Artificial Reproductive Technology (ART) Applied to Female Cervids Adapted from Domestic Ruminants
Abstract
:Simple Summary
Abstract
1. Historical Fluctuations in the Cervidae Population in Europe
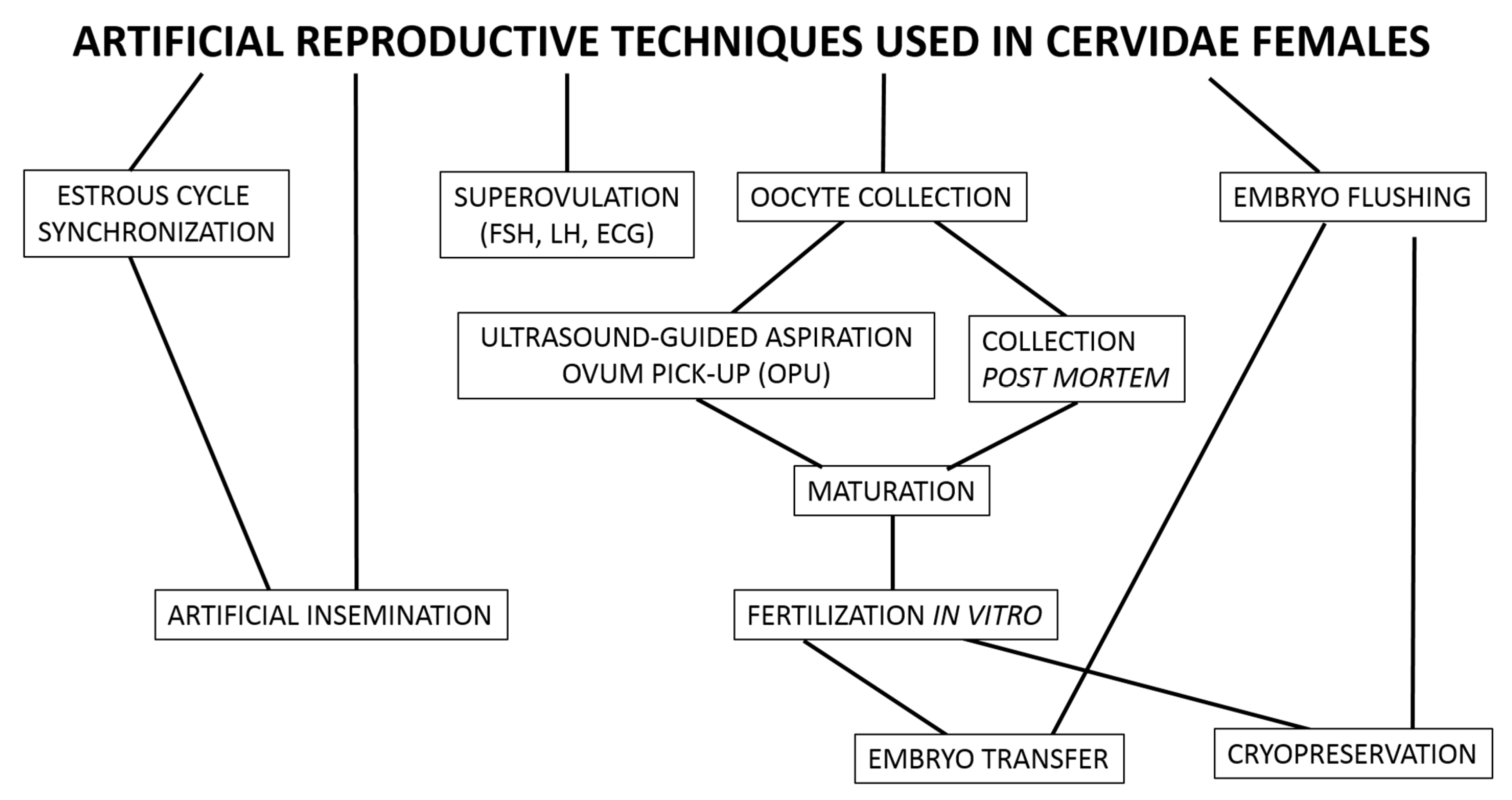
2. Reproductive Technology Applied to Female Deer
2.1. Cryopreservation of Oocytes and Embryos
2.2. Oocyte Collection
2.3. In Vitro Maturation (IVM)
2.4. Estrous Cycle Synchronization, IVF
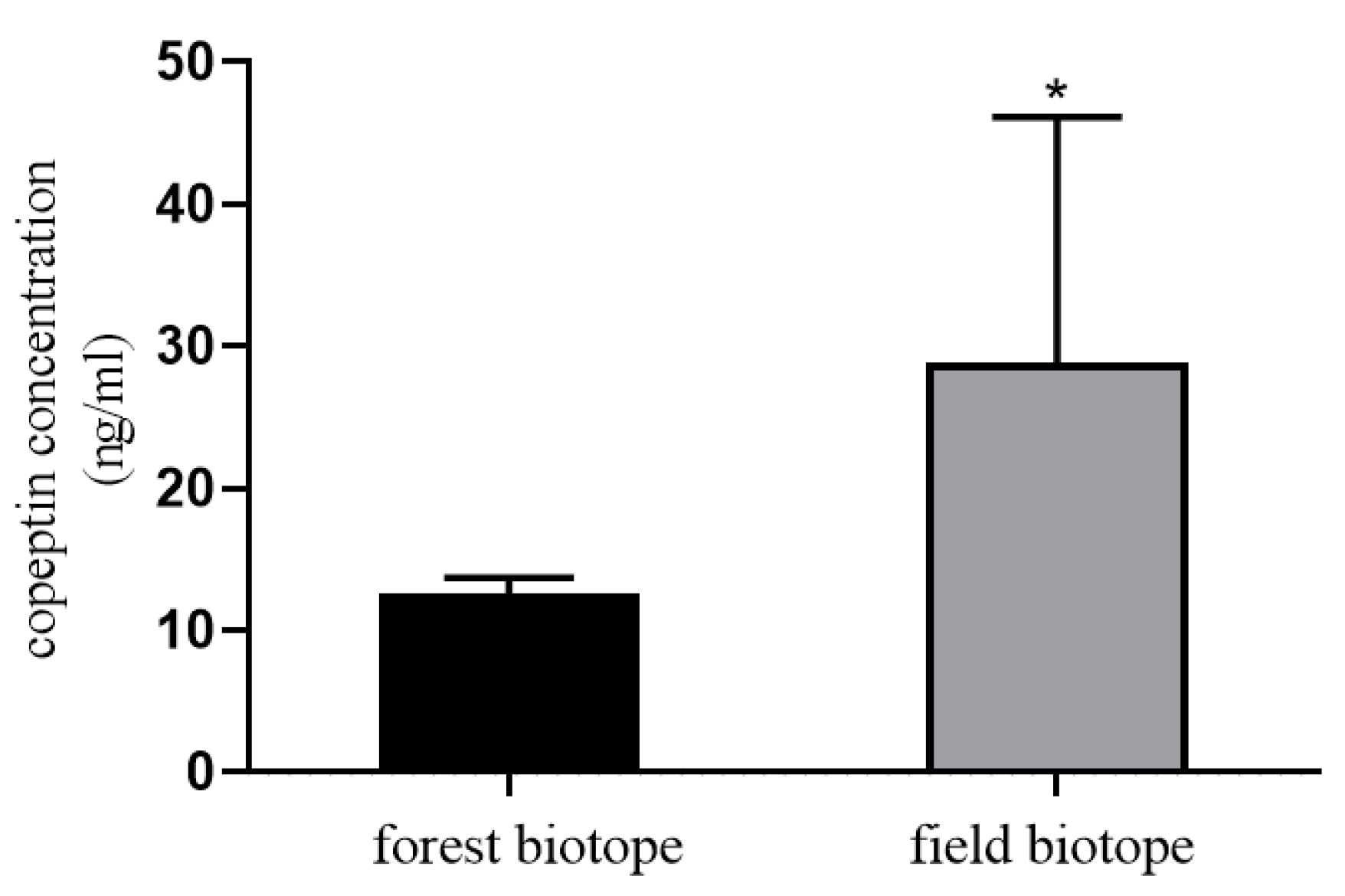
3. Measurement of Stress
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Benton, M.J. The red queen and the court jester: Species diversity and the role of biotic and abiotic factors through time. Science 2009, 323, 728–732. [Google Scholar] [CrossRef] [Green Version]
- Ludt, C.J.; Schroeder, W.; Rottmann, O.; Kuehn, R. Mitochondrial DNA phylogeography of red deer (Cervus elaphus). Mol. Phylogen. Evol. 2004, 31, 1064–1083. [Google Scholar] [CrossRef]
- Heckeberg, N.S. The systematics of the Cervidae: A total evidence approach. PeerJ 2020, 8, e8114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewitt, G. The genetic legacy of the quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef]
- Buzan, E.; Gerič, U.; Potušek, S.; Flajšman, K.; Pokorny, B. First Insights into the Population Genetic Structure and Heterozygosity-Fitness Relationship in Roe Deer Inhabiting the Area between the Alps and Dinaric Mountains. Animals 2020, 10, 2276. [Google Scholar] [CrossRef] [PubMed]
- Niedziałkowska, M.; Jędrzejewska, B.; Honnen, A.-C.; Otto, T.; Sidorovich, V.E.; Perzanowski, K.; Skog, A.; Hartl, G.B.; Borowik, T.; Bunevich, A.N.; et al. Molecular biogeography of red deer Cervus elaphus from eastern Europe: Insights from mitochondrial DNA sequences. Acta Theriol. 2011, 56, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, J.M.; Bonenfant, C.; Mysterud, A.; Gaillard, J.-M.; Csάnyi, S.; Stenseth, N.C. Temporal and spatial development of red deer harvesting in Europe: Biological and cultural factors. J. Appl. Ecol. 2006, 43, 721–734. [Google Scholar] [CrossRef]
- Burbaite, L.; Csάnyi, S. Red deer population and harvest changes in Europe. Acta Zool. Litu. 2010, 20, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Sfougaris, A.; Tsachalidis, E.; Giannakopoulos, A.; Pardalidis, T. Research on the ecology and management of the wild boar (Sus scrofa), roe deer (Capreolus capreolus), red deer (Cervus elaphus) and Balkan chamois (Rupicapra rupicapra balcanica) in Epirus, Greece. In Proceedings of the 24th Congress of IUGB, Thessaloniki, Greece, 20–24 September 1999. [Google Scholar]
