Raising Awareness of the Severity of “Contactless Stings” by Cassiopea Jellyfish and Kin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Information
- “How long (total) have you worked with Cassiopea or other Rhizostome jellyfishes?”
- “How many times have you felt “stinging water”?
- “In what role did you experience stinging water?”
3.2. Location
- “Where geographically (as specific as possible) did you feel “stinging water?”
- “What would you classify this location as?”
- “If you were close to a Cassiopea, how close to Cassiopea medusae were you?”
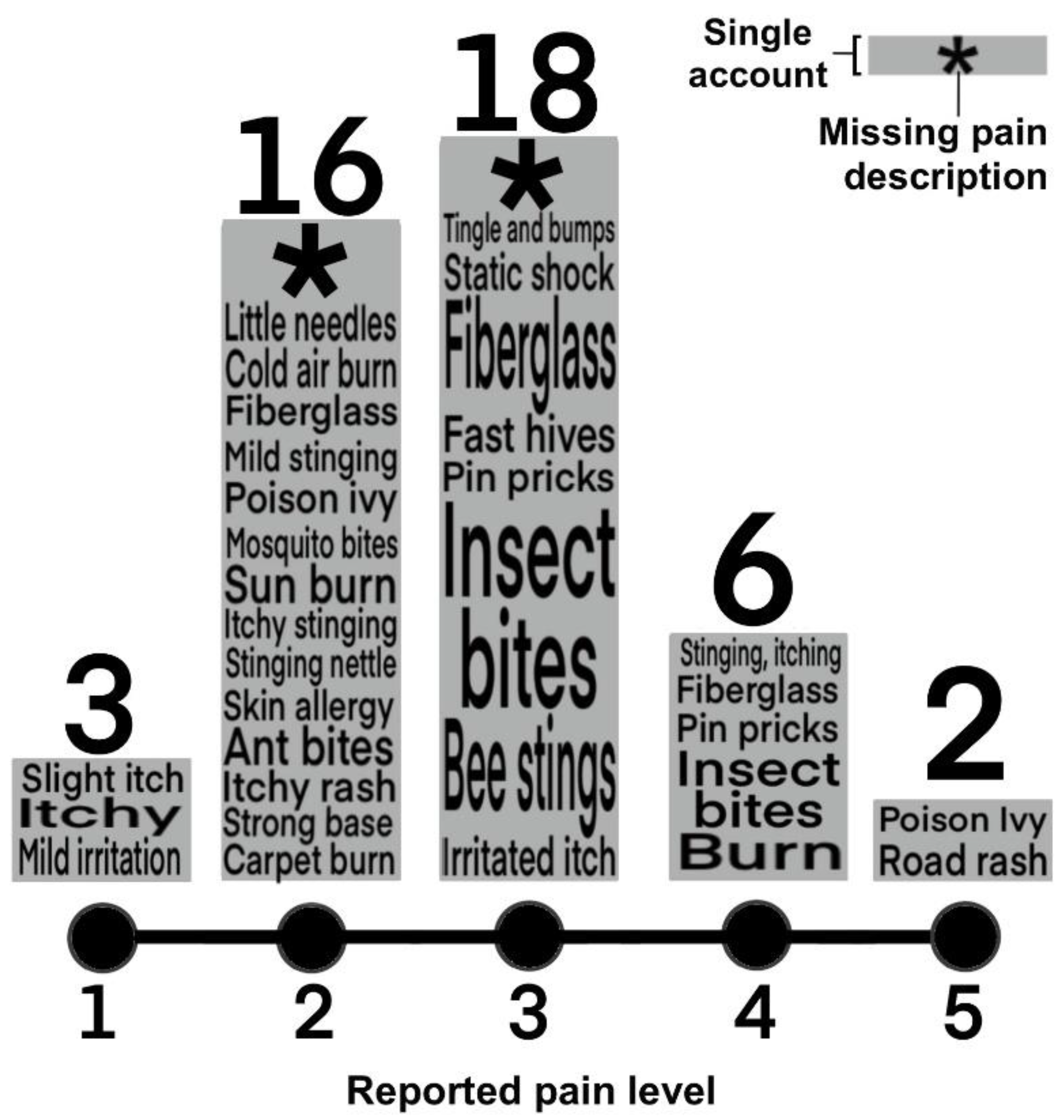
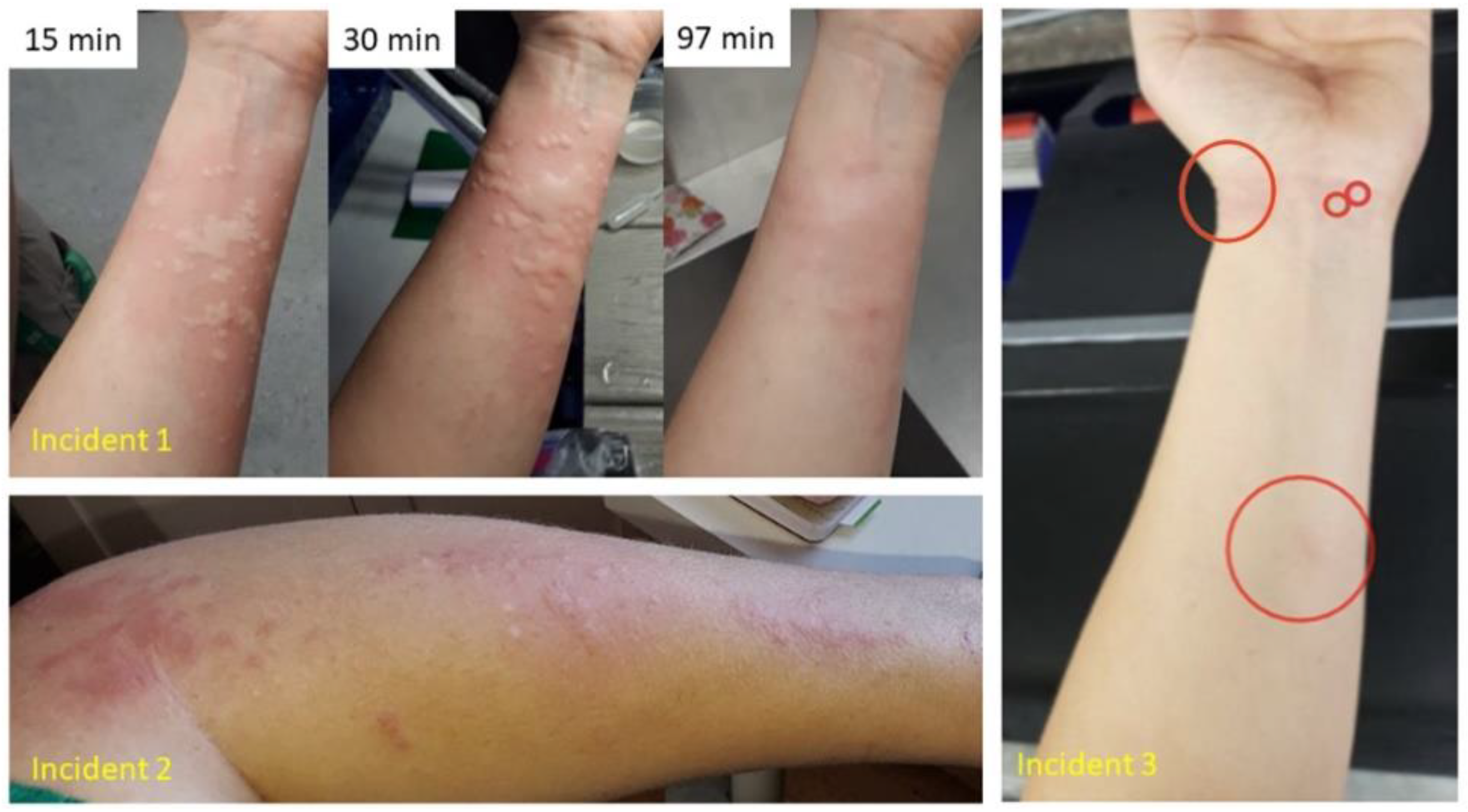
3.3. Stinging Water Effects
- “What level of discomfort did you experience?”
