A Methodology to Quantify Resilience in Growing Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
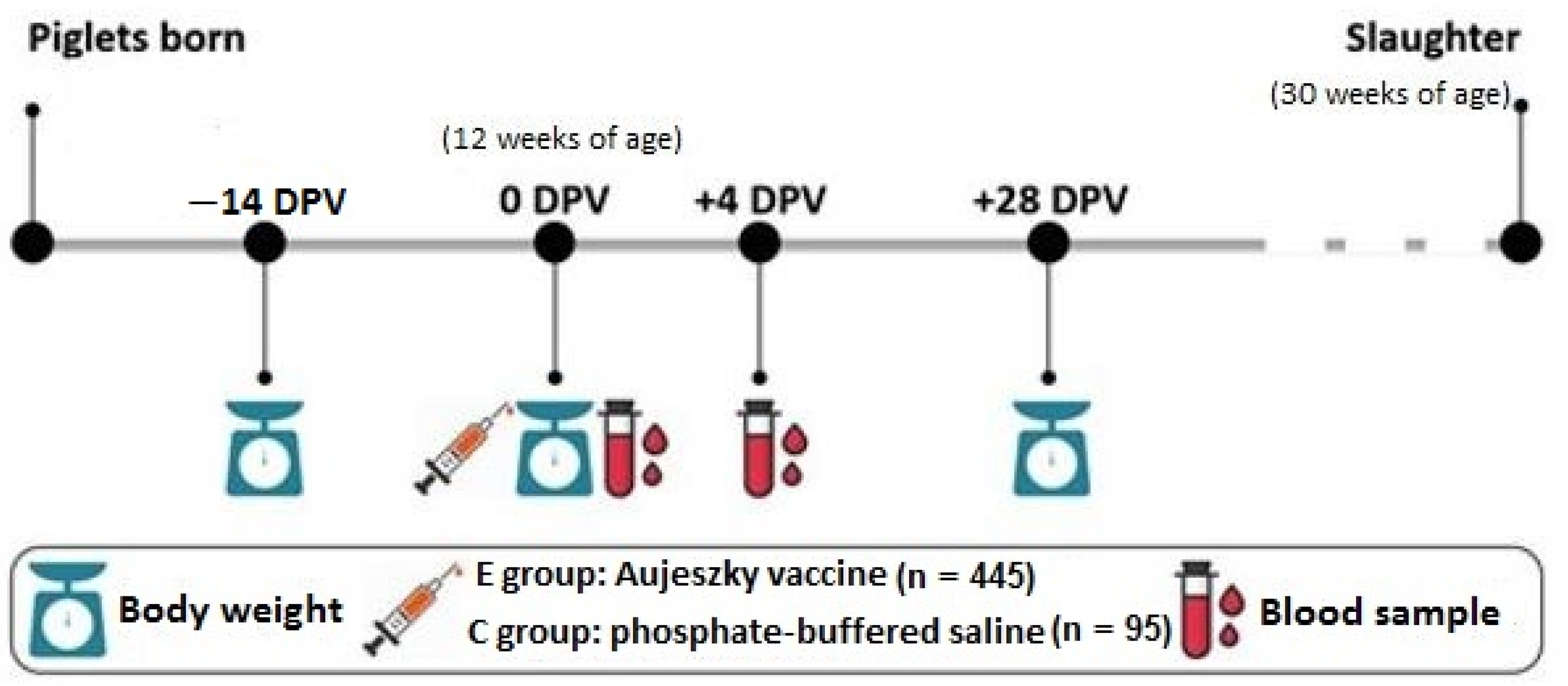
2.1. Animals and Experimental Design
2.2. Determination of the Acute-Phase Protein Haptoglobin
2.3. Proposal of Resilience Indicators
2.3.1. Body Weight Deviation
2.3.2. Increment of Haptoglobin after the Vaccine Challenge
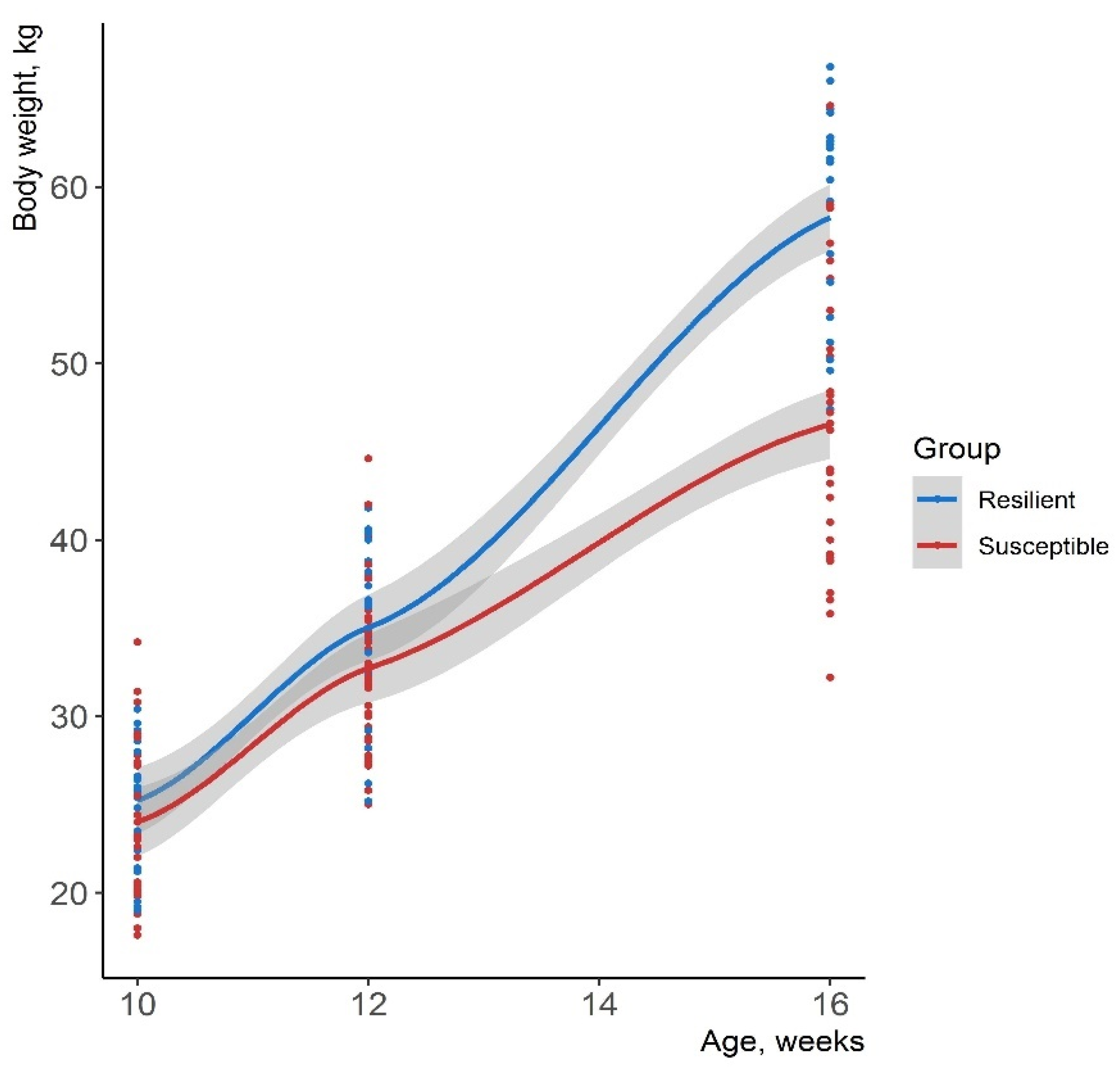
2.4. Classification of Pigs into Resilient and Susceptible Groups
- Pigs were classified based on the combination of resilience indicators ∆BW and ∆HP as suggested by Bai et al. [23]. Pigs with ∆BW above the third quartile (Q3) and ∆HP below the first quartile (Q1) were classified as R. In contrast, pigs with ∆BW below Q1 and ∆HP above Q3 were classified as S. The rest of the animals were considered as A.
- Pigs were classified based on %BW and %HP following the same previous quartile distribution to examine whether ∆BW and ∆HP are sensitive to the animal’s expected BW and the basal level of HP.
- Pigs were classified based on ∆BWADG and ∆HP following the same previous quartile distribution.