- Mertzanis, Y. Brown Bear and Wolf in Greece. In Protected Areas in the Southern Balkans–Legislation, Large Carnivores, Transborder Areas; Psadouras, S., Ed.; Arcturos & Hellenic Ministry of the Environment, Physical Planning, and Public Works Thessaloniki, Greece: Thessaloniki, Greece, 2002; pp. 115–133. [Google Scholar]
- IUCN. European Mammal Assessment. Capreolus Capreolus. Available online: http://ec.europa.eu/environment/nature/conservation/species/ema/ (accessed on 1 October 2007).
- Baleišis, R.; Bluzma, P.; Balčiauskas, L. Lietuvos Kanopiniai Žvėrys [Ungulates of Lithuania]; Akstis: Vilnius, Lithuania, 2003; 216p. [Google Scholar]
- Andersen, R.; Duncan, P.; Linnell, J.D.C. The European Roe Deer: The Biology of Success; Scandinavian University Press: Oslo, Norway, 1998. [Google Scholar]
- Lambert, R.T.; Ashworth, C.J.; Beattie, L.; Gebbie, F.E.; Hutchinson, J.S.; Kyle, D.J.; Racey, P.A. Temporal changes in reproductive hormones and conceptus-endometrial interactions during embryonic diapause and reactivation of the blastocyst in European roe deer (Capreolus capreolus). Reproduction 2001, 121, 863–871. [Google Scholar] [CrossRef]
- Arav, A. Cryopreservation of oocytes and embryos. Theriogenology 2014, 81, 96–102. [Google Scholar] [CrossRef]
- Díez, C.; Muñoz, M.; Caamaño, J.N.; Gómez, E. Cryopreservation of the bovine oocyte: Current status and perspectives. Reprod. Domest. Anim. 2012, 47, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Quan, G.; Wu, G.; Hong, Q. Oocyte cryopreservation based in sheep: The current status and future perspective. Biopreserv. Biobank. 2017, 15, 535–547. [Google Scholar] [CrossRef]
- Gastal, G.D.A.; Aguiar, F.L.N.; Rodrigues, A.P.R.; Scimeca, J.M.; Apgar, G.A.; Banz, W.J.; Feugang, J.M.; Gastal, E.L. Cryopreservation and in vitro culture of white-tailed deer ovarian tissue. Theriogenology 2018, 113, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.P.; Mucci, N.; Kaiser, G.G.; Aller, J.; Hunter, J.W.; Dixon, T.E.; Alberio, R.H. Multiple ovulation and embryo transfer with fresh, frozen and vitrified red deer (Cervus elaphus) embryos in Argentina. Anim. Reprod. Sci. 2007, 102, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.W. Current reproductive technology as applied to the New Zealand deer industry. In Proceedings of a Deer Course for Veterinarians; New Zealand Veterinary Association: Methuen, New Zealand, 1997; Volume 14, pp. 179–195. [Google Scholar]
- Dixon, T.E.; Hunter, J.W.; Beatson, N.S. Pregnancies following the export of frozen red deer embryos from New Zealand to Australia. Theriogenology 1991, 35, 193–198. [Google Scholar] [CrossRef]
