The Utilisation of Tannin Extract as a Dietary Additive in Ruminant Nutrition: A Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
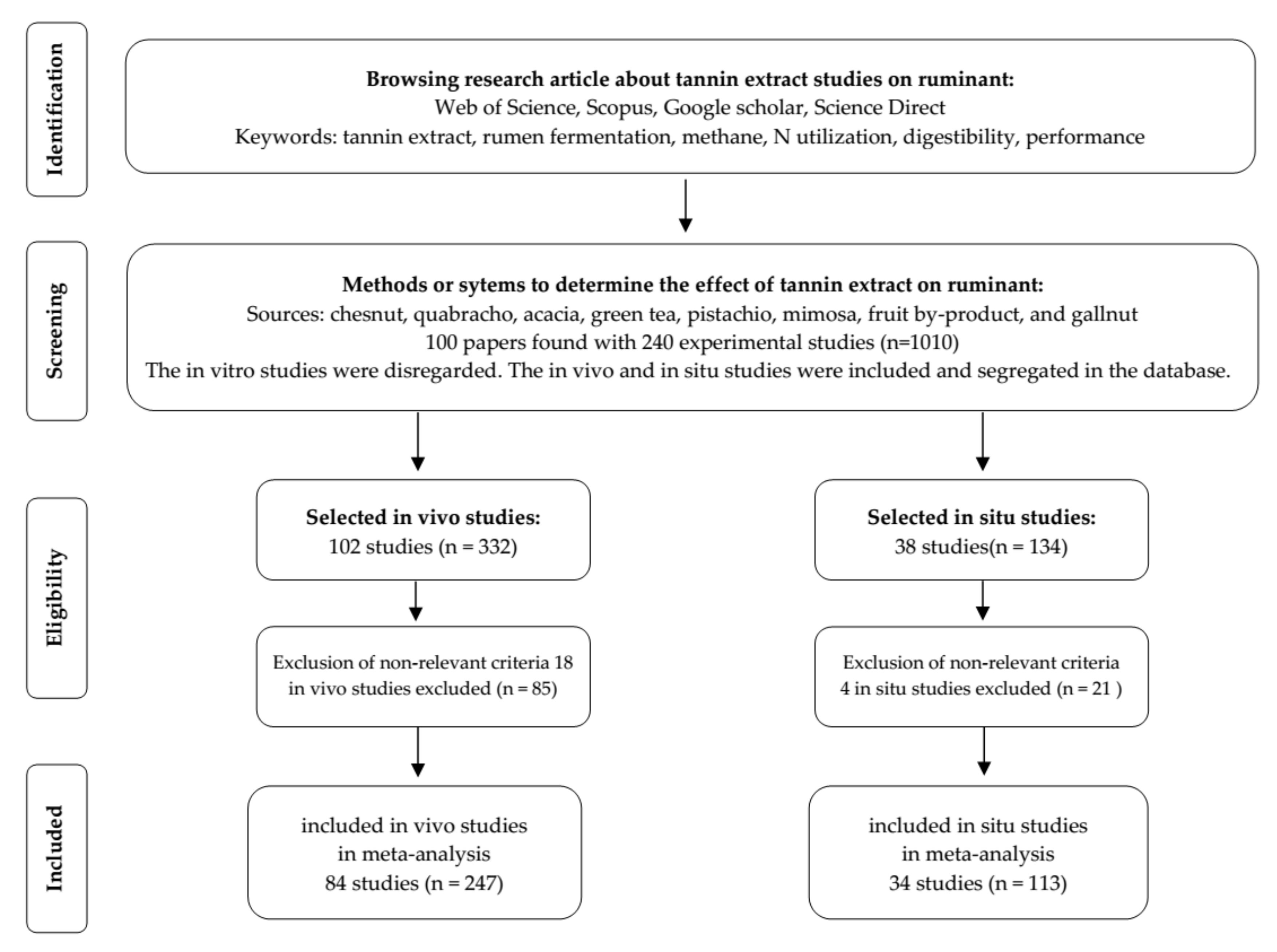
2. Materials and Methods
2.1. Database Development
2.2. Statistical Analysis
3. Results
4. Discussion
4.1. Influence of Tannin Extract on Performance, Digestibility, Rumen Parameters, Milk Production, and Methane Production
4.2. Influence of Tannin Extract on Ruminal N Digestibility, Blood Plasma, N Utilisation, and Urinary Purine Derivative of Ruminants
4.3. Influence of Tannin Extract on Kinetics Degradability In Situ
4.4. Noticeable Effect by the Divergence between Tannin Extracts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henke, A.; Westreicher-Kristen, E.; Molkentin, J.; Dickhoefer, U.; Knappstein, K.; Hasler, M.; Susenbeth, A. Effect of dietary quebracho tannin extract on milk fatty acid composition in cows. J. Dairy Sci. 2017, 100, 6229–6238. [Google Scholar] [CrossRef] [PubMed]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M.; Morgavi, D.P. Replacing urea with nitrate as a non-protein nitrogen source increases lambs’ growth and reduces methane production, whereas acacia tannin has no effect. Anim. Feed Sci. Technol. 2020, 259, 114360. [Google Scholar] [CrossRef]
- Avila, A.S.; Zambom, M.A.; Faccenda, A.; Fischer, M.L.; Anschau, F.A.; Venturini, T.; Tinini, R.C.R.; Dessbesell, J.G.; Faciola, A.P. Effects of black wattle (Acacia mearnsii) condensed tannins on intake, protozoa population, ruminal fermentation, and nutrient digestibility in jersey steers. Animals 2020, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Pal, K.; Patra, A.; Sahoo, A.; Soren, N.M. Effects of nitrate and fumarate in tree leaves-based diets on nutrient utilizaton, rumen fermentation, microbial protein supply and and blood profiles in sheep. Livest. Sci. 2015, 172, 5–15. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M. Effect of lipid-encapsulated acacia tannin extract on feed intake, nutrient digestibility and methane emission in sheep. Animals 2019, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2012, 96, 365–375. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; p. 1. [Google Scholar]
- Yanza, Y.R.; Szumacher-Strabel, M.; Jayanegara, A.; Kasenta, A.M.; Gao, M.; Huang, H.; Patra, A.K.; Warzych, E.; Cieślak, A. The effects of dietary medium-chain fatty acids on ruminal methanogenesis and fermentation in vitro and in vivo: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2021, 105, 874–889. [Google Scholar] [CrossRef]
- Zhang, F.; Li, B.; Ban, Z.; Liang, H.; Li, L.; Zhao, W.; Yan, X. Evaluation of origanum oil, hydrolysable tannins and tea saponin in mitigating ruminant methane: In vitro and in vivo methods. J. Anim. Physiol. Anim. Nutr. 2021, 105, 630–638. [Google Scholar] [CrossRef]
- Pineiro-Vazquez, A.T.; Jimenez-Ferrer, G.; Alayon-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Perez, C.F.; Ku-Vera, J.C. Effects of quebracho tannin extract on intake, digestibility, rumen fermentation, and methane production in crossbred heifers fed low-quality tropical grass. Trop. Anim. Health Prod. 2018, 50, 29–36. [Google Scholar] [CrossRef]
- Jayanegara, A.; Sujarnoko, T.U.P.; Ridla, M.; Kondo, M.; Kreuzer, M. Silage quality as influenced by concentration and type of tannins present in the material ensiled: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 456–465. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Capozzolo, M.C.; Lencioni, P.; Cabral, C.; Wattiaux, M.A. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 2016, 99, 4476–4486. [Google Scholar] [CrossRef]
- Ahnert, S.; Dickhoefer, U.; Schulz, F.; Susenbeth, A. Influence of ruminal Quebracho tannin extract infusion on apparent nutrient digestibility, nitrogen balance, and urinary purine derivatives excretion in heifers. Livest. Sci. 2015, 177, 63–70. [Google Scholar] [CrossRef]
- Alipour, D.; Rouzbehan, Y. Effects of several levels of extracted tannin from grape pomace on intestinal digestibility of soybean meal. Livest. Sci. 2010, 128, 87–91. [Google Scholar] [CrossRef]
- Al-Kindi, A.; Dickhoefer, U.; Schlecht, E.; Sundrum, A.; Schiborra, A. Effects of quebracho tannin extract (Schinopsis balansae Engl.) and activated charcoal on nitrogen balance, rumen microbial protein synthesis and faecal composition of growing Boer goats. Arch. Anim. Nutr. 2016, 70, 307–321. [Google Scholar] [CrossRef]
- Aprianita, A.; Donkor, O.N.; Moate, P.J.; Williams, S.R.O.; Auldist, M.J.; Greenwood, J.S.; Hannah, M.C.; Wales, W.J.; Vasiljevic, T. Effects of dietary cottonseed oil and tannin supplements on protein and fatty acid composition of bovine milk. J. Dairy Res. 2014, 81, 183–192. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef]
- Abo-Donia, F.M.; Yang, L.Y.; Hristov, A.N.; Wang, M.; Tang, S.X.; Zhou, C.S.; Han, X.F.; Kang, J.H.; Tan, Z.L.; He, Z.X. Effects of tannins on the fatty acid profiles of rumen fluids and milk from lactating goats fed a total mixed ration containing rapeseed oil. Livest. Sci. 2017, 204, 16–24. [Google Scholar] [CrossRef]
- Ávila, S.C.; Kozloski, G.V.; Orlandi, T.; Mezzomo, M.P.; Stefanello, S. Impact of a tannin extract on digestibility, ruminal fermentation and duodenal flow of amino acids in steers fed maize silage and concentrate containing soybean meal or canola meal as protein source. J. Agric. Sci. 2015, 153, 943–953. [Google Scholar] [CrossRef]
- Baah, J.; Ivan, M.; Hristov, A.N.; Koenig, K.M.; Rode, L.M.; McAllister, T.A. Effects of potential dietary antiprotozoal supplements on rumen fermentation and digestibility in heifers. Anim. Feed Sci. Technol. 2007, 137, 126–137. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef]
- Benchaar, C.; McAllister, T.A.; Chouinard, P.Y. Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schidigera saponin extracts. J. Dairy Sci. 2008, 91, 4765–4777. [Google Scholar] [CrossRef]
- Buccioni, A.; Pauselli, M.; Viti, C.; Minieri, S.; Pallara, G.; Roscini, V.; Rapaccini, S.; Marinucci, M.T.; Lupi, P.; Conte, G.; et al. Milk fatty acid composition, rumen microbial population, and animal performances in response to diets rich in linoleic acid supplemented with chestnut or quebracho tannins in dairy ewes. J. Dairy Sci. 2015, 98, 1145–1156. [Google Scholar] [CrossRef]