- Pigs were classified based on the observed BW28 to examine whether ∆BW and ∆HP captured more information than the differences in the observed BW28.
2.5. Heritability Estimates
3. Results
3.1. Descriptive Statistics for the Novel Resilience Indicators
3.2. Classification of Pigs’ Resilience
3.3. Heritability Estimates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations; FAOSTAT Statistical Database: Rome, Italy, 2019. [Google Scholar]
- Colditz, I.G.; Hine, B.C. Resilience in farm animals: Biology, management, breeding and implications for animal welfare. Anim. Prod. Sci. 2016, 56, 1961–1983. [Google Scholar] [CrossRef]
- Iung, L.H.D.S.; Carvalheiro, R.; Neves, H.H.D.R.; Mulder, H.A. Genetics and genomics of uniformity and resilience in livestock and aquaculture species: A review. J. Anim. Breed. Genet. 2020, 137, 263–280. [Google Scholar] [CrossRef]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Berghof, T.V.L.; Bovenhuis, H.; Mulder, H.A. Body Weight Deviations as Indicator for Resilience in Layer Chickens. Front. Genet. 2019, 10, 1216. [Google Scholar] [CrossRef] [Green Version]
- Putz, A.M.; Harding, J.C.S.; Dyck, M.K.; Fortin, F.; Plastow, G.S.; Dekkers, J.C.M. Novel Resilience Phenotypes Using Feed Intake Data From a Natural Disease Challenge Model in Wean-to-Finish Pigs. Front. Genet. 2019, 9, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argente, M.J.; García, M.L.; Zbyňovská, K.; Petruška, P.; Capcarová, M.; Blasco, A. Correlated response to selection for litter size environmental variability in rabbits’ resilience. Animal 2019, 13, 2348–2355. [Google Scholar] [CrossRef]
- Elgersma, G.G.; de Jong, G.; van der Linde, R.; Mulder, H.A. Fluctuations in milk yield are heritable and can be used as a resilience indicator to breed healthy cows. J. Dairy Sci. 2018, 101, 1240–1250. [Google Scholar] [CrossRef]
- Poppe, M.; Bonekamp, G.; van Pelt, M.L.; Mulder, H.A. Genetic analysis of resilience indicators based on milk yield records in different lactations and at different lactation stages. J. Dairy Sci. 2021, 104, 1967–1981. [Google Scholar] [CrossRef]
- Dantzer, R.; Cohen, S.; Russo, S.J.; Dinan, T.G. Resilience and immunity. Brain. Behav. Immun. 2018, 74, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.I.; Tonelli, L.; Sternberg, E.M. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 2002, 20, 125–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef]
- Guy, S.Z.Y.; Li, L.; Thomson, P.C.; Hermesch, S. Quantifying the health challenges in an Australian piggery using medication records for the definition of disease resilience1. J. Anim. Sci. 2019, 97, 1076–1089. [Google Scholar] [CrossRef]
- Chen, Y.; Tibbs Cortes, L.E.; Ashley, C.; Putz, A.M.; Lim, K.S.; Dyck, M.K.; Fortin, F.; Plastow, G.S.; Dekkers, J.C.M.; Harding, J.C.S.; et al. The genetic basis of natural antibody titers of young healthy pigs and relationships with disease resilience. BMC Genom. 2020, 21, 648. [Google Scholar] [CrossRef]
- Murata, H.; Shimada, N.; Yoshioka, M. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef]
- Cray, C. Acute phase proteins in animals. In Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2012; Volume 105, pp. 113–150. [Google Scholar]
- Klauke, T.N.; Piñeiro, M.; Schulze-Geisthövel, S.; Plattes, S.; Selhorst, T.; Petersen, B. Coherence of animal health, welfare and carcass quality in pork production chains. Meat Sci. 2013, 95, 704–711. [Google Scholar] [CrossRef]