- Mapletoft, R.J.; Steward, K.B.; Adams, G.P. Recent advances in the superovulation in cattle. Reprod. Nutr. Dev. 2002, 42, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Graff, K.J.; Meintjes, M.; Dyer, V.W.; Paul, J.B.; Denniston, R.S.; Ziomek, C.; Godke, R.A. Transvaginal ultrasound-guided oocyte retrieval following FSH stimulation of domestic goats. Theriogenology 1999, 51, 1099–1119. [Google Scholar] [CrossRef]
- Locatelli, Y.; Hendriks, A.; Vallet, J.C.; Baril, G.; Duffard, N.; Bon, N.; Ortiz, K.; Scala, C.; Maurel, M.C.; Mermillod, P.; et al. Assessment LOPU-IVF in Japanese sika deer (Cervus nippon nippon) and application to Vietnamese sika deer (Cervus nippon pseudaxis) a related subspecies threatened with extinction. Theriogenology 2012, 78, 2039–2049. [Google Scholar] [CrossRef]
- Korzekwa, A.J.; Kotlarczyk, A.M.; Szczepańska, A.A.; Grzyb, M.; Siergiej, A.; Wocławek-Potocka, I. Antioxidative potential of red deer embryos depends on reproductive stage of hind as a oocyte donor. Animals 2020, 10, 1190. [Google Scholar] [CrossRef]
- Nivet, A.-L.; Bunel, A.; Labrecque, R.; Belanger, J.; Vigneault, C.; Blondin, P.; Sirard, M.-A. FSH withdrawal improves developmental competence of oocytes in the bovine model. Reproduction 2012, 143, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Ginther, O.J.; Kot, K.; Kulick, L.J.; Wiltbank, M.C. Emergence and deviation of follicles during the development of follicular waves in cattle. Theriogenology 1997, 48, 75–87. [Google Scholar] [CrossRef]
- Girard, A.; Dufort, I.; Douville, G.; Sirard, M.-A. Global gene expression in granulosa cells of growing, plateau and atretic dominant follicles in cattle. Reprod. Biol. Endocrinol. 2015, 13, 17. [Google Scholar] [CrossRef] [Green Version]
- Blondin, P.; Bousquet, D.; Twagiramungu, H.; Barnes, F.; Sirard, M.A. Manipulation of follicular development to produce developmentally competent bovine oocytes. Biol. Reprod. 2002, 66, 38–43. [Google Scholar] [CrossRef]
- Locatelli, Y.; Vallet, J.C.; Huyghe, F.P.; Cognié, Y.; Legendre, X.; Mermillod, P. Laparoscopic ovum pick-up and in vitro production of sika deer embryos: Effect of season and culture conditions. Theriogenology 2006, 66, 1334–1342. [Google Scholar] [CrossRef]