- Buccioni, A.; Serra, A.; Minieri, S.; Mannelli, F.; Cappucci, A.; Benvenuti, D.; Rapaccini, S.; Conte, G.; Mele, M. Milk production, composition, and milk fatty acid profile from grazing sheep fed diets supplemented with chestnut tannin extract and extruded linseed. Small Rumin. Res. 2015, 130, 200–207. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Henke, A.; Molkentin, J.; Knappstein, K.; Susenbeth, A.; Dickhoefer, U. Relationship between milk odd and branched-chain fatty acids and urinary purine derivatives in dairy cows supplemented with quebracho tannins-A study to test milk fatty acids as predictors of rumen microbial protein synthesis. Anim. Feed Sci. Technol. 2016, 214, 22–33. [Google Scholar] [CrossRef]
- Cieslak, A.; Zmora, P.; Pers-Kamczyc, E.; Szumacher-Strabel, M. Effects of tannins source (Vaccinium vitis idaea L.) on rumen microbial fermentation in vivo. Anim. Feed Sci. Technol. 2012, 176, 102–106. [Google Scholar] [CrossRef]
- Colombini, S.; Colombari, G.; Crovetto, G.M.; Galassi, G.; Rapetti, L. Tannin treated lucerne silage in dairy cow feeding. Ital. J. Anim. Sci. 2009, 8, 289–291. [Google Scholar] [CrossRef]
- Dallastra, L.J.H.; Alves, T.P.; Dal-Pizzol, J.; Fonseca, B.L.; Camera, M.; Raupp, G.T.; Nunes Ribeiro-Filho, H.M. Tannin extract of Acacia mearnsii for lactating ewes. Semin. Agrar. 2018, 39, 2741–2748. [Google Scholar] [CrossRef]
- Deaville, E.R.; Givens, D.I.; Mueller-Harvey, I. Chestnut and mimosa tannin silages: Effects in sheep differ for apparent digestibility, nitrogen utilisation and losses. Anim. Feed Sci. Technol. 2010, 157, 129–138. [Google Scholar] [CrossRef]
- Denninger, T.M.; Schwarm, A.; Birkinshaw, A.; Terranova, M.; Dohme-Meier, F.; Münger, A.; Eggerschwiler, L.; Bapst, B.; Wegmann, S.; Clauss, M.; et al. Immediate effect of Acacia mearnsii tannins on methane emissions and milk fatty acid profiles of dairy cows. Anim. Feed Sci. Technol. 2020, 261, 114388. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Moreira, O.C.; Pereira, M.S.; Bessa, R.J.B. The use of a tannin crude extract from Cistus ladanifer L. to protect soya-bean protein from degradation in the rumen. Animal 2007, 1, 645–650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dentinho, M.T.P.; Belo, A.T.; Bessa, R.J.B. Digestion, ruminal fermentation and microbial nitrogen supply in sheep fed soybean meal treated with Cistus ladanifer L. tannins. Small Rumin. Res. 2014, 119, 57–64. [Google Scholar] [CrossRef]
- Dentinho, M.T.P.; Paulos, K.; Francisco, A.; Belo, A.T.; Jerónimo, E.; Almeida, J.; Bessa, R.J.B.; Santos-Silva, J. Effect of soybean meal treatment with Cistus ladanifer condensed tannins in growth performance, carcass and meat quality of lambs. Livest. Sci. 2020, 236, 104021. [Google Scholar] [CrossRef]
- Dickhoefer, U.; Ahnert, S.; Susenbeth, A. Effects of quebracho tannin extract on rumen fermentation and yield and composition of microbial mass in heifers. J. Anim. Sci. 2016, 94, 1561–1575. [Google Scholar] [CrossRef]
- Dschaak, C.M.; Williams, C.M.; Holt, M.S.; Eun, J.S.; Young, A.J.; Min, B.R. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef]
- Duval, B.D.; Aguerre, M.; Wattiaux, M.; Vadas, P.A.; Powell, J.M. Potential for Reducing On-Farm Greenhouse Gas and Ammonia Emissions from Dairy Cows with Prolonged Dietary Tannin Additions. Water. Air. Soil Pollut. 2016, 227, 329. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Fernández, M.; Mantecón, A.R. Digestive utilisation of quebracho-treated soya bean meals in sheep. J. Agric. Sci. 2000, 134, 101–108. [Google Scholar] [CrossRef][Green Version]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef]
- Griffiths, W.M.; Clark, C.E.F.; Clark, D.A.; Waghorn, G.C. Supplementing lactating dairy cows fed high-quality pasture with black wattle (Acacia mearnsii) tannin. Animal 2013, 7, 1789–1795. [Google Scholar] [CrossRef][Green Version]
- Henke, A.; Dickhoefer, U.; Westreicher-Kristen, E.; Knappstein, K.; Molkentin, J.; Hasler, M.; Susenbeth, A. Effect of dietary Quebracho tannin extract on feed intake, digestibility, excretion of urinary purine derivatives and milk production in dairy cows. Arch. Anim. Nutr. 2016, 71, 37–53. [Google Scholar] [CrossRef]
- Herremans, S.; Decruyenaere, V.; Cantalapiedra-Hijar, G.; Beckers, Y.; Froidmont, E. Effects of hydrolysable tannin-treated grass silage on milk yield and composition, nitrogen partitioning and nitrogen isotopic discrimination in lactating dairy cows. Animal 2020, 14, 771–779. [Google Scholar] [CrossRef]
- Hervas, G.; Frutos, P.; Serrano, E.; Mantecon, A.R.; Giraldez, F.J. Effect of tannic acid on rumen degradation and intestinal digestion of treated soya bean meals in sheep. J. Agric. Sci. 2000, 135, 305–310. [Google Scholar] [CrossRef][Green Version]
- Hervás, G.; Frutos, P.; Ramos, G.; Giráldez, F.J.; Mantecón, A.R. Intraruminal administration of two doses of quebracho tannins to sheep: Effect on rumen degradation and total tract digestibility, faecal recovery and toxicity. J. Anim. Feed Sci. 2004, 13, 111–120. [Google Scholar] [CrossRef][Green Version]
- Jolazadeh, A.R.; Dehghan-banadaky, M.; Rezayazdi, K. Effects of soybean meal treated with tannins extracted from pistachio hulls on performance, ruminal fermentation, blood metabolites and nutrient digestion of Holstein bulls. Anim. Feed Sci. Technol. 2015, 203, 33–40. [Google Scholar] [CrossRef]
- Perna Junior, F.; Vásquez, D.C.Z.; Gardinal, R.; Meyer, P.M.; Berndt, A.; Friguetto, R.T.S.; de Abreu Demarchi, J.J.A.; Rodrigues, P.H.M. Short-term use of monensin and tannins as feed additives on digestibility and methanogenesis in cattle. Rev. Bras. Zootec. 2020, 49. [Google Scholar] [CrossRef]
- Koenig, K.M.; Beauchemin, K.A. Effect of feeding condensed tannins in high protein finishing diets containing corn distillers grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. J. Anim. Sci. 2018, 96, 4398–4413. [Google Scholar] [CrossRef]
- Komolong, M.K.; Barber, D.G.; McNeill, D.M. Post-ruminal protein supply and N retention of weaner sheep fed on a basal diet of lucerne hay (Medicago sativa) with increasing levels of quebracho tannins. Anim. Feed Sci. Technol. 2001, 92, 59–72. [Google Scholar] [CrossRef]