- Fraile, L.; Saco, Y.; Grau-Roma, L.; Nofrarías, M.; López-Soria, S.; Sibila, M.; Callén, A.; Bassols, A.; Segalés, J. Serum haptoglobin dynamics in pigs vaccinated or not vaccinated against porcine circovirus type 2. Porc. Health Manag. 2015, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Lipowski, A. Evaluation of efficacy and safety of Aujeszky’s disease vaccines. Pol. J. Vet. Sci. 2006, 9, 75–79. [Google Scholar] [PubMed]
- Saco, Y.; Fraile, L.; Giménez, M.; Pato, R.; Montoya, M.; Bassols, A. Haptoglobin serum concentration is a suitable biomarker to assess the efficacy of a feed additive in pigs. Animal 2010, 4, 1561–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, M.D.; Fuentes, P.; Tecles, F.; Martínez-Subiela, S.; Martínez, J.S.; Muñoz, A.; Cerón, J.J. Porcine acute phase protein concentrations in different diseases in field conditions. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2006, 53, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Carpintero, R.; Alonso, C.; Piñeiro, M.; Iturralde, M.; Andrés, M.; Le Potier, M.F.; Madec, F.; Álava, M.Á.; Piñeiro, A.; Lampreave, F. Pig major acute-phase protein and apolipoprotein A-I responses correlate with the clinical course of experimentally induced African swine fever and Aujeszky’s disease. Vet. Res. 2007, 38, 741–753. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Putz, A.M.; Wang, Z.; Fortin, F.; Harding, J.C.S.; Dyck, M.K.; Dekkers, J.C.M.; Field, C.J.; Plastow, G.S.; Canada, P.G. Exploring Phenotypes for Disease Resilience in Pigs Using Complete Blood Count Data From a Natural Disease Challenge Model. Front. Genet. 2020, 11, 216. [Google Scholar] [CrossRef]
- Thrusfield, M.; Christley, R.; Brown, H.; Diggle, P.J.; French, N.; Howe, K.; Kelly, L.; O’Connor, A.; Sargeant, J.; Wood, H. Veterinary Epidemiology; John Wiley & Sons, Ltd.: Chichester, UK, 2018; ISBN 9781118280249. [Google Scholar]
- Legarra, A.; Varona, L.; de Maturana, E.L. TM: Threshold Model. 2008. Available online: http://genoweb.toulouse.inra.fr/~alegarra/tm_folder/ (accessed on 1 September 2021).
- Friggens, N.C.; Blanc, F.; Berry, D.P.; Puillet, L. Review: Deciphering animal robustness. A synthesis to facilitate its use in livestock breeding and management. Animal 2017, 11, 2237–2251. [Google Scholar] [CrossRef] [Green Version]
- Rothschild, M.F.; Hill, H.T.; Christian, L.L.; Warner, C.M. Genetic differences in serum-neutralization titers of pigs after vaccination with pseudorabies modified live-virus vaccine. Am. J. Vet. Res. 1984, 45, 1216–1218. [Google Scholar] [PubMed]
- Zanella, R.; Gava, D.; de Oliveira Peixoto, J.; Schaefer, R.; Ciacci-Zanella, J.R.; Biondo, N.; da Silva, M.V.G.B.; Cantão, M.E.; Ledur, M.C. Unravelling the genetic components involved in the immune response of pigs vaccinated against influenza virus. Virus Res. 2015, 210, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Abella, G.; Pena, R.N.; Nogareda, C.; Armengol, R.; Vidal, A.; Moradell, L.; Tarancon, V.; Novell, E.; Estany, J.; Fraile, L. A WUR SNP is associated with European Porcine Reproductive and Respiratory Virus Syndrome resistance and growth performance in pigs. Res. Vet. Sci. 2016, 104, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeker, D.L.; Rothschild, M.F.; Christian, L.L.; Warner, C.M.; Hill, H.T. Genetic Control of Immune Response to Pseudorabies and Atrophic Rhinitis Vaccines: II: Comparison of Additive Direct and Maternal Genetic Effects. J. Anim. Sci. 1987, 64, 414–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauw, W.M. Immune response from a resource allocation perspective. Front. Genet. 2012, 3, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros-Freixedes, R.; Reixach, J.; Tor, M.; Estany, J. Expected genetic response for oleic acid content in pork. J. Anim. Sci. 2012, 90, 4230–4238. [Google Scholar] [CrossRef]