- Pincus, G.; Enzmann, E.V. The comparative behavior of mammalian eggs in vivo and in vitro: I. The activation of ovarian eggs. J. Exp. Med. 1935, 62, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Ward, F.; Enright, B.; Rizos, D.; Boland, M.; Lonergan, P. Optimization of in vitro bovine embryo production: Effect of duration of maturation, length of gamete co-incubation, sperm concentration and sire. Theriogenology 2002, 57, 2105–2117. [Google Scholar] [CrossRef]
- Berg, D.K.; Thongphakdee, A. In vitro culture of deer embryos. Methods Mol. Biol. 2019, 2006, 191–207. [Google Scholar]
- Van Soom, A.; Mateusen, B.; Leroy, J.; De Kruif, A. Assessment of mammalian embryo quality: What can we learn from embryo morphology? Reprod. Biomed. Online 2003, 7, 664–670. [Google Scholar] [CrossRef]
- Wrenzycki, C.; Herrmann, D.; Niemann, H. Messenger RNA in oocytes and embryos in relation to embryo viability. Theriogenology 2007, 68, 77–83. [Google Scholar] [CrossRef]
- Lonergan, P.; Khatir, H.; Piumi, F.; Rieger, D.; Humblot, P.; Boland, M.P. Effect of time interval from insemination to first cleavage on the developmental characteristics, sex ratio and pregnancy rate after transfer of bovine embryos. J. Reprod. Fertil. 1999, 117, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Fair, T.; Murphy, M.; Rizos, D.; Moss, C.; Martin, F.; Boland, M.P.; Lonergan, P. Analysis of differential maternal mRNA expression in developmentally competent and incompetent bovine two-cell embryos. Mol. Reprod. Dev. 2004, 67, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; García-Álvarez, O.; Martín-Maestro, A.; Peris-Frau, P.; Ortiz, J.A.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Influence of foetal calf serum supplementation during in vitro embryo culture in Iberian red deer. Reprod. Domest. Anim. 2019, 54, 69–71. [Google Scholar] [CrossRef] [PubMed]
- García-Alvarez, O.; Maroto-Morales, A.; Berlinguer, F.; Fernández-Santos, M.R.; Esteso, M.C.; Mermillod, P.; Ortiz, J.A.; Ramon, M.; Pérez-Guzmán, M.D.; Garde, J.J.; et al. Effect of storage temperature during transport of ovaries on in vitro embryo production in Iberian red deer (Cervus elaphus hispanicus). Theriogenology 2011, 75, 65–72. [Google Scholar] [CrossRef]
- Locatelli, Y.; Cognié, Y.; Vallet, J.C.; Baril, G.; Verdier, M.; Poulin, N.; Legendre, X.; Mermillod, P. Successful use of oviduct epithelial cell coculture for in vitro production of viable red deer (Cervus elaphus) embryos. Theriogenology 2005, 64, 1729–1739. [Google Scholar] [CrossRef]
- Berg, D.K.; Asher, G.W. New developments reproductive technologies in deer. Theriogenology 2003, 59, 189–205. [Google Scholar] [CrossRef]
