Non-Invasive Determination of Annual Fecal Cortisol, Androstenedione, and Testosterone Variations in a Herd of Male Asian Elephants (Elephas maximus) and Their Relation to Some Climatic Variables
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Collection of Fecal Samples
2.2. Fecal Extraction and Enzyme Immunoassays for Steroid Hormones
2.3. Climatic Data
2.4. Statistical Study
3. Results
3.1. Climatic Data
3.2. Hormone Concentrations
3.2.1. Testosterone
3.2.2. Androstenedione
3.2.3. Cortisol
3.3. Correlations
3.4. Musth Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wasser, S.K.; Papageorge, S.; Foley, C.; Brown, J.L. Excretory fate of estradiol and progesterone in the African elephant (Loxodonta africana) and patterns of fecal steroid concentrations throughout the estrous cycle. Gen. Comp. Endocrinol. 1996, 102, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Barja, I.; Silvan, G.; Illera, J.C. Relationships between sex and stress hormone levels in feces and marking behavior in a wild population of Iberian wolves (Canis lupus signatus). J. Chem. Ecol. 2008, 34, 697–701. [Google Scholar] [CrossRef]
- Barja, I.; Silvan, G.; Rosellini, S.; Pineiro, A.; Gonzalez-Gil, A.; Camacho, L.; Illera, J.C. Stress physiological responses to tourist pressure in a wild population of European pine marten. J. Steroid Biochem. Mol. Biol. 2007, 104, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Casares, M.; Silvan, G.; Carbonell, M.D.; Gerique, C.; Martinez-Fernandez, L.; Caceres, S.; Illera, J.C. Circadian rhythm of salivary cortisol secretion in female zoo-kept African elephants (Loxodonta africana). Zoo Biol. 2016, 35, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.J.; Stabenfeldt, G.H.; Cragun, J.R.; Addiego, L.A.; Overstreet, J.W.; Lasley, B.L. Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clin. Chem. 1991, 37, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.J.; Kolver, E.S.; Verkerk, G.A.; Matthews, L.R. Fecal glucocorticoid metabolites as a measure of adrenal activity in dairy cattle. Gen. Comp. Endocrinol. 2002, 126, 229–241. [Google Scholar] [CrossRef]
- Yon, L.; Faulkner, B.; Kanchanapangka, S.; Chaiyabutr, N.; Meepan, S.; Lasley, B. A safer method for studying hormone metabolism in an Asian elephant (Elephas maximus): Accelerator mass spectrometry. Zoo Biol. 2010, 29, 760–766. [Google Scholar] [CrossRef]
- Wong, E.P.; Yon, L.; Purcell, R.; Walker, S.L.; Othman, N.; Saaban, S.; Campos-Arceiz, A. Concentrations of faecal glucocorticoid metabolites in Asian elephant’s dung are stable for up to 8 h in a tropical environment. Conserv. Physiol. 2016, 4, cow070. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Bubenik, A.B.; Schams, D.; Leatherland, J.F. Circadian and circannual rhythms of LH, FSH, testosterone (T), prolactin, cortisol, T3 and T4 in plasma of mature, male white-tailed deer. Comp. Biochem. Physiol. Part A Physiol. 1983, 76, 37–45. [Google Scholar] [CrossRef]