- “What would you consider this discomfort most comparable to?”
3.4. Jellyfish Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayha, K.M.; Dawson, M.N.; Collins, A.G.; Barbeitos, M.S.; Haddock, S.H.D. Evolutionary relationships among scyphozoan jellyfish families based on complete taxon sampling and phylogenetic analyses of 18S and 28S ribosomal DNA. Integr. Comp. Biol. 2010, 50, 436–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.C.; Collins, A.G. Cladistic analysis of Medusozoa and cnidarian evolution. Invertebr. Biol. 2004, 123, 23–42. [Google Scholar] [CrossRef]
- Dawson, M.N. Morphological variation and systematics in the Scyphozoa: Mastigias (Rhizostomeae, Mastigiidae)—A golden unstandard? Hydrobiologia 2005, 537, 185–206. [Google Scholar] [CrossRef] [Green Version]
- Morandini, A.C.; Stampar, S.N.; Maronna, M.M.; Da Silveira, F.L. All non-indigenous species were introduced recently? the case study of Cassiopea (Cnidaria: Scyphozoa) in Brazilian waters. J. Mar. Biol. Assoc. U. K. 2017, 97, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Phillips, P.J.; Burke, W.D.; Keener, E.J. Observations on the trophic significance of jellyfishes in Mississippi Sound with quantitative data on the associative behavior of small fishes with medusae. Trans. Am. Fish. Soc. 1969, 98, 703–712. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Kondo, Y.; Sakai, Y.; Shimazu, T. In-situ observations of symbionts on medusae occurring in Japan, Thailand, Indonesia and Malaysia. Bull. Hiroshima Univ. Mus. 2010, 2, 9–18. [Google Scholar]
- Muffett, K.; Miglietta, M.P. Planktonic associations between medusae (classes Scyphozoa and Hydrozoa) and epifaunal crustaceans. PeerJ 2021, 9, e11281. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, D.; Keesing, J.K. Jellyfish blooms in China: Dominant species, causes and consequences. Mar. Pollut. Bull. 2010, 60, 954–963. [Google Scholar] [CrossRef]
- Omori, M.; Nakano, E. Jellyfish fisheries in southeast Asia. Hydrobiologia 2001, 451, 19–26. [Google Scholar] [CrossRef]
- Getino Mamet, L.N.; Daglio, L.G.; De-León, F.J.G. High genetic differentiation in the edible cannonball jellyfish (cnidaria: Scyphozoa: Stomolophus spp.) from the Gulf of California, Mexico. Fish. Res. 2019, 219, 105328. [Google Scholar] [CrossRef]
- Brotz, L.; Schiariti, A.; López-Martínez, J.; Álvarez-Tello, J.; Peggy Hsieh, Y.H.; Jones, R.P.; Quiñones, J.; Dong, Z.; Morandini, A.C.; Preciado, M.; et al. Jellyfish fisheries in the Americas: Origin, state of the art, and perspectives on new fishing grounds. Rev. Fish Biol. Fish. 2017, 27, 1–29. [Google Scholar] [CrossRef]
- Lucas, C.H.; Gelcich, S.; Uye, S.I. Living with jellyfish: Management and adaptation strategies. In Jellyfish Blooms; Springer: Berlin/Heidelberg, Germany, 2014; pp. 129–150. ISBN 9789400770157. [Google Scholar]
- Ames, C.L.; Klompen, A.M.L.; Badhiwala, K.; Muffett, K.; Reft, A.J.; Kumar, M.; Janssen, J.D.; Schultzhaus, J.N.; Field, L.D.; Muroski, M.E.; et al. Cassiosomes are stinging-cell structures in the mucus of the upside-down jellyfish Cassiopea xamachana. Commun. Biol. 2020, 3, 1–15. [Google Scholar] [CrossRef]
- Shanks, A.L.; Graham, W.M. Chemical defense in a scyphomedusa. Mar. Ecol. Prog. Ser. 1988, 45, 81–86. [Google Scholar] [CrossRef]
