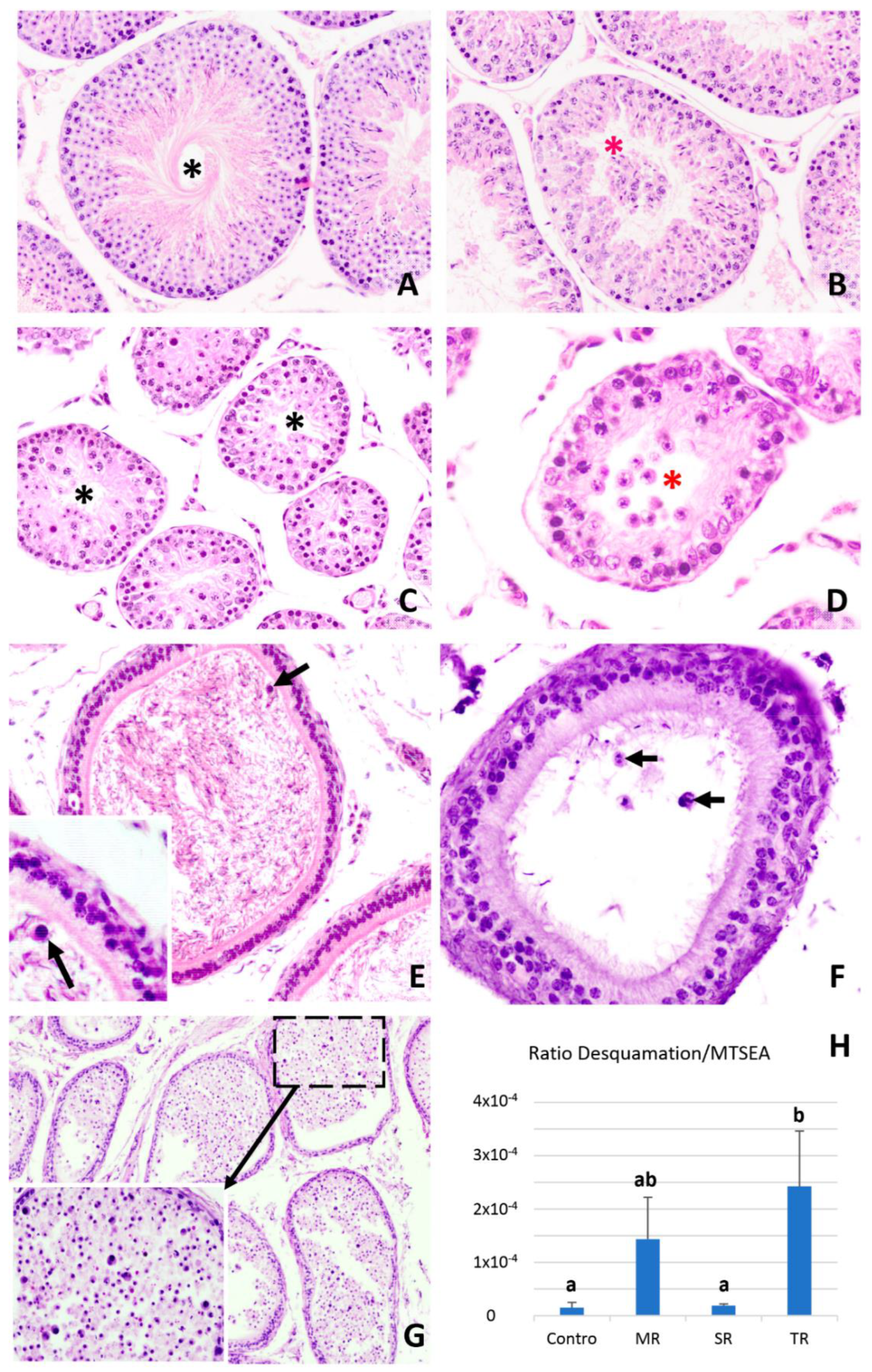
2.4. Cell Desquamation of Germinal Cells in the Regression of the Seminiferous Epithelium of Mammals Related to Seasonal Reproduction
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beltran-Frutos, E.; Casarini, L.; Santi, D.; Brigante, G. Seasonal reproduction and gonadal function: A focus on humans starting from animal studies. Biol. Reprod. 2022, 106, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, T.; Aurich, J.E. Regulation of seasonal reproductive activity in the stallion, ram and hamster. Anim. Reprod. Sci. 2000, 58, 197–213. [Google Scholar] [CrossRef]
- Lincoln, G.A. Seasonal aspects of testicular function. In The Testis; Burger, H., de Kretser, D., Eds.; Raven Press: New York, NY, USA, 1981; pp. 255–302. [Google Scholar]
- Hochereau-de Reviers, M.T.; Perreau, C.; Lincoln, G.A. Photoperiodic variations of somatic and germ cell populations in the Soay ram testis. J. Reprod. Fertil. 1985, 74, 329–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochereau-de Reviers, M.T.; Perreau, C.; Pisselet, C.; Pelletier, J. Effect of a 2-month light cycle regimen on testicular parameters of adult Ile-de-France rams. Microsc. Res. Tech. 1992, 20, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Hochereau-de Reviers, M.T.; Lincoln, G.A. Seasonal variation in the histology of the testis of the red deer, Cervus elaphus. J. Reprod. Fertil. 1978, 54, 209–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berndtson, W.E.; Squires, E.L.; Thompson, D.L. Spermatogenesis, testicular composition and the concentration of testosterone in the equine testis as influenced by season. Theriogenology 1983, 20, 449–457. [Google Scholar] [CrossRef]
- Gottreich, A.; Hammel, I.; Yogev, L.; Terkel, J. Quantitative microscopic changes in the mole rat testes during an annual cycle. Anat. Rec. 1995, 243, 195–199. [Google Scholar] [CrossRef]
- Schön, J.; Göritz, F.; Streich, J.; Blottner, S. Histological organization of roe deer testis throughout the seasonal cycle: Variable and constant components of tubular and interstitial compartment. Anat. Embryol. 2004, 208, 151–159. [Google Scholar] [CrossRef]
- Tsubota, T.; Howell-Skalla, L.; Nitta, H.; Osawa, Y.; Mason, J.I.; Meiers, P.G.; Nelson, R.A.; Bahr, J.M. Seasonal changes in spermatogenesis and testicular steroidogenesis in the male black bear Ursus americanus. J. Reprod. Fertil. 1997, 109, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Tähkä, K.M.; Zhuang, Y.H.; Tähkä, S.; Tuohimaa, P. Photoperiod-induced changes in androgen receptor expression in testes and accessory sex glands of the bank vole, Clethrionomys glareolus. Biol. Reprod. 1997, 56, 898–908. [Google Scholar] [CrossRef] [Green Version]
- Foreman, D. Seminiferous tubule stages in the prairie dog (Cynomys ludovicianus) during the annual breeding cycle. Anat. Rec. 1997, 247, 355–367. [Google Scholar] [CrossRef]