- Kozloski, G.V.; Härter, C.J.; Hentz, F.; de ávila, S.C.; Orlandi, T.; Stefanello, C.M. Intake, digestibility and nutrients supply to wethers fed ryegrass and intraruminally infused with levels of Acacia mearnsii tannin extract. Small Rumin. Res. 2012, 106, 125–130. [Google Scholar] [CrossRef]
- Krueger, W.K.; Gutierrez-Bañuelos, H.; Carstens, G.E.; Min, B.R.; Pinchak, W.E.; Gomez, R.R.; Anderson, R.C.; Krueger, N.A.; Forbes, T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 2010, 159, 1–9. [Google Scholar] [CrossRef]
- Lima, P.R.; Apdini, T.; Freire, A.S.; Santana, A.S.; Moura, L.M.L.; Nascimento, J.C.S.; Rodrigues, R.T.S.; Dijkstra, J.; Garcez Neto, A.F.; Queiroz, M.A.Á.; et al. Dietary supplementation with tannin and soybean oil on intake, digestibility, feeding behavior, ruminal protozoa and methane emission in sheep. Anim. Feed Sci. Technol. 2019, 249, 10–17. [Google Scholar] [CrossRef]
- Liu, H.W.; Zhou, D.W.; Li, K. Effects of chestnut tannins on performance and antioxidative status of transition dairy cows. J. Dairy Sci. 2013, 96, 5901–5907. [Google Scholar] [CrossRef] [PubMed]
- Martínez, T.F.; Moyano, F.J.; Díaz, M.; Barroso, F.G.; Alarcón, F.J. Ruminal degradation of tannin-treated legume meals. J. Sci. Food Agric. 2004, 84, 1979–1987. [Google Scholar] [CrossRef]
- Mezzomo, R.; Paulino, P.V.R.; Detmann, E.; Valadares Filho, S.C.; Paulino, M.F.; Monnerat, J.P.I.S.; Duarte, M.S.; Silva, L.H.P.; Moura, L.S. Influence of condensed tannin on intake, digestibility, and efficiency of protein utilisation in beef steers fed high concentrate diet. Livest. Sci. 2011, 141, 1–11. [Google Scholar] [CrossRef]
- Mezzomo, R.; Paulino, P.V.R.; Barbosa, M.M.; da Silva Martins, T.; Paulino, M.F.; Alves, K.S.; Gomes, D.I.; dos Santos Monnerat, J.P.I. Performance and carcass characteristics of young cattle fed with soybean meal treated with tannins. Anim. Sci. J. 2016, 87, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarpour, A.; Naserian, A.A.; Pourmollae, F.; Ghaffari, M.H. Effect of treating alfalfa silage with pistachio by-products extract on Saanen dairy goats performance and microbial nitrogen synthesis. J. Anim. Physiol. Anim. Nutr. 2016, 100, 758–767. [Google Scholar] [CrossRef]
- Nasehi, M.; Torbatinejad, N.M.; Rezaie, M.; Ghoorchi, T. The effect of green tea waste extract on ruminal degradability and intestinal digestibility of barley grain. Turk. J. Vet. Anim. Sci. 2018, 42, 624–632. [Google Scholar] [CrossRef]
- Norris, A.B.; Crossland, W.L.; Tedeschi, L.O.; Foster, J.L.; Muir, J.P.; Pinchak, W.E.; Fonseca, M.A. Inclusion of quebracho tannin extract in a high-roughage cattle diet alters digestibility, nitrogen balance, and energy partitioning. J. Anim. Sci. 2020, 98, skaa047. [Google Scholar] [CrossRef]
- Orlandi, T.; Kozloski, G.V.; Alves, T.P.; Mesquita, F.R.; Ávila, S.C. Digestibility, ruminal fermentation and duodenal flux of amino acids in steers fed grass forage plus concentrate containing increasing levels of Acacia mearnsii tannin extract. Anim. Feed Sci. Technol. 2015, 210, 37–45. [Google Scholar] [CrossRef]
- Orlandi, T.; Stefanello, S.; Mezzomo, M.P.; Pozo, C.A.; Kozloski, G.V. Impact of a tannin extract on digestibility and net flux of metabolites across splanchnic tissues of sheep. Anim. Feed Sci. Technol. 2020, 261, 114384. [Google Scholar] [CrossRef]
- Orlandi, T.; Pozo, C.A.; Mezzomo, M.P.; Kozloski, G.V. Acacia mearnsii tannin extract as a feed additive: Impact on feed intake, digestibility and nitrogen excretion by sheep fed a tropical grass-based diet. Cienc. Rural 2020, 50, 1–6. [Google Scholar] [CrossRef]
- Perna Junior, F.; Cassiano, E.C.O.; Martins, M.F.; Romero, L.A.; Zapata, D.C.V.; Pinedo, L.A.; Marino, C.T.; Rodrigues, P.H.M. Effect of tannins-rich extract from Acacia mearnsii or monensin as feed additives on ruminal fermentation efficiency in cattle. Livest. Sci. 2017, 203, 21–29. [Google Scholar] [CrossRef]
- Poncet, C.; Rémond, D. Rumen digestion and intestinal nutrient flows in sheep consuming pea seeds: The effect of extrusion or chestnut tannin addition. Anim. Res. 2002, 51, 201–216. [Google Scholar] [CrossRef]
- Salami, S.A.; Valenti, B.; Bella, M.; O’Grady, M.N.; Luciano, G.; Kerry, J.P.; Jones, E.; Priolo, A.; Newbold, C.J. Characterisation of the ruminal fermentation and microbiome in lambs supplemented with hydrolysable and condensed tannins. FEMS Microbiol. Ecol. 2018, 94, 1–13. [Google Scholar] [CrossRef]
- Salawu, M.B.; Acamovic, T.; Stewart, C.S.; Hovell, F.D.D.B. Quebracho tannins with or without Browse Plus (a commercial preparation of polyethylene glycol) in sheep diets: Effect on digestibility of nutrients in vivo and degradation of grass hay in sacco and in vitro. Anim. Feed Sci. Technol. 1997, 69, 67–78. [Google Scholar] [CrossRef]
- Salawu, M.B.; Acamovic, T.; Stewart, C.S.; Hovell, F.D.D.B.; McKay, I. Assessment of the nutritive value of Calliandra calothyrsus: In sacco degradation and in vitro gas production in the presence of Quebracho tannins with or without Browse Plus. Anim. Feed Sci. Technol. 1997, 69, 219–232. [Google Scholar] [CrossRef]
- Salawu, M.B.; Acamovic, T.; Stewart, C.S.; Hvelplund, T.; Weisbjerg, M.R. The use of tannins as silage additives: Effects on silage composition and mobile bag disappearance of dry matter and protein. Anim. Feed Sci. Technol. 1999, 82, 243–259. [Google Scholar] [CrossRef]
- Shakeri, P.; Reiasi, A.; Tahmasbi, R. The effect of pistachio by-product extracts treatment in protecting soybean meal and canola meal protein from rumen microbial degradation. J. Sci. Food Agric. 2020, 100, 5222–5229. [Google Scholar] [CrossRef]
- Sharifi, A.; Chaji, M. Effects of processed recycled poultry bedding with tannins extracted from pomegranate peel on the nutrient digestibility and growth performance of lambs. S. Afr. J. Anim. Sci. 2019, 49, 291–300. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Kreuzer, M.; Wettstein, H.R.; Machmüller, A. Rumen fermentation and nitrogen balance of lambs fed diets containing plant extracts rich in tannins and saponins, and associated emissions of nitrogen and methane. Arch. Anim. Nutr. Tierernahr. 2002, 56, 379–392. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Kreuzer, M.; Sutter, F.; Machmüller, A.; Wettstein, H.R. Performance, body nitrogen conversion and nitrogen emission from manure of dairy cows fed diets supplemented with different plant extracts. J. Anim. Feed Sci. 2004, 13, 73–91. [Google Scholar] [CrossRef]