- Al-Rubiay, K.K.; Al-Musaoi, H.A.; Alrubaiy, L.; Al-Freje, M.G. Skin and systemic manifestations of jellyfish stings in Iraqi fishermen. Libyan J. Med. 2009, 4, 75–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segura-Puertas, L.; Ramos, M.E.; Aramburo, C.; Heimer de la Cotera, E.P.; Burnett, J.W. One Linuche mystery solved: All 3 stages of the coronate scyphomedusa Linuche unguiculata cause seabather’s eruption. J. Am. Acad. Dermatol. 2001, 44, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Avian, M.; Spanier, E.; Galil, B. Nematocysts of Rhopilema Nomadica (Scyphozoa: Rhizostomeae), an Immigrant Jellyfish in the Eastern Mediterranean. J. Morphol. 1995, 224, 221–231. [Google Scholar] [CrossRef]
- Glatstein, M.; Adir, D.; Galil, B.; Scolnik, D.; Rimon, A.; Pivko-Levy, D.; Hoyte, C. Pediatric Jellyfish Envenomation in the Mediterranean Sea. Eur. J. Emerg. Med. 2018, 25, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Fleck, J.; Fitt, W.K. Degrading mangrove leaves of Rhizophora mangle Linne provide a natural cue for settlement and metamorphosis of the upside down jellyfish Cassiopea xamachana Bigelow. J. Exp. Mar. Bio. Ecol. 1999, 234, 83–94. [Google Scholar] [CrossRef]
- Bordehore, C.; Alonso, C.; Sánchez-Fernández, L.; Canepa, A.; Acevedo, M.; Nogué, S.; Fuentes, V.L. Lifeguard Assistance at Spanish Mediterranean Beaches: Jellyfish Prevail and Proposals for Improving Risk Management. Ocean Coast. Manag. 2016, 131, 45–52. [Google Scholar] [CrossRef] [Green Version]
- National Ocean Service. What Are the Most Common Jellies in the Keys? Available online: https://floridakeys.noaa.gov/animals/commonkeysjellies.html (accessed on 30 June 2021).
- Gershwin, L.A.; Dabinett, K. Comparison of eight types of protective clothing against Irukandji jellyfish stings. J. Coast. Res. 2009, 25, 117–130. [Google Scholar] [CrossRef]
- Ohdera, A.H.; Abrams, M.J.; Ames, C.L.; Baker, D.M.; Suescún-Bolívar, L.P.; Collins, A.G.; Freeman, C.J.; Gamero-Mora, E.; Goulet, T.L.; Hofmann, D.K.; et al. Upside-Down but Headed in the Right Direction: Review of the Highly Versatile Cassiopea xamachana System. Front. Ecol. Evol. 2018, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cañas, J.A.; Rodrigo-Muñoz, J.M.; Rondon-Cepeda, S.H.; Bordehore, C.; Fernández-Nieto, M.; Del Pozo, V. Jellyfish Collagen: A New Allergen in the Beach. Ann. Allergy Asthma Immunol. 2018, 120, 430–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases (accessed on 14 September 2021).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muffett, K.M.; Klompen, A.M.L.; Collins, A.G.; Lewis Ames, C. Raising Awareness of the Severity of “Contactless Stings” by Cassiopea Jellyfish and Kin. Animals 2021, 11, 3357. https://doi.org/10.3390/ani11123357
Muffett KM, Klompen AML, Collins AG, Lewis Ames C. Raising Awareness of the Severity of “Contactless Stings” by Cassiopea Jellyfish and Kin. Animals. 2021; 11(12):3357. https://doi.org/10.3390/ani11123357
Chicago/Turabian StyleMuffett, Kaden McKenzie, Anna M. L. Klompen, Allen G. Collins, and Cheryl Lewis Ames. 2021. "Raising Awareness of the Severity of “Contactless Stings” by Cassiopea Jellyfish and Kin" Animals 11, no. 12: 3357. https://doi.org/10.3390/ani11123357