- Nicolau, G.Y.; Lakatua, D.; Sackett-Lundeen, L.; Haus, E. Circadian and circannual rhythms of hormonal variables in elderly men and women. Chronobiol. Int. 1984, 1, 301–319. [Google Scholar] [CrossRef]
- Shivatcheva, T.M.; Ankov, V.K.; Hadjioloff, A.I. Circannual fluctuations of the serum cortisol in the European ground squirrel, Citellus citellus L. Comp. Biochem. Physiol. A Comp. Physiol. 1988, 90, 515–518. [Google Scholar] [CrossRef]
- Cordero, M.; Brorsen, B.W.; McFarlane, D. Circadian and circannual rhythms of cortisol, ACTH, and alpha-melanocyte-stimulating hormone in healthy horses. Domest. Anim. Endocrinol. 2012, 43, 317–324. [Google Scholar] [CrossRef]
- Jainudeen, M.R.; Katongole, C.B.; Short, R.V. Plasma testosterone levels in relation to musth and sexual activity in the male asiatic elephant, Elephas maximus. J. Reprod. Fertil. 1972, 29, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Lincoln, G.A.; Ratnasooriya, W.D. Testosterone secretion, musth behaviour and social dominance in captive male Asian elephants living near the equator. J. Reprod. Fertil. 1996, 108, 107–113. [Google Scholar] [CrossRef]
- Rasmussen, L.E.; Buss, I.O.; Hess, D.L.; Schmidt, M.J. Testosterone and dihydrotestosterone concentrations in elephant serum and temporal gland secretions. Biol. Reprod. 1984, 30, 352–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.L.; Somerville, M.; Riddle, H.S.; Keele, M.; Duer, C.K.; Freeman, E.W. Comparative endocrinology of testicular, adrenal and thyroid function in captive Asian and African elephant bulls. Gen. Comp. Endocrinol. 2007, 151, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Brown, J. Reproductive endocrinology. In Biology, Medicine and Sugery of Elephants, 1st ed.; Susan, K., Mikota, M.E.F., Eds.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 377–388. [Google Scholar]
- Chave, E.; Edwards, K.L.; Paris, S.; Prado, N.; Morfeld, K.A.; Brown, J.L. Variation in metabolic factors and gonadal, pituitary, thyroid, and adrenal hormones in association with musth in African and Asian elephant bulls. Gen. Comp. Endocrinol. 2019, 276, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Niemuller, C.A.; Liptrap, R.M. Altered androstenedione to testosterone ratios and LH concentrations during musth in the captive male Asian elephant (Elephas maximus). J. Reprod. Fertil. 1991, 91, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ganswindt, A.; Palme, R.; Heistermann, M.; Borragan, S.; Hodges, J.K. Non-invasive assessment of adrenocortical function in the male African elephant (Loxodonta africana) and its relation to musth. Gen. Comp. Endocrinol. 2003, 134, 156–166. [Google Scholar] [CrossRef]