- Barnes, B.M.; Kretzmann, M.; Licht, P.; Zucker, I. The influence of hibernation on testis growth and spermatogenesis in the golden-mantled ground squirrel, Spermophilus lateralis. Biol. Reprod. 1986, 35, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Simeunovic, B.; Strbenc, M.; Bavdek, S.V. Position and histological structure of the testes in the brown hare (Lepus europaeus) during seasonal regression and recrudescence. Anat. Histol. Embryol. 2000, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Saez, F.J.; Madrid, J.F.; Canteras, M.; Pastor, L.M. Testicular histomorphometry and the proliferative and apoptotic activities of the seminiferous epithelium in Syrian hamster (Mesocricetus auratus) during regression owing to short photoperiod. Andrology 2015, 3, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Luaces, J.P.; Rossi, L.F.; Merico, V.; Zuccotti, M.; Redi, C.A.; Solari, A.J.; Merani, M.S.; Garagna, S. Spermatogenesis is seasonal in the large hairy armadillo, Chaetophractus villosus (Dasypodidae, Xenarthra, Mammalia). Reprod. Fertil. Dev. 2013, 25, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.M. Cyclic formation and decay of the blood-testis barrier in the mink (Mustela vison), a seasonal breeder. Am. J. Anat. 1986, 175, 91–117. [Google Scholar] [CrossRef]
- Blottner, S.; Schön, J.; Jewgenow, K. Seasonally activated spermatogenesis is correlated with increased testicular production of testosterone and epidermal growth factor in mink (Mustela vison). Theriogenology 2006, 66, 1593–1598. [Google Scholar] [CrossRef]
- Araújo, R.A.; Amaro, B.D.; Talamoni, S.A.; Godinho, H.P. Seasonal reproduction of yellowish myotis, Myotis levis (Chiroptera: Vespertilionidae), from a Neotropical highland. J. Morphol. 2013, 274, 1230–1238. [Google Scholar] [CrossRef]
- Madekurozwa, M.C.; Chabvepi, T.S.; Matema, S.; Teerds, K.J. Relationship between seasonal changes in spermatogenesis in the juvenile ostrich (Stuthio camelus) and the presence of the LH receptor and 3beta-hydroxysteroid dehydrogenase. Reproduction 2002, 123, 735–742. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhu, X.B.; Zhu, Z.B.; Aoyama, M.; Sugita, S. Histological and morphometric analyses of seasonal testicular variations in the Jungle Crow (Corvus macrorhynchos). Anat. Sci. Int. 2010, 85, 121–129. [Google Scholar] [CrossRef]
- Young, K.A.; Nelson, R.J. Mediation of seasonal testicular regression by apoptosis. Reproduction 2001, 122, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rodríguez, J.; Martínez-García, C. Spontaneous germ cell death in the testis of the adult rat takes the form of apoptosis: Re-evaluation of cell types that exhibit the ability to die during spermatogenesis. Cell Prolif. 1996, 29, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Loveland, K.L.; Schlatt, S. Stem cell factor and c-kit in the mammalian testis: Lessons originating from Mother Nature’s gene knockouts. J. Endocrinol. 1997, 153, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell. Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.Y.; Mruk, D.D. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 2002, 82, 825–874. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, L.; Nicholls, P.K.; O’Bryan, M.K.; McLachlan, R.I.; Stanton, P.G. Spermiation: The process of sperm release. Spermatogenesis 2011, 1, 14–35. [Google Scholar] [CrossRef]
- Sinha Hikim, A.P.; Swerdloff, R.S. Hormonal and genetic control of germ cell apoptosis in the testis. Rev. Reprod. 1999, 4, 38–47. [Google Scholar] [CrossRef]
- Hikim, A.P.; Wang, C.; Leung, A.; Swerdloff, R.S. Involvement of apoptosis in the induction of germ cell degeneration in adult rats after gonadotropin-releasing hormone antagonist treatment. Endocrinology 1995, 136, 2770–2775. [Google Scholar] [CrossRef]