- Uccheddu, S.; Pintus, E.; Garde, J.J.; Fleba, L.; Muzzeddu, M.; Pudda, F.; Bogliolo, L.; Strina, A.; Nieddu, S.; Ledda, S. Post-mortem recovery, in vitro maturation and fertilization of fallow deer (Dama dama, Linnaeus 1758) oocytes collected during reproductive and no reproductive season. Reprod. Domest. Anim. 2020, 55, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.J.; Barton, N.H.; Swanson, G.; Abernethy, K.; Pemberton, J.M. Introgression through rare hybridization: A genetic study of a hybrid zone between red and sika deer (genus Cervus) in Argyll, Scotland. Genetics 1999, 152, 355–371. [Google Scholar] [CrossRef]
- Macek, M.B.; Shur, B.D. Protein-carbohydrate complementarity in mammalian gamete recognition. Gamete Res. 1988, 20, 93–109. [Google Scholar] [CrossRef]
- Wassarman, P.M. Regulation of mammalian fertilization by zona pellucida glycoproteins. J. Reprod. Fertil. Suppl. 1990, 42, 79–87. [Google Scholar]
- Wassarman, P.M. Towards molecular mechanisms for gamete adhesion and fusion during mammalian fertilization. Curr. Opinion Cell Biol. 1995, 7, 658–664. [Google Scholar] [CrossRef]
- Parillo, F.; Diverio, S.; Romeo, G.; Fagioli, O. Variations in lectin-binding on the zona pellucida during oocyte growth in some wild ungulates. Ann. Anat. 2003, 185, 109–115. [Google Scholar] [CrossRef]
- Baird, D.T.; McNeilly, A.S. Gonadotrophic control of follicular development and function during the oestrous cycle of the ewe. J. Reprod. Fertil. Suppl. 1981, 30, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.W. Gestation length in red deer: Genetically determined or environmentally controlled? Soc. Reprod. Fertil. Suppl. 2007, 64, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.W.; Scott, I.C.; Archer, J.A.; Ward, J.F.; Littlejohn, R.P. Seasonal luteal cyclicity of pubertal and adult red deer. J. Anim. Reprod. Sci. 2011, 125, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Korzekwa, A.J.; Szczepańska, A.; Bogdaszewski, M.; Nadolski, P.; Malż, P.; Giżejewski, Z. Production of prostaglandins in placentae and corpus luteum in pregnant hinds of red deer (Cervus elaphus). Theriogenology 2016, 85, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.W.; Fisher, M.W.; Jabbour, H.N.; Smith, J.F.; Mulley, R.C.; Morrow, C.J.; Veldhuizen, F.A.; Langridge, M. Relationship between the onset of oestrus, the preovulatory surge in luteinizing hormone and ovulation following oestrous synchronisation and superovulation of farmed red deer (Cervus elaphus). J. Reprod. Fertil. 2002, 96, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Korzekwa, A.J.; Kordan, W.; Kotlarczyk, A.M.; Kozdrowski, R. The Effectiveness of pharmacological synchronization of the estrous cycle in hinds (Cervus elaphus L.): A pilot field trial. Animals 2020, 10, 2148. [Google Scholar] [CrossRef]