- Wingate, L.; Lasley, B. Is musth a reproductive event: An examination of arguments for and against this view. In Proceedings of the International Elephant and Rhino Research Symposium, A Research Update on Elephants and Rhinos, Vienna, Austria, 7–11 June 2001; Schuling Verlag: Münster, Germany, 2002; pp. 150–156. [Google Scholar]
- Clauss, M.; Zerbe, P.; Bingaman Lackey, L.; Codron, D.; Müller, D.W.H. Basic considerations on seasonal breeding in mammals including their testing by comparing natural habitats and zoos. Mamm. Biol. 2021, 101, 373–386. [Google Scholar] [CrossRef]
- Heldstab, S.A.; van Schaik, C.P.; Muller, D.W.H.; Rensch, E.; Lackey, L.B.; Zerbe, P.; Hatt, J.M.; Clauss, M.; Matsuda, I. Reproductive seasonality in primates: Patterns, concepts and unsolved questions. Biol. Rev. 2021, 96, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Hufenus, R.; Schiffmann, C.; Hatt, J.M.; Müller, D.H.W.; Bingaman Lackey, L.; Clauss, M.; Zerbe, P. Seasonality of reproduction in Asian elephants Elephas maximus and African elephants Loxodonta africana: Underlying photoperiodic cueing? Mammal Rev. 2018, 48, 261–276. [Google Scholar] [CrossRef]
- Thitaram, C.; Brown, J.L.; Pongsopawijit, P.; Chansitthiwet, S.; Wongkalasin, W.; Daram, P.; Roongsri, R.; Kalmapijit, A.; Mahasawangkul, S.; Rojansthien, S.; et al. Seasonal effects on the endocrine pattern of semi-captive female Asian elephants (Elephas maximus): Timing of the anovulatory luteinizing hormone surge determines the length of the estrous cycle. Theriogenology 2008, 69, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyffels, J.T.; George, R.; Adams, L.; Adams, C.; Clauss, T.; Newton, A.; Hyatt, M.W.; Yach, C.; Penfold, L.M. Testosterone and semen seasonality for the sand tiger shark Carcharias taurusdagger. Biol. Reprod. 2020, 102, 876–887. [Google Scholar] [CrossRef]
- Zerbe, P.; Clauss, M.; Codron, D.; Bingaman Lackey, L.; Rensch, E.; Streich, J.W.; Hatt, J.M.; Muller, D.W. Reproductive seasonality in captive wild ruminants: Implications for biogeographical adaptation, photoperiodic control, and life history. Biol. Rev. 2012, 87, 965–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, C.A.H.; Papageorge, S.; Wasser, S.K. Noninvasive stress and reproductive measures of social and ecological pressures in free-ranging African elephants. Conserv. Biol. 2001, 15, 1134–1142. [Google Scholar] [CrossRef]
- Ataallahi, M.; Nejad, J.G.; Takahashi, J.; Song, Y.; Sung, K.; Yun, J.; Park, K. Effects of environmental changes during different seasons on hair cortisol concentration as a biomarker of chronic stress in Korean native cattle. Int. J. Agric. Biol. 2019, 21, 1166–1172. [Google Scholar]