- Sinha Hikim, A.P.; Rajavashisth, T.B.; Sinha Hikim, I.; Lue, Y.; Bonavera, J.J.; Leung, A.; Wang, C.; Swerdloff, R.S. Significance of apoptosis in the temporal and stage-specific loss of germ cells in the adult rat after gonadotropin deprivation. Biol. Reprod. 1997, 57, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Lue, Y.; Hikim, A.P.; Wang, C.; Bonavera, J.J.; Baravarian, S.; Leung, A.; Swerdloff, R.S. Early effects of vasectomy on testicular structure and on germ cell and macrophage apoptosis in the hamster. J. Androl. 1997, 18, 166–173. [Google Scholar] [PubMed]
- Hikim, A.P.; Wang, C.; Lue, Y.; Johnson, L.; Wang, X.H.; Swerdloff, R.S. Spontaneous germ cell apoptosis in humans: Evidence for ethnic differences in the susceptibility of germ cells to programmed cell death. J. Clin. Endocrinol. Metab. 1998, 83, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; McKinnell, C.; Kivlin, C.; Fisher, J.S. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhu, W.J.; Hao, S.F.; Liang, W.B.; Li, J. Stereological analysis of age-related changes of testicular peritubular cells in men. Arch. Gerontol. Geriatr. 2012, 55, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.S.; Dym, M. Ultrastructural differentiation of rat Sertoli cells. Biol. Reprod. 1979, 21, 909–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berensztein, E.B.; Sciara, M.I.; Rivarola, M.A.; Belgorosky, A. Apoptosis and proliferation of human testicular somatic and germ cells during prepuberty: High rate of testicular growth in newborns mediated by decreased apoptosis. J. Clin. Endocrinol. Metab. 2002, 87, 5113–5118. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Kageyama, Y.; Ishizaka, K.; Kihara, K.; Oshima, H. Involvement of apoptosis in the control of Sertoli and pre-meiotic germ cell numbers in the developing rabbit testis. Andrologia 2002, 34, 34–40. [Google Scholar] [CrossRef]
- Pinart, E.; Sancho, S.; Briz, M.D.; Bonet, S.; Garcia, N.; Badia, E. Ultrastructural study of the boar seminiferous epithelium: Changes in cryptorchidism. J. Morphol. 2000, 244, 190–202. [Google Scholar] [CrossRef]
- Nistal, M.; González-Peramato, P.; Paniagua, R. Lipomembranous fat necrosis in three cases of testicular torsion. Histopathology 2001, 38, 443–447. [Google Scholar] [CrossRef]
- Martínez-Hernández, J.; Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Serrano-Sánchez, M.I.; Pastor, L.M. Proliferation, apoptosis, and number of Sertoli cells in the Syrian hamster during recrudescence after exposure to short photoperiod. Biol. Reprod. 2020, 102, 588–597. [Google Scholar] [CrossRef]
- Blottner, S.; Schön, J.; Roelants, H. Apoptosis is not the cause of seasonal testicular involution in roe deer. Cell. Tissue Res. 2007, 327, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Tiba, T.; Takahashi, M.; Igura, M.; Kita, I. Enhanced proliferation of undifferentiated spermatogonia after treatment of short photoperiod exposure in the Syrian hamster, Mesocricetus auratus. Anat. Histol. Embryol. 1992, 21, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Pastor, L.M.; Martinez-Hernandez, J.; Seco-Rovira, V.; Ferrer, C.; Beltran-Frutos, E. C-kit expression in testicular cells of Syrian hamster during spontaneous recrudescence after exposure to short photoperiod. Reprod. Domest. Anim. 2017, 52, 93. [Google Scholar]