- Szczechowiak, J.; Szumacher-Strabel, M.; El-Sherbiny, M.; Pers-Kamczyc, E.; Pawlak, P.; Cieslak, A. Rumen fermentation, methane concentration and fatty acid proportion in the rumen and milk of dairy cows fed condensed tannin and/or fish-soybean oils blend. Anim. Feed Sci. Technol. 2016, 216, 93–107. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Bichi, E.; Belenguer, Á.; Frutos, P. Tannins as feed additives to modulate ruminal biohydrogenation: Effects on animal performance, milk fatty acid composition and ruminal fermentation in dairy ewes fed a diet containing sunflower oil. Anim. Feed Sci. Technol. 2011, 164, 199–206. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Belenguer, A.; Bichi, E.; Frutos, P. Effect of the inclusion of quebracho tannins in a diet rich in linoleic acid on milk fatty acid composition in dairy ewes. J. Dairy Sci. 2013, 96, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, S.A.; Cibils, A.F.; Estell, R.E.; Soto-Navarro, S.A.; Chen, L.; Hallford, D.M. Effects of adding protein, condensed tannins, and polyethylene glycol to diets of sheep and goats fed one-seed juniper and low quality roughage. Small Rumin. Res. 2013, 112, 56–68. [Google Scholar] [CrossRef]
- Wischer, G.; Greiling, A.M.; Boguhn, J.; Steingass, H.; Schollenberger, M.; Hartung, K.; Rodehutscord, M. Effects of long-term supplementation of chestnut and valonea extracts on methane release, digestibility and nitrogen excretion in sheep. Animal 2014, 8, 938–948. [Google Scholar] [CrossRef]
- Zimmer, N.; Cordesse, R. Digestibility and ruminal digestion of non-nitrogenous compounds in adult sheep and goats: Effects of chestnut tannins. Anim. Feed Sci. Technol. 1996, 61, 259–273. [Google Scholar] [CrossRef]
- Orskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- St-Pierre, N.R. Invited review. Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 2001, 84, 741–755. [Google Scholar] [CrossRef]
- Sauvant, D.; Schmidely, P.; Daudin, J.J.; St-Pierre, N.R. Metaanalyses of experimental data in animal nutrition. Animal 2008, 2, 1203–1214. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Estrada-Angulo, A.; Castro-Pérez, B.I.; Urías-Estrada, J.D.; Ríos-Rincón, F.G.; Arteaga-Wences, Y.J.; Barreras, A.; López-Soto, M.A.; Plascencia, A.; Zinn, R.A. Influence of protein level on growth performance, dietary energetics and carcass characteristics of Pelibuey × Katahdin lambs finished with isocaloric diets. Small. Rum. Res. 2018, 160, 59–64. [Google Scholar] [CrossRef]
- Yanza, Y.R.; Szumacher-Strabel, M.; Bryszak, M.; Gao, M.; Kolodziejski, P.; Stochmal, A.; Slusarczyk, S.; Patra, A.K.; Cieslak, A. Coleus amboinicus (Lour.) leaves as a modulator of ruminal methanogenesis and biohydrogenation in vitro. J. Anim. Sci. 2018, 96, 4868–4881. [Google Scholar] [CrossRef]
- Ślusarczyk, S.; Cieślak, A.; Yanza, Y.R.; Szumacher-Strabel, M.; Varadyova, Z.; Stafiniak, M.; Wojnicz, D.; Matkowski, A. Phytochemical Profile and Antioxidant Activities of Coleus amboinicus Lour. Cultivated in Indonesia and Poland. Molecules 2021, 26, 2915. [Google Scholar] [CrossRef]
- Cieslak, A.; Zmora, P.; Stochmal, A.; Pecio, L.; Oleszek, W.; Pers-Kamczyc, E.; Szczechowiak, J.; Nowak, A.; Szumacher-Strabel, M. Rumen antimethanogenic effect of Saponaria officinalis L. phytochemicals in vitro. J. Agric. Sci. 2014, 152, 981–983. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total Environ. 2016, 545–546, 556–558. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, 2–16. [Google Scholar] [CrossRef]
- Niderkorn, V.; Jayanegara, A. Opportunities Offered by Plant Bioactive Compounds to Improve Silage Quality, Animal Health and Product Quality for Sustainable Ruminant Production: A Review. Agronomy 2021, 11, 86. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the Conundrum of Tannins in Animal Nutrition and Health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Silanikove, N.; Perevolotsky, A.; Provenza, F.D. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim. Feed Sci. Technol. 2001, 91, 69–81. [Google Scholar] [CrossRef]
- Woodward, S.L.; Waghorn, G.C.; Laboyrie, P.G. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) reduce methane emissions from dairy cows. Proc. N. Z. Soc. Anim. Prod. 2004, 64, 160–164. [Google Scholar]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. De Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Ren, H.; Su, X.; Bai, H.; Yang, Y.; Wang, H.; Dan, Z.; Lu, J.; Wu, S.; Cai, C.; Cao, Y.; et al. Specific enrichment of microbes and increased ruminal propionate production: The potential mechanism underlying the high energy efficiency of Holstein heifers fed steam-flaked corn. AMB Express 2019, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Bae, H.D.; Jones, G.A.; Cheng, K.J. Microbial attachment and feed digestion in the rumen. J. Anim. Sci. 1994, 72, 3004–3018. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Irawan, A.; Noviandi, C.T.; Widyobroto, B.P.; Astuti, A.; Ates, S. Effect of Leucaena leucocephala and corn oil on ruminal fermentation, methane production and fatty acid profile: An in vitro study. Anim. Prod. Sci. 2021, 61, 459–469. [Google Scholar] [CrossRef]
- O’donovan, L.; Brooker, J.D. Effect of hydrolysable and condensed tannins on growth, morphology and metabolism of Streptococcus gallolyticus (S. caprinus) and Streptococcus bovis. Microbiology 2001, 147, 1025–1033. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Giráldez, F.J.; Mantecón, Á.R.; Álvarez Del Pino, M.C. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 2004, 109, 65–78. [Google Scholar] [CrossRef]
- Hill, G.D. Plant antinutritional factors. In Encyclopedia of Food Science & Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4578–4587. [Google Scholar] [CrossRef]
- Schofield, P.; Mbagua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
| Nr. | References Nr. | Experiment | Animal | Species and Status | Tannin Source | Tannin Type | Tannin Level (g/kg DM) | Adaptation/Exp.day (d) | Tannin Applied | Basal Feed |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [2] | in vivo | sheep | Merino | Silvafeed and A. mearnsii | CT | 0–50 | 14/26 | mixed in diet (TMR) | eragrostis and lucerne hay, and concentrate |