- Mumby, H.S.; Mar, K.U.; Thitaram, C.; Courtiol, A.; Towiboon, P.; Min-Oo, Z.; Htut-Aung, Y.; Brown, J.L.; Lummaa, V. Stress and body condition are associated with climate and demography in Asian elephants. Conserv. Physiol. 2015, 3, cov030. [Google Scholar] [CrossRef] [Green Version]
- Menargues Marcilla, A.; Urios, V.; Liminana, R. Seasonal rhythms of salivary cortisol secretion in captive Asian elephants (Elephas maximus). Gen. Comp. Endocrinol. 2012, 176, 259–264. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Lee, B.H.; Kim, J.Y.; Chemere, B.; Sung, K.I.; Lee, H.G. Effect of alpine grazing on plasma and hair cortisol, serotonin, and DHEA in dairy cows and its welfare impact. Domest. Anim. Endocrinol. 2021, 75, 106581. [Google Scholar] [CrossRef]
- Rathwa, S.D.; Vasava, A.A.; Pathan, M.M.; Madhira, S.P.; Patel, Y.G.; Pande, A.M. Effect of season on physiological, biochemical, hormonal, and oxidative stress parameters of indigenous sheep. Vet. World 2017, 10, 650–654. [Google Scholar] [CrossRef] [Green Version]
- Clubb, R.; Mason, G. A Review of the Welfare of Zoo Elephants in Europe: A Report Commissioned by the RSPCA; Animal Behaviour Research Group, Department of Zoology, University of Oxford: Oxford, UK, 2002. [Google Scholar]
- Ratnasooriya, W.D.; Ratnayake, S.S.; Jayatunga, Y.N. Effects of pyrethroid insecticide ICON (lambda cyhalothrin) on reproductive competence of male rats. Asian J. Androl. 2002, 4, 35–41. [Google Scholar]
- Barth, A.D.; Waldner, C.L. Factors affecting breeding soundness classification of beef bulls examined at the Western College of Veterinary Medicine. Can. Vet. J. 2002, 43, 274–284. [Google Scholar] [PubMed]
- Sukumar, R. The Living Elephants: Evolutionary Ecology, Behavior, and Conservation; Oxford University Press: New York, NY, USA, 2003; p. 478. [Google Scholar]
- Weissenbock, N.M.; Schwammer, H.M.; Ruf, T. Estrous synchrony in a group of African elephants (Loxodonta africana) under human care. Anim. Reprod. Sci. 2009, 113, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.E.; Schulte, B.A. Chemical signals in the reproduction of Asian (Elephas maximus) and African (Loxodonta africana) elephants. Anim. Reprod. Sci. 1998, 53, 19–34. [Google Scholar] [CrossRef]
- Bolechova, P.C.M.; De Man, D.; Galeffi, C.; Hofman, S.; Kappelhof, J.; Kfir, G.; Kjellson, B.; Kölpin, T.; Lawrenz, A.; Lüders, I.; et al. EAZA Best Practice Guidelines for Elephants; EAZA Executive Office: Amsterdam, The Netherlands, 2020; pp. 1–214. [Google Scholar]
- Gippoliti, S. Ex situ conservation programmes in European zoological gardens: Can we afford to lose them? Biodivers. Conserv. 2012, 21, 1359–1364. [Google Scholar] [CrossRef] [Green Version]