- Roelants, H.; Schneider, F.; Göritz, F.; Streich, J.; Blottner, S. Seasonal changes of spermatogonial proliferation in roe deer, demonstrated by flow cytometric analysis of c-kit receptor, in relation to follicle-stimulating hormone, luteinizing hormone, and testosterone. Biol. Reprod. 2002, 66, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raucci, F.; Di Fiore, M.M. The c-kit receptor protein in the testis of green frog Rana esculenta: Seasonal changes in relationship to testosterone titres and spermatogonial proliferation. Reproduction 2007, 133, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanehzad, M.; Abbaszadeh, R.; Holakuyee, M.; Modarressi, M.H.; Nourashrafeddin, S.M. FSH regulates RA signaling to commit spermatogonia into differentiation pathway and meiosis. Reprod. Biol. Endocrinol. 2021, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Furuta, I.; Porkka-Heiskanen, T.; Scarbrough, K.; Tapanainen, J.; Turek, F.W.; Hsueh, A.J. Photoperiod regulates testis cell apoptosis in Djungarian hamsters. Biol. Reprod. 1994, 51, 1315–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, K.A.; Zirkin, B.R.; Nelson, R.J. Short photoperiods evoke testicular apoptosis in white-footed mice (Peromyscus leucopus). Endocrinology 1999, 140, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Zirkin, B.R.; Nelson, R.J. Testicular regression in response to food restriction and short photoperiod in white-footed mice (Peromyscus leucopus) is mediated by apoptosis. Biol. Reprod. 2000, 62, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Young, K.A.; Zirkin, B.R.; Nelson, R.J. Testicular apoptosis is down-regulated during spontaneous recrudescence in white-footed mice (Peromyscus leucopus). J. Biol. Rhythms 2001, 16, 479–488. [Google Scholar] [CrossRef]
- Strbenc, M.; Fazarinc, G.; Bavdek, S.V.; Pogacnik, A. Apoptosis and proliferation during seasonal testis regression in the brown hare (Lepus europaeus L.). Anat. Histol. Embryol. 2003, 32, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Tachiwana, T.; Takata, K.; Tay, T.W.; Ishii, M.; Nakamura, R.; Kimura, S.; Kanai, Y.; Kurohmaru, M.; Hayashi, Y. Testicular dynamics in Syrian hamsters exposed to both short photoperiod and low ambient temperature. Anat. Histol. Embryol. 2005, 34, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Meguro, K.; Komatsu, K.; Ohdaira, T.; Shoji, R.; Yamada, T.; Sugimura, S.; Fujishima, Y.; Nakata, A.; Fukumoto, M.; et al. Seasonal changes in the spermatogenesis of the large Japanese field mice (Apodemus speciosus) controlled by proliferation and apoptosis of germ cells. Anim. Reprod. Sci. 2020, 214, 106288. [Google Scholar] [CrossRef]
- Massoud, D.; Lao-Pérez, M.; Ortega, E.; Burgos, M.; Jiménez, R.; Barrionuevo, F.J. Divergent Seasonal Reproductive Patterns in Syntopic Populations of Two Murine Species in Southern Spain. Animals 2021, 11, 243. [Google Scholar] [CrossRef]
- Bonda-Ostaszewska, E.; Włostowski, T. Apoptosis, proliferation, and cell size in seasonal changes of body and organ weight in male bank voles Myodes glareolus. Mamm. Res. 2015, 60, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Profaska-Szymik, M.; Galuszka, A.; Korzekwa, A.J.; Hejmej, A.; Gorowska-Wojtowicz, E.; Pawlicki, P.; Kotula-Balak, M.; Tarasiuk, K.; Tuz, R. Implication of Membrane Androgen Receptor (ZIP9) in Cell Senescence in Regressed Testes of the Bank Vole. Int. J. Mol. Sci. 2020, 21, 6888. [Google Scholar] [CrossRef]