| 2 | [3] | in vivo | steer | Jersey | A. mearnsii | CT | 0–20 | 14/20 | mixed in diet | Tifton hay, corn, soybean meal |
| 3 | [6] | in vivo | lamb | South African Mutton × Merino | A. mearnsii | CT | 0–42 | 21/60 | mixed in diet | eragrostis, lucerne hay, sunflower meal, ground maize |
| 4 | [10] | in vivo | sheep | Han × Dorper, small tailed castrated | Gallnut | HT | 0–60 | 14/24 | added in diet | corn, soybean meal, wheat bran, rapeseed meal, rice bran, cottonseed meal, DDGS, alfalfa hay, and Chinese wildrye grass |
| 5 | [11] | in vivo | heifer | Crossbred | Quebracho | CT | 0–40 | 14/23 | added in diet | Pennisetum purpureum grass |
| 6 | [13] | in vivo | dairy cow | FH | Chestnut and Quebracho | CT and HT | 0–18 | 14/21 | mixed with TMR | alfalfa silage, corn silage, cottonseed, rice hulls (replaced with tannin) |
| 7 | [14] | in vivo | heifer | FH | Quebracho | CT | 0–60 | 42,248 | infusion intraruminally | hay and concentrate |
| 8 | [15] | in situ | sheep | Ghezel | Grape pomace | CT | 0–60 | 10/ns | mixed with feed | lucerne hay, wheat bran, and barley grain |
| 9 | [16] | in vivo | goat | Boer | Quebracho | CT | 0–40 | 21/27 | mixed with feed | grass hay, concentrate |
| 10 | [17] | in vivo | dairy cow | FH, lactating, multiparous | A. mearnsii | CT | 0–16 | 44,256 | administered via rumen-fistula | alfalfa hay, concentrate |
| 11 | [18] | in vivo | steer | Weaned Crossbred | Chestnut | HT | 0–15 | ns/114 | supplemented in diet | alfalfa and barley silage |
| 12 | [19] | in vivo | dairy goat | Liuyang black nannies, lactating, multiparous | Gallnut | HT | 0–9 | 14/42 | mixed in diet (TMR) | forage and concentrate (TMR) |
| 13 | [20] | in vivo | steer | FH | A. mearnsii | CT | 0–50 | 42,278 | mixed in diet | maize silage, soybean meal, canola meal (TMR) |
| 14 | [21] | in vivo | heifer | Jersey | Quebracho | CT | 0–6 | 14/47 | supplemented in diet | barley grain, barley silage, and canola meal |
| 15 | [22] | in vivo | heifer | Angus | Quebracho | CT | 0–20 | ns/28 | supplemented in diet | barley silage, barley grain, soybean meal, and corn gluten meal |
| 16 | [23] | in vivo and in situ | dairy cow | FH, lactating | Quebracho | CT | 0–4.5 | 15/28 | supplemented in diet | grass silage, corn, beet pulp, corn gluten meal, and wheat bran |
| 17 | [24] | in vivo | ewe | Comisana, multiparous | Chestnut and Quebracho | CT and HT | 0–52.8 | 15/28 | mixed in diet | barley, corn, wheat bran, soybean mela, beet pulp, and soybean oil |
| 18 | [25] | in vivo | ewe | Sarda, multiparous | Chestnut | HT | 0–80 | 21/49 | mixed in diet | ryegrass, oat, and white clover |
| 19 | [26] | in vivo | dairy cow | FH, lactating | Quebracho | CT | 0–30 | 13/21 | added to basal diet | grass silage, maize silage, rapeseed expeller, wheat grain, and concentrate |
| 20 | [27] | in vivo | dairy cow | Polish FH | Vaccinium vitis idaea | CT | 0–140 | 21/24 | supplemented in diet | corn silage, lucerne silage, meadow hay, wheat grain, corn grain, and rapeseed meal |
| 21 | [28] | in situ | dairy cow | FH | Chestnut | HT | 0–46 | 21/28 | added in diet | lucerne silage, maize silage, grass hay, maize meal, soybean meal, and barley meal |
| 22 | [29] | in vivo | ewe | TexelxLacaune crossbreed | A. mearnsii | CT | 0–20 | 14/19 | added to basal diet | corn silage, pre-dried alfalfa, and soybean meal |
| 23 | [30] | in vivo | sheep | - | Cheestnut and Mimosa | CT and HT | 0–76.1 | 15/21 | added to diet and mixed with silage | ryegrass |
| 24 | [31] | in vivo | dairy cow | Brown-Swiss | A. mearnsii | CT | 0–14.7 | 19/23 | in pellet form (acacia pellet) | corn silage, grass silage, grass hay, and concentrate |
| 25 | [32] | in situ | ram sheep | - | Cistus ladanifer L. | CT | 0–117 | ns | added (mixed) with soybean meal | wheat, barley, maize gluten feed, sunflower meal, and soybean meal |
| 26 | [33] | in vivo and in situ | ram sheep | Merino | Cistus ladanifer L. | CT | 0–30 | 14/29 | added (mixed) with soybean meal | oat straw, manioc, and soybean meal |
| 27 | [34] | in vivo | lamb | Merino Branco | Cistus ladanifer L. | CT | 0–30 | 15,523 | added (mixed) with soybean meal | grass hay, maize, citrus pulp, and soybean meal |
| 28 | [35] | in vivo | heifer | Jersey × German Black Pied Lowland | Quebracho | CT | 0–60 | 43,709 | infusion intraruminally | grass hay and concentrate |
| 29 | [36] | in vivo | dairy cow | FH | Quebracho | CT | 0–30 | 14/21 | supplemented in diet | alfalfa hay, corn silage, barley, beet pulp, corn, canola meal, and wheat |
| 30 | [37] | in vivo | dairy cow | FH | Quebracho | CT | 0–18 | 33,055 | added in diet | alfalfa silage, corn silage, rolled HMSC, corn grain, canola meal, ESMB, soybean meal, cottonseed, soy hulls, and rice hulls |
| 31 | [38] | in vivo | sheep | - | Quebracho | CT | 0–36.5 | 22,190 | intraruminal infusion and treated soybean meal | alfalfa and grass hay |
| 32 | [39] | in vivo | dairy cow | FH | A. mearnsii | CT | 0–19 | 2/8 and 14/49 | mixed with water, grazing, and stall | ryegrass |
| 33 | [40] | in vivo | dairy cow | FH | A. mearnsii | CT | 0–29 | 46,813 | oral drench and mixed in barley pellet | ryegrass (pasture), barley, and molasses |
| 34 | [41] | in vivo | dairy cow | FH | Quebracho | CT | 0–30 | 13/21 | mixed in diet | grass silage, maize silage, wheat, rapeseed, and concentrate |
| 35 | [42] | in vivo | dairy cow | FH | Oak | HT | 0–26 | 14/21 | mixed in grass silage | grass silage, corn silage, beet pulp, rapeseed, and wheat |
| 36 | [43] | in situ | ewe | Merino | Tannic acid | HT | 0–200 | 10/ns | treated with soybean meal | grass hay and soybean meal |
| 37 | [44] | in situ | ewe | Merino | Quebracho | CT | 0–70 | ns/51 | infusion intraruminally | lucerne hay |
| 38 | [45] | in vivo | bull | FH | Pistachio | HT | 0–15 | 14/98 | treated with soybean meal | alfalfa hay, corn silage, corn, barley, wheat, soybean meal, and rice bran |
| 39 | [46] | in vivo | cattle | FH | A. mearnsii | CT | 0–6 | 14/21 | mixed in diet | corn silage, corn grain, and soybean meal |
| 40 | [47] | in vivo and in situ | heifer | Crossbred, beef heifer | A. mearnsii | CT | 0–25 | 21/35 | mixed in diet (substituted barley grain) | barley silage, barley grain, and corn DDGS |
| 41 | [48] | in vivo | sheep | LeicesterxMerinoxDorset crossbreed | Quebracho | CT | 0–60 | 27/34 | oral drench | lucerne hay |