- Pineiro, A.; Hernandez, M.C.; Silvan, G.; Illera, J.C.; Barja, I. Reproductive hormones monthly variation in free-ranging European wildcats: Lack of association with faecal marking. Reprod. Domest. Anim. 2020, 55, 1784–1793. [Google Scholar] [CrossRef]
- Van Wees, M.; Damen, M. Asian Elephant EEP Studbook. 2016. Available online: https://www.diergaardeblijdorp.nl/wp-content/uploads/2015/01/Asian-elephant-EEP-2014-Studbook.pdf (accessed on 13 September 2021).
- Ghosal, R.; Ganswindt, A.; Seshagiri, P.B.; Sukumar, R. Endocrine correlates of musth in free-ranging Asian elephants (Elephas maximus) determined by non-invasive faecal steroid hormone metabolite measurements. PLoS ONE 2013, 8, e84787. [Google Scholar] [CrossRef] [Green Version]
- Ganswindt, A.; Heistermann, M.; Hodges, K. Physical, physiological, and behavioral correlates of musth in captive African elephants (Loxodonta africana). Physiol. Biochem. Zool. 2005, 78, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, N.; Das, S.; Sukumar, R. Population, reproduction and management of captive Asian elephants (Elephas maximus) in Jaldapara Wildlife Sanctuary, West Bengal, India. Indian For. 2009, 135, 1545–1555. [Google Scholar]
- Sukumar, R.; Krishnamurthy, V.; Wemmer, C.; Rodden, M. Demography of captive Asian elephants (Elephas maximus) in southern India. Zoo Biol. 1997, 16, 263–272. [Google Scholar] [CrossRef]
- Mumby, H.S.; Courtiol, A.; Mar, K.U.; Lummaa, V. Birth seasonality and calf mortality in a large population of Asian elephants. Ecol. Evol. 2013, 3, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Thongtip, N.; Saikhun, J.; Mahasawangkul, S.; Kornkaewrat, K.; Pongsopavijitr, P.; Songsasen, N.; Pinyopummin, A. Potential factors affecting semen quality in the Asian elephant (Elephas maximus). Reprod. Biol. Endocrinol. 2008, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongtip, N.; Mahasawangkul, S.; Thitaram, C.; Pongsopavijitr, P.; Kornkaewrat, K.; Pinyopummin, A.; Angkawanish, T.; Jansittiwate, S.; Rungsri, R.; Boonprasert, K.; et al. Successful artificial insemination in the Asian elephant (Elephas maximus) using chilled and frozen-thawed semen. Reprod. Biol. Endocrinol. 2009, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Sukumar, R. A brief review of the status, distribution and biology of wild Asian elephants. Elephas maximus. Int. Zoo Yearb. 2006, 40, 1–8. [Google Scholar] [CrossRef]
- Lueders, I.; Hildebrandt, T.B.; Gray, C.; Botha, S.; Rich, P.; Niemuller, C. Supression of testicular function in a male Asian elephant (Elephas maximus) treated with gonadotropin-releasing hormone vaccines. J. Zoo Wildl. Med. 2014, 45, 611–619. [Google Scholar] [CrossRef]