- Lao-Pérez, M.; Massoud, D.; Real, F.M.; Hurtado, A.; Ortega, E.; Burgos, M.; Jiménez, R.; Barrionuevo, F.J. Mediterranean Pine Vole. Animals 2021, 11, 1639. [Google Scholar] [CrossRef]
- Young, K.A.; Ball, G.F.; Nelson, R.J. Photoperiod-induced testicular apoptosis in European starlings (Sturnus vulgaris). Biol. Reprod. 2001, 64, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, L.K.; Ross, W.L.; Young, K.A. Increases in apoptosis and declines in Bcl-XL protein characterise testicular regression in American crows (Corvus brachyrhynchos). Reprod. Fertil. Dev. 2007, 19, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Morales, E.; Pastor, L.M.; Ferrer, C.; Zuasti, A.; Pallarés, J.; Horn, R.; Calvo, A.; Santamaría, L.; Canteras, M. Proliferation and apoptosis in the seminiferous epithelium of photoinhibited Syrian hamsters (Mesocricetus auratus). Int. J. Androl. 2002, 25, 281–287. [Google Scholar] [CrossRef]
- Pastor, L.M.; Zuasti, A.; Ferrer, C.; Bernal-Mañas, C.M.; Morales, E.; Beltrán-Frutos, E.; Seco-Rovira, V. Proliferation and apoptosis in aged and photoregressed mammalian seminiferous epithelium, with particular attention to rodents and humans. Reprod. Domest. Anim. 2011, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Sáez, F.J.; Madrid, J.F.; Pastor, L.M. The death of sertoli cells and the capacity to phagocytize elongated spermatids during testicular regression due to short photoperiod in Syrian hamster (Mesocricetus auratus). Biol. Reprod. 2014, 90, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán-Frutos, E.; Seco-Rovira, V.; Ferrer, C.; Madrid, J.F.; Sáez, F.J.; Canteras, M.; Pastor, L.M. Cellular changes in the hamster testicular interstitium with ageing and after exposure to short photoperiod. Reprod. Fertil. Dev. 2016, 28, 838–851. [Google Scholar] [CrossRef]
- Beltrán-Frutos, E.; Seco-Rovira, V.; Martínez-Hernández, J.; Ferrer, C.; Pastor, L.M. Loss of hamster Leydig cells during regression after exposure to a short photoperiod. Reprod. Fertil. Dev. 2018, 30, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Tae, H.J.; Jang, B.G.; Ahn, D.C.; Choi, E.Y.; Kang, H.S.; Kim, N.S.; Lee, J.H.; Park, S.Y.; Yang, H.H.; Kim, I.S. Morphometric studies on the testis of Korean ring-necked pheasant (Phasianus colchicus karpowi) during the breeding and non-breeding seasons. Vet. Res. Commun. 2005, 29, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Seco-Rovira, V.; Peral-Aguilar, S.; Beltrán-Frutos, E.; Martínez-Hernández, J.; Ferrer, C.; Pastor, L.M. Desquamation of germ cells in the seminiferous epithelium during testicular regression due to exposure to short photoperiod in the Syrian hamster (Mesocricetus auratus). In Proceedings of the 20th Annual Conference of the European Society for Domestic Animal Reproduction (ESDAR) and the 13th Conference of the Spanish Association for Animal Reproduction (AERA), Lisbon, Portugal, 27–29 October 2017; Wiley Blackwell: Hoboken, NJ, USA, 2017; Volume 51, p. 226. [Google Scholar]
- Beltran-Frutos, E.; Martinez-Hernandez, J.; Seco-Rovira, V.; Serrano-Sanchez, M.; Salmerón, D.; Ferrer, C.; Pastor, L.M. Population changes in myoid cells during spontaneous recrudescence after exposure to short photoperiod in Syrian hámster in Histol Histopathol. In Proceedings of the XX Congreso de la SEHIT. VI Congreso Iberoamericano de Histología VIII International Congress of Histology and Tissue Engineering, Murcia, Spain, 4–6 September 2019; Volume 34, p. 140. [Google Scholar]