| 42 | [49] | in vivo | sheep | PolwarthxTexel wethers crossbreed | A. mearnsii | CT | 0–60 | 42,278 | infusion intraruminally | ryegrass |
| 43 | [50] | in vivo | steer | - | Mimosa and Chestnut | CT and HT | 0–15 | 30/42 | supplemented in diet | corn, hay–sorghum, cottonseed hulls, cottonseed meal, and molasses |
| 44 | [51] | in vivo | sheep | Santa Inês crossbred | Tannin | CT | 0–30 | 42,278 | supplemented in diet | elephant grass, corn, and soybean meal |
| 45 | [52] | in vivo | dairy cow | Chinese FH, transition | Chestnut | HT | 0–10 | ns/42 | supplemented in diet | corn silage, alfalfa silage, wheat straw, soybean meal, and corn DDGS |
| 46 | [53] | in situ | ewe | Segurena, nonlactating | Tannic acid | HT | 0–50 | ns | treated with soybean meal | oat hay and barley grain |
| 47 | [54] | in vivo | steer | Non-castrated | Quebracho | CT | 0–40 | 14/21 | treated with soybean meal | sugar cane bagasse, corn grain, soybean meal, urea, and cottonseed |
| 48 | [55] | in vivo | bull | Nellore intact | Tannin | CT | 0–75 | 14/28 | treated with soybean meal | sugar cane bagasse, corn grain, soybean meal, urea, and cottonseed |
| 49 | [56] | in vivo | dairy goat | - | Pistachio | HT | 0–10 | 14/21 | mixed with silage (alfalfa) | alfalfa silage, barley grain, cottonseed meal, and wheat bran |
| 50 | [57] | in situ | steer | Talyshi | Green tea | HT and CT | 0–19 | ns | treated with barley grain | alfalfa hay, wheat straw, and concentrate |
| 51 | [58] | in vivo | steer | - | Quebracho | CT | 0–45 | 44,166 | added in diet | cottonseed hulls, corn, alfalfa pellet, bermuda-grass hay, and molasses |
| 52 | [59] | in vivo | steer | FH | A. mearnsii | CT | 0–27 | 42,675 | added in diet | oat and concentrate |
| 53 | [60] | in vivo | sheep | Santa Ines, male | A. mearnsii | CT | 0–10 | 14/21 | added in diet | Tifton hay and concentrate |
| 54 | [61] | in vivo | sheep | Texel, male | A. mearnsii | CT | 0–20 | 14/21 | added in diet | oat–ryegrass hay, soybean meal, cracked corn, and wheat bran |
| 55 | [62] | in vivo | dairy cow | FH | A. mearnsii | CT | 0–100 | 15/21 | added in diet | corn silage, corn grain, and soybean meal |
| 56 | [63] | in vivo and in situ | sheep | Texel | Chestnut | HT | 0–30 | 14/42 | supplemented in diet | orchard grass hay and concentrate based on pea seed |
| 57 | [64] | in vivo | lamb | Sarda × Comisana crossbreed, male | Chestnut, Tara, Mimosa A. nigraa, and Gambier | CT and HT | 0–40 | 27,638 | supplemented in diet | barley, alfalfa, wheat bran, molasses, and soybean meal |
| 58 | [65] | in situ | sheep | - | Quebracho | CT | 0–50 | 21/28 | added in diet | grass hay, grass cube, and whole barley |
| 59 | [66] | in situ | sheep | - | Quebracho | CT | 0–50 | 21/28 | added in diet | grass hay, grass cube, and whole barley |
| 60 | [67] | in situ | dairy cow | FH | Mimosa and Quebracho | CT | 0–50 | ns | added in silage | ryegrass, grass hay, grass silage, and concentrate |
| 61 | [68] | in situ | steer | Taleshi | Pistachio | HT | 0–10 | ns | added in diet (canola and soya bean meal) | alfalfa hay, wheat straw, barley grain, corn grain, wheat bran, and cottonseed meal |
| 62 | [69] | in vivo | lamb | Arabi, fat-tailed, male | Pomagranate-peel | CT | 0–33.5 | 14/78 | treated with recycle poultry bedding | alfalfa hay, wheat straw, corn silage, recycled poultry bedding, soybean meal, corn grain, barley, and wheat bran |
| 63 | [70] | in vivo | lamb | Swiss White Hill | Chestnut | HT | 0–2 | 44,531 | mixed in diet | hay and concentrate |
| 64 | [71] | in vivo | dairy cow | - | Tannin | HT | 0–4.9 | 14/21 | mixed in diet | grass–clover silage, meadow hay, and pelleted concentrate |
| 65 | [72] | in vivo | dairy cow | Polish FH | Lingonberry leaves | CT | 0–4.83 | 21/26 | added in diet | maize silage, lucerne silage, grass silage, beet pulp, brewer grain, rapeseed meal, and concentrate |
| 66 | [73] | in vivo | ewe | Assaf ewes | Tannin | Mixed and CT | 0–10 | 14/28 | supplemented in diet | alfalfa hay, concentrate |
| 67 | [74] | in vivo | ewe | Assaf ewes | Quebracho | CT | 0–40 | 14/28 | supplemented in diet | alfalfa hay, concentrate |
| 68 | [75] | in vivo | sheep and goat (boar) | Rambouillet and Spanish Boer, ewe | Quebracho | CT | 0–100 | 15/18 | mixed in diet | Sudan grass hay, corn, soybean meal, fish meal, and wheat straw |
| 69 | [76] | in vivo | sheep | Merino-Landschaf Crossbreed | Chestnut and Valonea | HT | 0–20 | ns/190 | mixed in diet | ryegrass-based hay, barley grain, wheat grain, soybean meal, and molasses |
| 70 | [77] | in vivo and in situ | sheep and goat | - | Tannin | HT | 0–110 | 14/24 | sprayed to hay | grassland hay |
| Response Variables | Unit | n | Mean | SEM | Min | Max | Response Variables | Unit | n | Mean | SEM | Min | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intake | IsoC5 | mol/100 mol | 81 | 2.16 | 5.82 | 0.09 | 53 | ||||||
| DMI | kg/d | 172 | 8.12 | 8.17 | 0.4 | 27.7 | C2:C3 | 109 | 3.82 | 1.3 | 1.34 | 8.3 | |
| OMI | kg/d | 80 | 6.8 | 7.46 | 0.3 | 24.6 | Bacteria | log 10 | 12 | 6.77 | 0.26 | 6.47 | 7.2 |
| CPI | kg/d | 86 | 1.03 | 1.45 | 0 | 5.15 | Protozoa | log 10 | 42 | 5.48 | 0.83 | 3.03 | 6.3 |
| NDFI | kg/d | 75 | 3.05 | 2.68 | 0.2 | 10 | Feed disappearance | ||||||
| DMI/BW0.75 | g/kg | 161 | 101 | 46.5 | 24 | 205 | Ruminal protein | g/100 g | 22 | 61.9 | 10.4 | 51.3 | 83 |
| OMI/BW0.75 | g/kg | 83 | 85.5 | 39.6 | 27 | 188 | Digested ruminal DM-N | g/100 g | 22 | 61.7 | 16.9 | 15 | 85 |
| CPI/BW0.75 | g/kg | 89 | 13.7 | 9.64 | 2.1 | 41.5 | Digested ruminal OM-N | g/100 g | 14 | 54.9 | 13.6 | 42 | 82 |
| NDFI/BW0.75 | g/kg | 78 | 38.9 | 15.5 | 15 | 74.1 | Duodenum protein | g/100 g | 22 | 74.6 | 13.6 | 55.8 | 90 |
| Digestibility | Intestinal protein | g/100 g | 22 | 89.9 | 6.21 | 80 | 96 | ||||||
| DMD | g/100 g | 144 | 60.8 | 11.8 | 26 | 82.6 | Blood plasma | ||||||
| OMD | g/100 g | 135 | 68.4 | 7.82 | 44 | 83.9 | PUN | mg/dL | 31 | 19 | 14 | 7.28 | 58 |
| CPD | g/100 g | 134 | 65.2 | 14.1 | 8 | 89.5 | Albumin | g/dL | 14 | 4.04 | 0.83 | 3.08 | 5.4 |
| NDFD | g/100 g | 165 | 50.3 | 17 | 0.2 | 79 | N utilisation | ||||||
| Performance | Milk N | g/100 g N | 22 | 27.6 | 4.21 | 18.7 | 34 | ||||||
| ADG | g/d | 45 | 591 | 497 | 109 | 1920 | Urine N | g/100 g N | 85 | 47.7 | 46.9 | 16.4 | 459 |