- Davey, G. Visitors’ effects on the welfare of animals in the zoo: A review. J. Appl. Anim. Welf. Sci. 2007, 10, 169–183. [Google Scholar] [CrossRef]
- Hadlow, N.; Brown, S.; Wardrop, R.; Conradie, J.; Henley, D. Where in the world? Latitude, longitude and season contribute to the complex co-ordinates determining cortisol levels. Clin. Endocrinol. 2018, 89, 299–307. [Google Scholar] [CrossRef]
- Ganswindt, A.; Muenscher, S.; Henley, M.; Henley, S.; Heistermann, M.; Palme, R.; Thompson, P.; Bertschinger, H. Endocrine correlates of musth and the impact of ecological and social factors in free-ranging African elephants (Loxodonta africana). Horm. Behav. 2010, 57, 506–514. [Google Scholar] [CrossRef]
- Yon, L.; Kanchanapangka, S.; Chaiyabutr, N.; Meepan, S.; Stanczyk, F.Z.; Dahl, N.; Lasley, B. A longitudinal study of LH, gonadal and adrenal steroids in four intact Asian bull elephants (Elephas maximus) and one castrate African bull (Loxodonta africana) during musth and non-musth periods. Gen. Comp. Endocrinol. 2007, 151, 241–245. [Google Scholar] [CrossRef]



| Name of Elephant | Age as on 2016 (Years) | Name of Father | Name of Mother | Place of Birth | Period of Time in La Reserva as on 2016 (Years) |
|---|---|---|---|---|---|
| Tse Pyang | 18 | Naing Thein | Thiha Phyu | Emmen | 13 |
| Aung Si | 14 | Naing Thein | Thiha Phyu | Emmen | 9 |
| Kan Kaung | 14 | Naing Thein | Yu Zin | Emmen | 3 |
| Than Myan | 14 | Naing Thein | Htoo Yin Aye | Emmen | 3 |
| Toomai | 12 | Emmet | Azizah | Whipsnade | 5 |
| Hunt Bwe | 10 | Radza | Swe San Thay | Emmen | 5 |
| Dimas | 6 | Ankhor | Cynthia | Berlin TP | 2 |
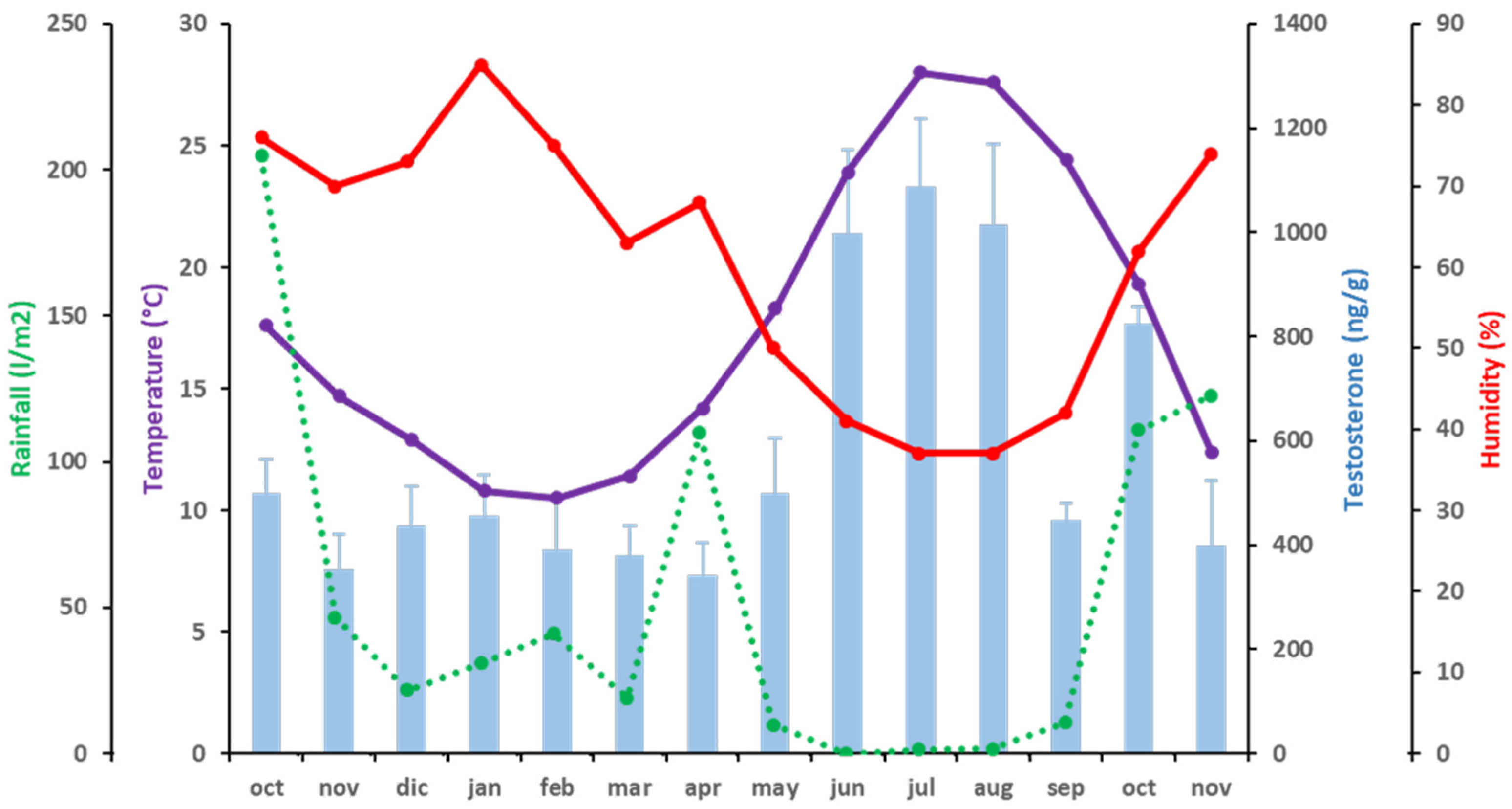
| Month 1 | Temperature (°C) | Rainfall (L/m2) | Humidity (%) |
|---|---|---|---|
| Oct 15 | 17.6 | 204.8 | 76 |
| Nov 15 | 14.7 | 46.8 | 70 |
| Dec 15 | 12.9 | 21.8 | 73 |
| Jan 16 | 10.8 | 31 | 85 |
| Feb 16 | 10.5 | 41.2 | 75 |
| Mar 16 | 11.4 | 19 | 63 |
| Apr 16 | 14.2 | 109.8 | 68 |
| May 16 | 18.3 | 10 | 50 |
| Jun 16 | 23.9 | 0 | 41 |
| Jul 16 | 28 | 1.4 | 37 |
| Aug 16 | 27.6 | 1.6 | 37 |
| Sep 16 | 24.4 | 10.6 | 42 |
| Oct 18 | 19.3 | 110.8 | 62 |
| Nov 16 | 12.4 | 122.8 | 74 |
| Month 1 | T (ng/g) | A4 (ng/g) | C (ng/g) |
|---|---|---|---|
| Oct 15 | 498.92 ± 65.83 | 224.33 ± 21.59 | 25.12 ± 13.42 |
| Nov 15 | 352.81 ± 68.57 | 162.66 ± 23.00 | 33.09 ± 18.81 |
| Dec 15 | 437.10 ± 74.72 | 195.05 ± 27.50 | 27.26 ± 12.85 |
| Jan 16 | 455.53 ± 77.88 | 197.38 ± 33.93 | 31.41 ± 13.37 |
| Feb 16 | 391.15 ± 102.06 | 173.30 ± 16.55 | 29.82 ± 16.03 |
| Mar 16 | 379.70 ± 57.79 | 150.69 ± 18.22 | 45.37 ±19.40 |
| Apr 16 | 340.94 ± 63.82 | 152.66 ± 21.22 | 29.43 ± 13.49 |
| May 16 | 499.00 ± 105.99 | 225.85 ± 47.10 | 13.97 ± 10.53 |
| Jun 16 | 997.34 ± 161.44 | 389.54 ± 54.41 | 15.62 ± 11.56 |