- Martínez-Hernández, J.; Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Canteras, M.; Sánchez-Huertas, M.D.M.; Pastor, L.M. Testicular histomorphometry and the proliferative and apoptotic activities of the seminiferous epithelium in Syrian hamster during spontaneous recrudescence after exposure to short photoperiod. Reprod. Domest. Anim. 2018, 53, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhan, J.; Xu, Y.; Zhang, F.; Yuan, Z.; Weng, Q. Proliferation and apoptosis processes in the seasonal testicular development of the wild Daurian ground squirrel (Citellus dauricus Brandt, 1844). Reprod. Fertil. Dev. 2017, 29, 1680–1688. [Google Scholar] [CrossRef]
- Banerjee, S.; Tsutsui, K.; Chaturvedi, C.M. Apoptosis-mediated testicular alteration in Japanese quail (Coturnix coturnix japonica) in response to temporal phase relation of serotonergic and dopaminergic oscillations. J. Exp. Biol. 2016, 219, 1476–1487. [Google Scholar] [CrossRef] [Green Version]
- Morales, E.; Ferrer, C.; Zuasti, A.; Garcia-Borron, J.C.; Canteras, M.; Pastor, L.M. Apoptosis and molecular pathways in the seminiferous epithelium of aged and photoinhibited Syrian hamsters (Mesocricetus auratus). J. Androl. 2007, 28, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Huang, Y.; Liu, T.; Haseeb, A.; Ahmed, N.; Zhang, L.; Bian, X.; Chen, Q. Characteristics of seasonal spermatogenesis in the soft-shelled turtle. Anim. Reprod. Sci. 2020, 214, 106307. [Google Scholar] [CrossRef]
- Falvo, S.; Rosati, L.; Di Fiore, M.M.; Di Giacomo Russo, F.; Chieffi Baccari, G.; Santillo, A. Proliferative and Apoptotic Pathways in the Testis of Quail. Animals 2021, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Sasso-Cerri, E.; Cerri, P.S.; Freymüller, E.; Miraglia, S.M. Apoptosis during the seasonal spermatogenic cycle of Rana catesbeiana. J. Anat. 2006, 209, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.N.; Tsukahara, N.; Sugita, S. Apoptosis-mediated seasonal testicular regression in the Japanese Jungle Crow (Corvus macrorhynchos). Theriogenology 2012, 77, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Meachem, S.J.; Stanton, P.G.; Schlatt, S. Follicle-stimulating hormone regulates both Sertoli cell and spermatogonial populations in the adult photoinhibited Djungarian hamster testis. Biol. Reprod. 2005, 72, 1187–1193. [Google Scholar] [CrossRef] [Green Version]
- Sinha Hikim, A.P.; Bartke, A.; Russell, L.D. Morphometric studies on hamster testes in gonadally active and inactive states: Light microscope findings. Biol. Reprod. 1988, 39, 1225–1237. [Google Scholar] [CrossRef] [Green Version]
- Tarulli, G.A.; Stanton, P.G.; Meachem, S.J. Is the adult Sertoli cell terminally differentiated? Biol. Reprod. 2012, 87, 13. [Google Scholar] [CrossRef] [Green Version]
- Heiskanen, P.; Billig, H.; Toppari, J.; Kaleva, M.; Arsalo, A.; Rapola, J.; Dunkel, L. Apoptotic cell death in the normal and cryptorchid human testis: The effect of human chorionic gonadotropin on testicular cell survival. Pediatr. Res. 1996, 40, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Sasso-Cerri, E.; Miraglia, S.M. In situ demonstration of both TUNEL-labeled germ cell and Sertoli cell in the cimetidine-treated rats. Histol. Histopathol. 2002, 17, 411–417. [Google Scholar] [CrossRef]