- Berg, D.K.; Thompson, J.G.; Asher, G.W. Development of in vitro embryo production systems for red deer (Cervus elaphus) Part 1. Effect of epithelial oviductal monolayers and heparin on in vitro sperm motility and penetration of in vitro matured oocytes. Anim. Reprod. Sci. 2002, 70, 65–76. [Google Scholar] [CrossRef]
- Carbillet, J.; Rey, B.; Palme, R.; Morellet, N.; Bonnot, N.; Chaval, Y.; Cargnelutti, B.; Hewison, A.J.M.; Gilot-Fromont, E.; Verheyden, H. Under cover of the night: Context-dependency of anthropogenic disturbance on stress levels of wild roe deer Capreolus capreolus. Conserv. Physiol. 2020, 8, coaa086. [Google Scholar] [CrossRef]
- Vera, F.; Zenuto, R.; Antenucci, C.D. Expanding the actions of cortisol and corticosterone in wild vertebrates: A necessary step to overcome the emerging challenges. Gen. Comp. Endocrinol. 2017, 246, 337–353. [Google Scholar] [CrossRef]
- Christ-Crain, M. Vasopressin and Copeptin in health and disease. Rev. Endocr. Metab. Disord. 2019, 20, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, D.; Elmi, A.; Barone, F.; Carnevali, G.; Govoni, N.; Bacci, M.L. Hair testosterone and cortisol concentrations in preand post-rut roe deer bucks: Correlations with blood levels and testicular morphometric parameters. Animals 2018, 8, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacey, M.J.; Delves, S.K.; Britland, S.E.; Allsopp, A.J.; Brett, S.J.; Fallowfield, J.L.; Woods, D.R. Copeptin reflects physiological strain during thermal stress. Eur. J. Appl. Physiol. 2017, 118, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzekwa, A.J.; Kotlarczyk, A.M. Artificial Reproductive Technology (ART) Applied to Female Cervids Adapted from Domestic Ruminants. Animals 2021, 11, 2933. https://doi.org/10.3390/ani11102933
Korzekwa AJ, Kotlarczyk AM. Artificial Reproductive Technology (ART) Applied to Female Cervids Adapted from Domestic Ruminants. Animals. 2021; 11(10):2933. https://doi.org/10.3390/ani11102933
Chicago/Turabian StyleKorzekwa, Anna J., and Angelika M. Kotlarczyk. 2021. "Artificial Reproductive Technology (ART) Applied to Female Cervids Adapted from Domestic Ruminants" Animals 11, no. 10: 2933. https://doi.org/10.3390/ani11102933
APA StyleKorzekwa, A. J., & Kotlarczyk, A. M. (2021). Artificial Reproductive Technology (ART) Applied to Female Cervids Adapted from Domestic Ruminants. Animals, 11(10), 2933. https://doi.org/10.3390/ani11102933