| Jul 16 | 1088.35 ± 131.04 | 480.40 ± 50.86 | 62.04 ± 29.55 |
| Aug 16 | 1015.11 ± 153.50 | 414.16 ± 75.18 | 141.92 ± 55.79 |
| Sep 16 | 446.71 ± 34.72 | 185.83 ± 41.60 | 156.67 ± 60.89 |
| Oct 16 | 825.09 ± 31.60 | 319.96 ± 32.69 | 58.59 ± 27.09 |
| Nov 16 | 398.76 ± 123.84 | 179.74 ± 36.52 | 24.70 ± 7.21 |
| Temperature (°C) | Rainfall (L/m2) | Humidity (%) | T (ng/g) | A4 (ng/g) | C (ng/g) | ||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Correlation value (ρ value) | −0.508 | −0.84 * | 0.723 * | 0.723 * | 0.349 | |
| p value | 0.064 | <0.01 | <0.01 | <0.01 | 0.221 | ||
| Rainfall (L/m2) | Correlation value (ρ value) | −0.508 | 0.748 * | −0.525 | −0.477 | −0.191 | |
| p value | 0.064 | <0.01 | 0.054 | 0.085 | 0.513 | ||
| Humidity (%) | Correlation value (ρ value) | −0.84 * | 0.748 * | −0.563 * | −0.524 | −0.383 | |
| p value | <0.01 | <0.01 | 0.036 | 0.055 | 0.177 | ||
| T (ng/g) | Correlation value (ρ value) | 0.723 * | −0.525 | −0.563 * | 0.982 * | 0.13 | |
| p value | <0.01 | 0.054 | 0.036 | <0.01 | 0.659 | ||
| A4 (ng/g) | Correlation value (ρ value) | 0.723 * | −0.477 | −0.524 | 0.982 * | 0.068 | |
| p value | <0.01 | 0.085 | 0.055 | <0.01 | 0.817 | ||
| C (ng/g) | Correlation value (ρ value) | 0.349 | −0.191 | −0.383 | 0.13 | 0.068 | |
| p value | 0.221 | 0.513 | 0.177 | 0.659 | 0.817 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Andrés, P.J.; Cáceres, S.; Crespo, B.; Silván, G.; Illera, J.C. Non-Invasive Determination of Annual Fecal Cortisol, Androstenedione, and Testosterone Variations in a Herd of Male Asian Elephants (Elephas maximus) and Their Relation to Some Climatic Variables. Animals 2021, 11, 2723. https://doi.org/10.3390/ani11092723
de Andrés PJ, Cáceres S, Crespo B, Silván G, Illera JC. Non-Invasive Determination of Annual Fecal Cortisol, Androstenedione, and Testosterone Variations in a Herd of Male Asian Elephants (Elephas maximus) and Their Relation to Some Climatic Variables. Animals. 2021; 11(9):2723. https://doi.org/10.3390/ani11092723
Chicago/Turabian Stylede Andrés, Paloma Jimena, Sara Cáceres, Belén Crespo, Gema Silván, and Juan Carlos Illera. 2021. "Non-Invasive Determination of Annual Fecal Cortisol, Androstenedione, and Testosterone Variations in a Herd of Male Asian Elephants (Elephas maximus) and Their Relation to Some Climatic Variables" Animals 11, no. 9: 2723. https://doi.org/10.3390/ani11092723
APA Stylede Andrés, P. J., Cáceres, S., Crespo, B., Silván, G., & Illera, J. C. (2021). Non-Invasive Determination of Annual Fecal Cortisol, Androstenedione, and Testosterone Variations in a Herd of Male Asian Elephants (Elephas maximus) and Their Relation to Some Climatic Variables. Animals, 11(9), 2723. https://doi.org/10.3390/ani11092723