- Clapperton, M.; Diack, A.B.; Matika, O.; Glass, E.J.; Gladney, C.D.; Mellencamp, M.A.; Hoste, A.; Bishop, S.C. Traits associated with innate and adaptive immunity in pigs: Heritability and associations with performance under different health status conditions. Genet. Sel. Evol. 2009, 41, 54. [Google Scholar] [CrossRef] [Green Version]
- Henryon, M.; Heegaard, P.M.H.; Nielsen, J.; Berg, P.; Juul-Madsen, H.R. Immunological traits have the potential to improve selection of pigs for resistance to clinical and subclinical disease. Anim. Sci. 2006, 82, 596–606. [Google Scholar] [CrossRef]


| Group | Experimental Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Batch | N | ADG | HP | N | ADG | HP |
| 1 | 84 | 594.3 (24.9) | 0.70 (70.5) | 20 | 556.2 (21.5) | 1.09 (54.7) |
| 2 | 95 | 624.8 (17.0) | 1.48 (58.0) | 16 | 707.7 (14.1) | 1.59 (31.9) |
| 3 | 86 | 756.2 (10.6) | 1.95 (42.0) | 21 | 768.4 (10.7) | 2.07 (33.1) |
| 4 | 86 | 756.0 (11.9) | 1.08 (55.3) | 23 | 794.7 (4.9) | 1.07 (53.5) |
| 5 | 94 | 662.4 (16.7) | 1.40 (37.3) | 15 | 749.3 (16.2) | 1.30 (39.3) |
| All batches | 445 | 677.7 (18.8) | 1.33 (59.4) | 95 | 716.9 (17.8) | 1.42 (49.1) |
| Trait | Mean | SD 1 | Min | Max |
|---|---|---|---|---|
| ∆BW 2 (kg) | −0.68 | 3.64 | −13.2 | +10.1 |
| %BW 3 (%) | −1.42 | 7.26 | −24.4 | +19.3 |
| ∆HP 4 (mg/mL) | +0.03 | 0.70 | −1.41 | +2.65 |
| %HP 5 (%) | +5.40 | 60.4 | −89.2 | +292 |
| Trait | ∆BW 1 | ∆HP 2 | %BW 3 | %HP 4 | ∆BWADG 5 |
|---|---|---|---|---|---|
| ∆BW | 1 | −0.09 * | 0.99 *** | −0.14 ** | 0.52 *** |
| ∆HP | 1 | −0.09 ns | 0.94 *** | −0.02 ns | |
| %BW | 1 | −0.14 ** | 0.51 *** | ||
| %HP | 1 | −0.05 ns |
| Trait | Group | Mean | SD |
|---|---|---|---|
| ∆BW 1 (kg) | R | +3.54 | 1.42 |
| S | −6.00 | 2.44 | |
| %BW 2 (%) | R | +6.60 | 2.81 |
| S | −11.7 | 5.13 | |
| ∆HP 3 (mg/mL) | R | −0.71 | 0.18 |
| S | +1.17 | 0.61 | |
| %HP 4 (%) | R | −61.2 | 18.9 |
| S | +108.4 | 68.2 |
| Trait | Mean 1 | P0.10 2 | HPD95% 3 |
|---|---|---|---|
| ∆BW 4 | 0.33 | 0.94 | 0.02–0.65 |
| %BW 5 | 0.37 | 0.93 | 0.02–0.74 |
| ∆HP 6 | 0.16 | 0.66 | 0.00–0.38 |
| %HP 7 | 0.13 | 0.53 | 0.00–0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laghouaouta, H.; Pena, R.N.; Ros-Freixedes, R.; Reixach, J.; Díaz, M.; Estany, J.; Armengol, R.; Bassols, A.; Fraile, L. A Methodology to Quantify Resilience in Growing Pigs. Animals 2021, 11, 2970. https://doi.org/10.3390/ani11102970
Laghouaouta H, Pena RN, Ros-Freixedes R, Reixach J, Díaz M, Estany J, Armengol R, Bassols A, Fraile L. A Methodology to Quantify Resilience in Growing Pigs. Animals. 2021; 11(10):2970. https://doi.org/10.3390/ani11102970
Chicago/Turabian StyleLaghouaouta, Houda, Ramona N. Pena, Roger Ros-Freixedes, Josep Reixach, Marta Díaz, Joan Estany, Ramon Armengol, Anna Bassols, and Lorenzo Fraile. 2021. "A Methodology to Quantify Resilience in Growing Pigs" Animals 11, no. 10: 2970. https://doi.org/10.3390/ani11102970