| ADG/DMI | g/kg | 45 | 101 | 66.3 | 0.2 | 241 | Faecal N | g/100 g N | 83 | 39.1 | 13.2 | 20.9 | 83 |
| GEI/BW0.75 | kcal/kg | 19 | 289 | 131 | 220 | 657 | N retention | g/100 g N | 67 | 21.6 | 10.3 | 0.6 | 39 |
| DEI/BW0.75 | kcal/kg | 19 | 186 | 105 | 132 | 507 | ENU | % | 14 | 30.1 | 6.94 | 20 | 41 |
| MEI/BW0.75 | kcal/kg | 40 | 56.4 | 61.2 | 0.1 | 142 | Urinary purine | ||||||
| Methane production | Allantoin | mmol/d | 37 | 69 | 94.8 | 8.9 | 408 | ||||||
| CH4 | L | 57 | 204 | 219 | 17 | 690 | Uric acids | mmol/d | 36 | 18.9 | 36.7 | 1 | 154 |
| CH4/DMI | L/kg | 51 | 24.7 | 7.87 | 6.7 | 42 | Purine derivative | mmol/d | 42 | 62.8 | 97.8 | 9.43 | 449 |
| CH4/BW0.75 | L/kg | 57 | 2.53 | 1.23 | 0.6 | 5.51 | Microbial N supply | g/d | 47 | 34.6 | 29.9 | 3.41 | 91 |
| Milk production and composition | EMPS | g/g | 38 | 51.1 | 42.7 | 9.5 | 164 | ||||||
| Milk | kg/d | 65 | 21.7 | 13.1 | 0.7 | 40.8 | DM kinetics degradability | ||||||
| Milk/BW0.75 | g/kg | 63 | 0.19 | 0.08 | 0 | 0.31 | A | % | 59 | 28.2 | 14.9 | 7.67 | 75 |
| Milk/DMI | kg/kg | 69 | 1.34 | 0.47 | 0.3 | 2.98 | B | % | 59 | 62.9 | 20.1 | 12.6 | 90 |
| FPCM | kg/d | 16 | 26.9 | 17.4 | 0.9 | 56 | a + b | % | 67 | 90 | 16 | 31 | 102 |
| Milk fat | g/100 g | 63 | 4.42 | 1.23 | 2.9 | 7.69 | C | /h | 65 | 0.06 | 0.02 | 0.02 | 0.1 |
| Milk protein | g/100 g | 65 | 3.79 | 0.99 | 2.8 | 6.41 | ERD 2% | kp0.02 | 24 | 72.2 | 7.76 | 53.4 | 82 |
| Milk lactose | g/100 g | 63 | 4.73 | 0.33 | 4 | 5.27 | ERD 5% | kp0.05 | 44 | 56 | 14.4 | 27.2 | 91 |
| Milk SNF | g/100 g | 38 | 8.47 | 2.61 | 3.5 | 11.1 | ERD 8% | kp0.08 | 24 | 47.6 | 10.7 | 27.2 | 63 |
| Milk TSC | g/100 g | 36 | 13.5 | 3.56 | 7.4 | 18.7 | CP kinetics degradability | ||||||
| Milk urea-N | mg/dL | 35 | 21.5 | 9.73 | 9.4 | 44.7 | a | % | 73 | 23.4 | 16.3 | 1.2 | 75 |
| Rumen fermentation | b | % | 73 | 72.8 | 18.5 | 2.75 | 97 | ||||||
| pH | 123 | 6.54 | 0.32 | 5.8 | 7.43 | a + b | % | 73 | 96.1 | 13 | 57.6 | 120 | |
| NH3 | mg/dL | 109 | 18 | 8.4 | 3.2 | 39.4 | c | /h | 71 | 0.05 | 0.03 | 0.01 | 0.1 |
| TVFA | mmol/L | 107 | 94.4 | 29.1 | 40 | 158 | ERD 2% | kp0.02 | 21 | 71 | 14.2 | 42.3 | 88 |
| C2 | mol/100 mol | 109 | 65.5 | 7.8 | 47 | 79.8 | ERD 5% | kp0.05 | 67 | 54.1 | 12.3 | 29.2 | 82 |
| C3 | mol/100 mol | 109 | 19.1 | 5.33 | 9.5 | 36.8 | ERD 8% | kp0.08 | 35 | 48.3 | 10.8 | 24.4 | 69 |
| IsoC4 | mol/100 mol | 77 | 2.38 | 3.45 | 0.1 | 15.2 | Ruminal N in situ degradability | ||||||
| C4 | mol/100 mol | 109 | 11 | 4.41 | 1.2 | 26.2 | ID | % | 22 | 62.9 | 17.5 | 39 | 91 |
| C5 | mol/100 mol | 86 | 1.24 | 0.81 | 0.2 | 3.82 | RUP | % | 13 | 40.6 | 11.4 | 24.4 | 60 |
| Response Variables | Unit | n | Model | Parameter Estimates | Model Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | SE Intercept | Slope | SE Slope | p-Value | RMSE | AIC | p-Value ct vs. ht | ||||
| Intake | |||||||||||
| DMI | kg/d | 170 | Q | 8.95 | 1.05 | −0.016 | 0.005 | 0.002 | 0.38 | 656 | 0.381 |
| 0.0001 | 0.00006 | 0.021 | |||||||||
| OMI | kg/d | 79 | L | 7.17 | 1.40 | −0.004 | 0.002 | 0.105 | 0.23 | 259 | 0.782 |
| CPI | kg/d | 84 | L | 1.11 | 0.27 | −0.0004 | 0.0003 | 0.149 | 0.05 | 108 | 0.379 |
| NDFI | kg/d | 74 | L | 3.00 | 0.52 | −0.003 | 0.001 | 0.025 | 0.14 | 142 | 0.280 |
| DMI/BW0.75 | g/kg | 165 | L | 106 | 6.07 | −0.09 | 0.02 | <0.001 | 3.89 | 1256 | 0.123 |
| OMI/BW0.75 | g/kg | 82 | L | 89.2 | 7.26 | −0.051 | 0.03 | 0.058 | 2.90 | 618 | 0.797 |
| CPI/BW0.75 | g/kg | 87 | L | 14.8 | 1.71 | −0.013 | 0.004 | 0.005 | 0.84 | 432 | 0.203 |
| NDFI/BW0.75 | g/kg | 77 | L | 39.5 | 2.92 | −0.05 | 0.02 | 0.003 | 1.59 | 471 | 0.096 |
| Digestibility | |||||||||||
| DMD | g/100 g | 116 | Q | 66.6 | 1.12 | −0.14 | 0.03 | <0.001 | 1.98 | 680 | 0.323 |
| 0.0008 | 0.0003 | 0.003 | |||||||||
| OMD | g/100 g | 134 | Q | 70.3 | 1.10 | −0.13 | 0.02 | <0.001 | 1.96 | 788 | 0.568 |
| 0.0007 | 0.0003 | 0.006 | |||||||||
| CPD | g/100 g | 124 | Q | 68.4 | 2.09 | −0.24 | 0.03 | <0.001 | 2.25 | 794 | 0.337 |
| 0.002 | 0.0005 | 0.001 | |||||||||
| NDFD | g/100 g | 137 | Q | 57.5 | 1.64 | −0.15 | 0.03 | <0.001 | 2.59 | 890 | 0.044 |
| 0.0009 | 0.0003 | 0.009 | |||||||||
| Performance | |||||||||||
| ADG | g/d | 45 | L | 558 | 145 | −0.48 | 0.84 | 0.575 | 70.4 | 579 | 0.135 |
| ADG/DMI | g/kg | 45 | Q | 99.5 | 19.2 | 0.96 | 0.55 | 0.092 | 19.4 | 458 | 0.376 |
| −0.03 | 0.01 | 0.007 | |||||||||
| GEI/BW0.75 | kcal/kg | 19 | L | 296 | 56.9 | −0.45 | 0.68 | 0.525 | 26.5 | 202 | N.a. |
| DEI/BW0.75 | kcal/kg | 19 | L | 200 | 44.5 | −0.85 | 0.73 | 0.264 | 28.3 | 201 | N.a. |
| MEI/BW0.75 | kcal/kg | 40 | L | 53.9 | 18.6 | 0.0008 | 0.008 | 0.919 | 1.21 | 224 | N.a. |
| Methane production | |||||||||||
| CH4 | L | 57 | L | 217 | 49.3 | −0.51 | 0.39 | 0.200 | 30.9 | 656 | 0.047 |
| CH4/DMI | L/kg | 51 | L | 26.4 | 1.94 | −0.10 | 0.02 | <0.001 | 1.87 | 292 | 0.051 |
| CH4/BW0.75 | L/kg | 57 | L | 2.74 | 0.27 | −0.009 | 0.003 | 0.007 | 0.25 | 111 | 0.046 |
| Milk production and composition | |||||||||||
| Milk yield | kg/d | 65 | Q | 21.7 | 2.61 | −0.04 | 0.02 | 0.081 | 1.02 | 385 | 0.999 |
| 0.0003 | 0.0002 | 0.083 | |||||||||
| Milk yield/BW0.75 | g/kg | 63 | L | 186 | 17.0 | 0.02 | 0.09 | 0.859 | 0.29 | −234 | 0.809 |
| Milk yield/DMI | g/kg | 69 | L | 1337 | 90.3 | 0.56 | 0.65 | 0.399 | 1.67 | 31.1 | 0.611 |
| FPCM | kg/d | 16 | Q | 24.9 | 6.37 | −0.09 | 0.02 | 0.002 | 0.31 | 95.7 | 0.300 |
| 0.002 | 0.0003 | <0.001 | |||||||||
| Milk fat | g/100 g | 63 | L | 4.55 | 0.25 | −0.0003 | 0.001 | 0.776 | 0.12 | 83.2 | 0.664 |
| Milk protein | g/100 g | 65 | L | 3.84 | 0.20 | −0.0003 | 0.001 | 0.582 | 0.06 | 21.1 | 0.094 |
| Milk lactose | g/100 g | 63 | L | 4.74 | 0.07 | −0.00005 | 0.0004 | 0.904 | 0.04 | −60.9 | 0.022 |
| Milk SNF | g/100 g | 38 | L | 8.75 | 0.72 | −0.009 | 0.002 | <0.001 | 0.14 | 78.2 | 0.650 |
| Milk TSC | g/100 g | 36 | L | 14.1 | 1.01 | −0.009 | 0.003 | 0.006 | 0.20 | 97.2 | 0.728 |
| Milk urea-N | mg/dL | 35 | L | 22.8 | 2.64 | −0.047 | 0.013 | 0.001 | 0.85 | 188 | 0.339 |
| Response Variables | Unit | n | Model | Parameter Estimates | Model Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | SE Intercept | Slope | SE Slope | p-Value | RMSE | AIC | p-Value ct vs. ht | ||||
| Rumen fermentation profile | |||||||||||
| pH | 122 | L | 6.50 | 0.05 | 0.0003 | 0.0004 | 0.502 | 0.10 | −33.5 | 0.104 | |