- Massoud, D.; Lao-Pérez, M.; Hurtado, A.; Abdo, W.; Palomino-Morales, R.; Carmona, F.D.; Burgos, M.; Jiménez, R.; Barrionuevo, F.J. Germ cell desquamation-based testis regression in a seasonal breeder, the Egyptian long-eared hedgehog, Hemiechinus auritus. PLoS ONE 2018, 13, e0204851. [Google Scholar] [CrossRef] [Green Version]
- Dadhich, R.K.; Real, F.M.; Zurita, F.; Barrionuevo, F.J.; Burgos, M.; Jiménez, R. Role of apoptosis and cell proliferation in the testicular dynamics of seasonal breeding mammals: A study in the Iberian mole, Talpa occidentalis. Biol. Reprod. 2010, 83, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Dadhich, R.K.; Barrionuevo, F.J.; Real, F.M.; Lupiañez, D.G.; Ortega, E.; Burgos, M.; Jiménez, R. Identification of live germ-cell desquamation as a major mechanism of seasonal testis regression in mammals: A study in the Iberian mole (Talpa occidentalis). Biol. Reprod. 2013, 88, 101. [Google Scholar] [CrossRef] [PubMed]
- Massoud, D.; Barrionuevo, F.J.; Ortega, E.; Burgos, M.; Jiménez, R. The testis of greater white-toothed shrew Crocidura russula in Southern European populations: A case of adaptive lack of seasonal involution? J. Exp. Zool. B Mol. Dev. Evol. 2014, 322, 304–315. [Google Scholar] [CrossRef]
- Luaces, J.P.; Rossi, L.F.; Sciurano, R.B.; Rebuzzini, P.; Merico, V.; Zuccotti, M.; Merani, M.S.; Garagna, S. Loss of Sertoli-germ cell adhesion determines the rapid germ cell elimination during the seasonal regression of the seminiferous epithelium of the large hairy armadillo Chaetophractus villosus. Biol. Reprod. 2014, 90, 48. [Google Scholar] [CrossRef] [PubMed]
- Merico, V.; Luaces, J.P.; Rossi, L.F.; Rebuzzini, P.; Merani, M.S.; Zuccotti, M.; Garagna, S. Sertoli-immature spermatids disengagement during testis regression in the armadillo. Reproduction 2019, 157, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Cao, G.; Zhang, Y.; Qu, J.; Li, W.; Wan, X.; Li, Y.X.; Zhang, Z.; Wang, Y.L.; Gao, F. Changes in the morphology and protein expression of germ cells and Sertoli cells in plateau pikas testes during non-breeding season. Sci. Rep. 2016, 6, 22697. [Google Scholar] [CrossRef] [Green Version]
- González, C.R.; Muscarsel Isla, M.L.; Vitullo, A.D. The balance between apoptosis and autophagy regulates testis regression and recrudescence in the seasonal-breeding South American plains vizcacha, Lagostomus maximus. PLoS ONE 2018, 13, e0191126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-Frutos, E.; Seco-Rovira, V.; Martínez-Hernández, J.; Ferrer, C.; Serrano-Sánchez, M.I.; Pastor, L.M. Cellular Modifications in Spermatogenesis during Seasonal Testicular Regression: An Update Review in Mammals. Animals 2022, 12, 1605. https://doi.org/10.3390/ani12131605
Beltrán-Frutos E, Seco-Rovira V, Martínez-Hernández J, Ferrer C, Serrano-Sánchez MI, Pastor LM. Cellular Modifications in Spermatogenesis during Seasonal Testicular Regression: An Update Review in Mammals. Animals. 2022; 12(13):1605. https://doi.org/10.3390/ani12131605
Chicago/Turabian StyleBeltrán-Frutos, Ester, Vicente Seco-Rovira, Jesús Martínez-Hernández, Concepción Ferrer, María Isabel Serrano-Sánchez, and Luis Miguel Pastor. 2022. "Cellular Modifications in Spermatogenesis during Seasonal Testicular Regression: An Update Review in Mammals" Animals 12, no. 13: 1605. https://doi.org/10.3390/ani12131605