| NH3 | mg/dL | 108 | L | 19.4 | 1.40 | −0.08 | 0.01 | <0.001 | 1.88 | 625 | 0.155 |
| VFA | mmol/L | 106 | L | 98.9 | 4.81 | −0.04 | 0.03 | 0.162 | 6.84 | 875 | 0.628 |
| C2 | mol/100mol | 108 | L | 65.3 | 1.30 | −0.020 | 0.006 | <0.001 | 1.36 | 573 | 0.016 |
| C3 | mol/100 mol | 108 | L | 19.0 | 0.88 | 0.017 | 0.005 | <0.001 | 1.15 | 522 | 0.287 |
| Iso-C4 | mol/100 mol | 77 | Q | 2.42 | 0.72 | 0.02 | 0.01 | 0.008 | 0.42 | 262 | 0.755 |
| −0.0002 | 0.0001 | 0.002 | |||||||||
| C4 | mol/100 mol | 108 | L | 11.3 | 0.72 | 0.001 | 0.006 | 0.815 | 1.38 | 533 | 0.010 |
| C5 | mol/100 mol | 85 | Q | 1.22 | 0.16 | −0.008 | 0.002 | 0.001 | 0.15 | 98.9 | 0.625 |
| 0.0001 | 0.00002 | <0.001 | |||||||||
| Iso-C5 | mol/100 mol | 81 | L | 2.47 | 0.94 | −0.0028 | 0.016 | 0.862 | 4.05 | 522 | 0.604 |
| C2:C3 | 108 | L | 3.83 | 0.21 | −0.006 | 0.001 | <0.001 | 0.27 | 214 | 0.202 | |
| Bacteria | log 10 | 12 | L | 6.71 | 0.11 | 0.00024 | 0.0005 | 0.663 | 0.06 | 9.20 | N.a. |
| Protozoa | log 10 | 42 | L | 5.31 | 0.22 | −0.0012 | 0.0006 | 0.058 | 0.10 | 30.7 | 0.714 |
| Feed disappearance | |||||||||||
| Ruminal protein | g/100 g | 22 | Q | 69.5 | 3.17 | −0.72 | 0.26 | 0.015 | 2.17 | 140 | N.a. |
| 0.01 | 0.01 | 0.022 | |||||||||
| Digested ruminal DM-N | g/100 g | 22 | L | 72.2 | 4.72 | −0.43 | 0.08 | <0.001 | 5.63 | 165 | N.a. |
| Digested ruminal OM-N | g/100 g | 14 | L | 62.1 | 4.98 | −0.31 | 0.07 | 0.002 | 3.61 | 98.2 | N.a. |
| Duodenum protein | g/100 g | 22 | L | 76.0 | 6.36 | −0.03 | 0.04 | 0.480 | 2.31 | 134 | N.a. |
| Intestinal protein | g/100 g | 22 | L | 91.9 | 2.88 | −0.03 | 0.01 | 0.038 | 0.73 | 91.3 | N.a. |
| Response Variables | Unit | n | Model | Parameter Estimates | Model Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | SE Intercept | Slope | SE Slope | p-Value | RMSE | AIC | p-Value ct vs. ht | ||||
| Blood plasma | |||||||||||
| PUN | mg/dL | 31 | Q | 20.7 | 3.33 | −0.19 | 0.05 | 0.002 | 0.98 | 177 | 0.089 |
| 0.005 | 0.001 | 0.002 | |||||||||
| Albumin | g/dL | 14 | L | 4.01 | 0.48 | −0.0013 | 0.003 | 0.698 | 0.14 | 20.7 | 0.060 |
| N utilisation | |||||||||||
| Milk N | g/100 g N | 22 | L | 26.9 | 1.46 | −0.0023 | 0.02 | 0.910 | 0.73 | 102 | 0.742 |
| Urine N | g/100 g N | 85 | L | 44.6 | 7.82 | 0.26 | 0.24 | 0.286 | 31.4 | 891 | 0.891 |
| Faecal N | g/100 g N | 83 | L | 35.0 | 2.53 | 0.18 | 0.02 | <0.0001 | 2.47 | 535 | 0.802 |
| N retention | g/100 g N | 67 | Q | 21.7 | 2.13 | 0.23 | 0.06 | <0.001 | 2.23 | 421 | 0.012 |
| −0.004 | 0.001 | <0.001 | |||||||||
| ENU | % | 14 | Q | 30.0 | 3.32 | 0.42 | 0.20 | 0.079 | 3.09 | 99.9 | N.a. |
| −0.008 | 0.004 | 0.070 | |||||||||
| Urinary purine | |||||||||||
| Allantoin | mmol/d | 37 | L | 76.0 | 29.6 | −0.40 | 0.23 | 0.105 | 17.94 | 374 | N.a. |
| Uric acids | mmol/d | 36 | L | 27.1 | 11.3 | −0.09 | 0.05 | 0.084 | 3.61 | 273 | <0.001 |
| Purine derivative | mmol/d | 42 | L | 65.6 | 29.2 | −0.40 | 0.23 | 0.097 | 18.83 | 427 | 0.869 |
| Microbial N supply | g/d | 47 | L | 36.9 | 7.71 | 0.02 | 0.03 | 0.539 | 2.48 | 332 | 0.676 |
| EMPS | g/g | 38 | L | 58.3 | 11.7 | −0.56 | 0.26 | 0.043 | 18.27 | 370 | <0.001 |
| Response Variables | Unit | n | Model | Parameter Estimates | Model Statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | SE Intercept | Slope | SE Slope | p-Value | RMSE | AIC | p-Value ct vs. ht | ||||
| DM kinetics degradability | |||||||||||
| a | % | 59 | Q | 30.7 | 3.73 | −0.14 | 0.03 | <0.001 | 2.85 | 402 | 0.006 |
| 0.0005 | 0.0002 | 0.020 | |||||||||
| b | % | 59 | Q | 56.7 | 5.08 | 0.14 | 0.03 | 0.001 | 2.95 | 418 | 0.072 |
| −0.0008 | 0.0002 | 0.001 | |||||||||
| a + b | % | 67 | L | 86.9 | 3.58 | −0.04 | 0.009 | <0.001 | 1.96 | 421 | 0.421 |
| c | %/h | 65 | L | 0.06 | 0.005 | −0.0002 | 0.00004 | <0.001 | 0.01 | −324 | 0.941 |
| ERD 2% | kp 0.02 | 24 | L | 79.0 | 1.85 | −0.36 | 0.03 | <0.001 | 2.19 | 133 | 0.025 |
| ERD 5% | kp 0.05 | 44 | Q | 61.7 | 4.04 | −0.33 | 0.05 | <0.001 | 3.45 | 311 | 0.160 |
| 0.001 | 0.0003 | <0.001 | |||||||||
| ERD 8% | kp 0.08 | 24 | L | 55.6 | 2.81 | −0.42 | 0.03 | <0.001 | 2.23 | 138 | 0.006 |
| CP kinetics degradability | |||||||||||
| a | % | 73 | Q | 30.9 | 3.80 | −0.30 | 0.03 | <0.001 | 2.79 | 485 | <0.001 |
| 0.001 | 0.0002 | <0.001 | |||||||||
| b | % | 73 | Q | 64.0 | 4.42 | 0.28 | 0.06 | <0.001 | 5.10 | 555 | <0.001 |
| −0.002 | 0.0003 | <0.001 | |||||||||
| a + b | % | 73 | L | 95.6 | 3.04 | −0.09 | 0.02 | <0.001 | 3.70 | 486 | 0.003 |
| c | %/h | 71 | Q | 0.06 | 0.01 | −0.0006 | 0.0001 | <0.001 | 0.01 | −320 | 0.822 |
| 0.000002 | 0.00 | 0.001 | |||||||||
| ERD 2% | kp 0.02 | 21 | Q | 81.1 | 6.69 | −0.72 | 0.09 | <0.001 | 1.64 | 128 | N.a. |
| 0.007 | 0.002 | 0.001 | |||||||||
| ERD 5% | kp 0.05 | 67 | Q | 60.8 | 2.72 | −0.37 | 0.04 | <0.001 | 3.37 | 456 | 0.281 |
| 0.0011 | 0.0002 | <0.001 | |||||||||
| ERD 8% | kp 0.08 | 35 | Q | 58.1 | 3.91 | −0.83 | 0.07 | <0.001 | 1.49 | 188 | N.a. |
| 0.007 | 0.001 | <0.001 | |||||||||
| Ruminal N in situ degradability | |||||||||||
| ID | % | 22 | L | 65.4 | 8.79 | −0.08 | 0.02 | 0.001 | 3.86 | 152 | 0.306 |
| RUP | % | 13 | L | 32.3 | 5.13 | 0.21 | 0.02 | <0.001 | 2.10 | 80.3 | N.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanza, Y.R.; Fitri, A.; Suwignyo, B.; Elfahmi; Hidayatik, N.; Kumalasari, N.R.; Irawan, A.; Jayanegara, A. The Utilisation of Tannin Extract as a Dietary Additive in Ruminant Nutrition: A Meta-Analysis. Animals 2021, 11, 3317. https://doi.org/10.3390/ani11113317
Yanza YR, Fitri A, Suwignyo B, Elfahmi, Hidayatik N, Kumalasari NR, Irawan A, Jayanegara A. The Utilisation of Tannin Extract as a Dietary Additive in Ruminant Nutrition: A Meta-Analysis. Animals. 2021; 11(11):3317. https://doi.org/10.3390/ani11113317
Chicago/Turabian StyleYanza, Yulianri Rizki, Ainissya Fitri, Bambang Suwignyo, Elfahmi, Nanik Hidayatik, Nur Rochmah Kumalasari, Agung Irawan, and Anuraga Jayanegara. 2021. "The Utilisation of Tannin Extract as a Dietary Additive in Ruminant Nutrition: A Meta-Analysis" Animals 11, no. 11: 3317. https://doi.org/10.3390/ani11113317
APA StyleYanza, Y. R., Fitri, A., Suwignyo, B., Elfahmi, Hidayatik, N., Kumalasari, N. R., Irawan, A., & Jayanegara, A. (2021). The Utilisation of Tannin Extract as a Dietary Additive in Ruminant Nutrition: A Meta-Analysis. Animals, 11(11), 3317. https://doi.org/10.3390/ani11113317









