Effect of Muscle Extract and Graphene Oxide on Muscle Structure of Chicken Embryos
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Graphene Oxide
2.2. Preparation and Analysis of Chicken Embryo Muscle Extract
2.3. In Vitro Study: Cell Culture
2.4. In Vitro Study: Proliferation of Cells
2.5. In Vitro Study: Viability of Cells
2.6. Hemotoxicity
2.7. Animal Model
2.8. Immunochistochemistry
2.9. Superoxide Dismutase (SOD) Activity
2.10. Statistical Analysis
3. Results
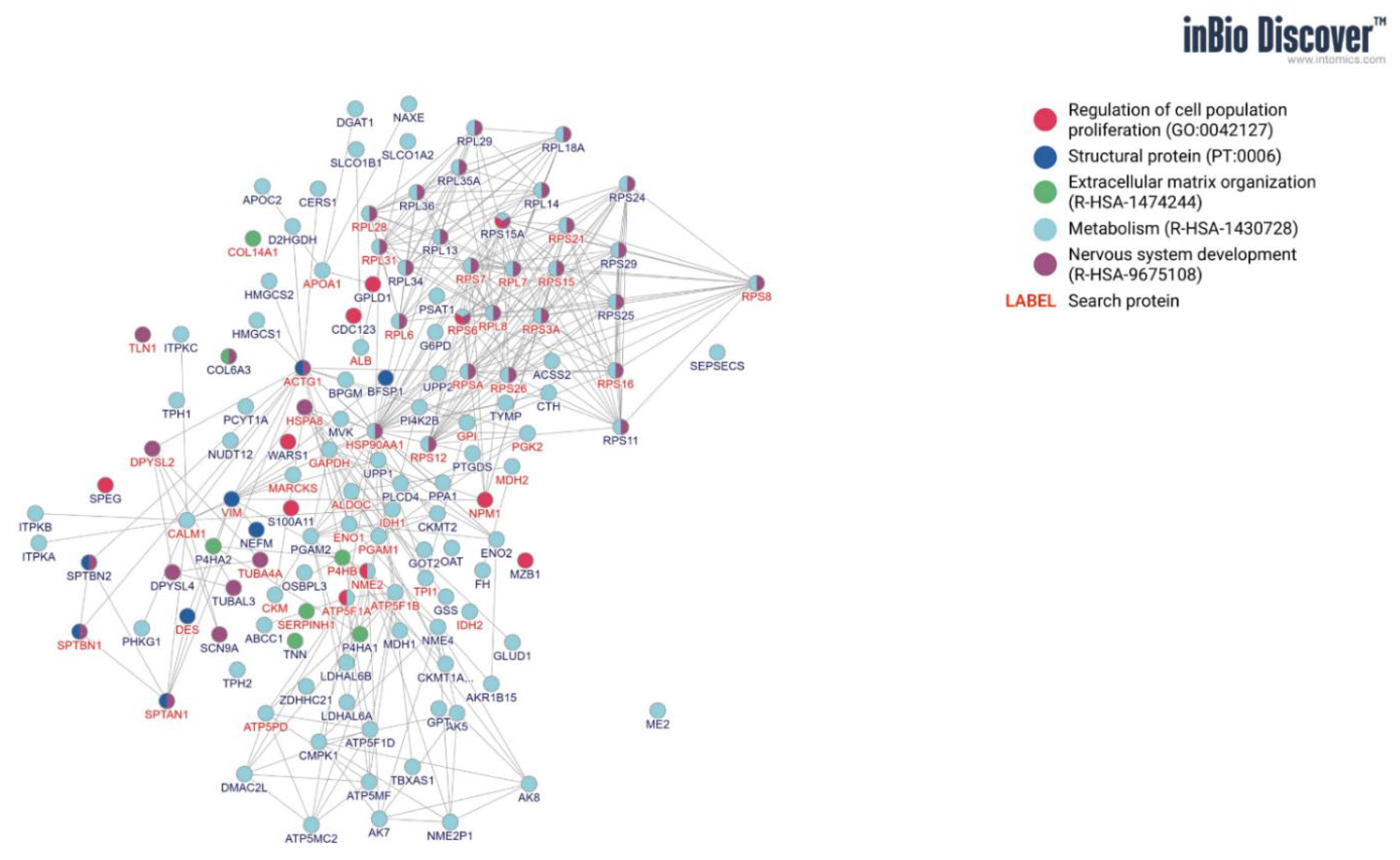
3.1. GO and CEME Characterization
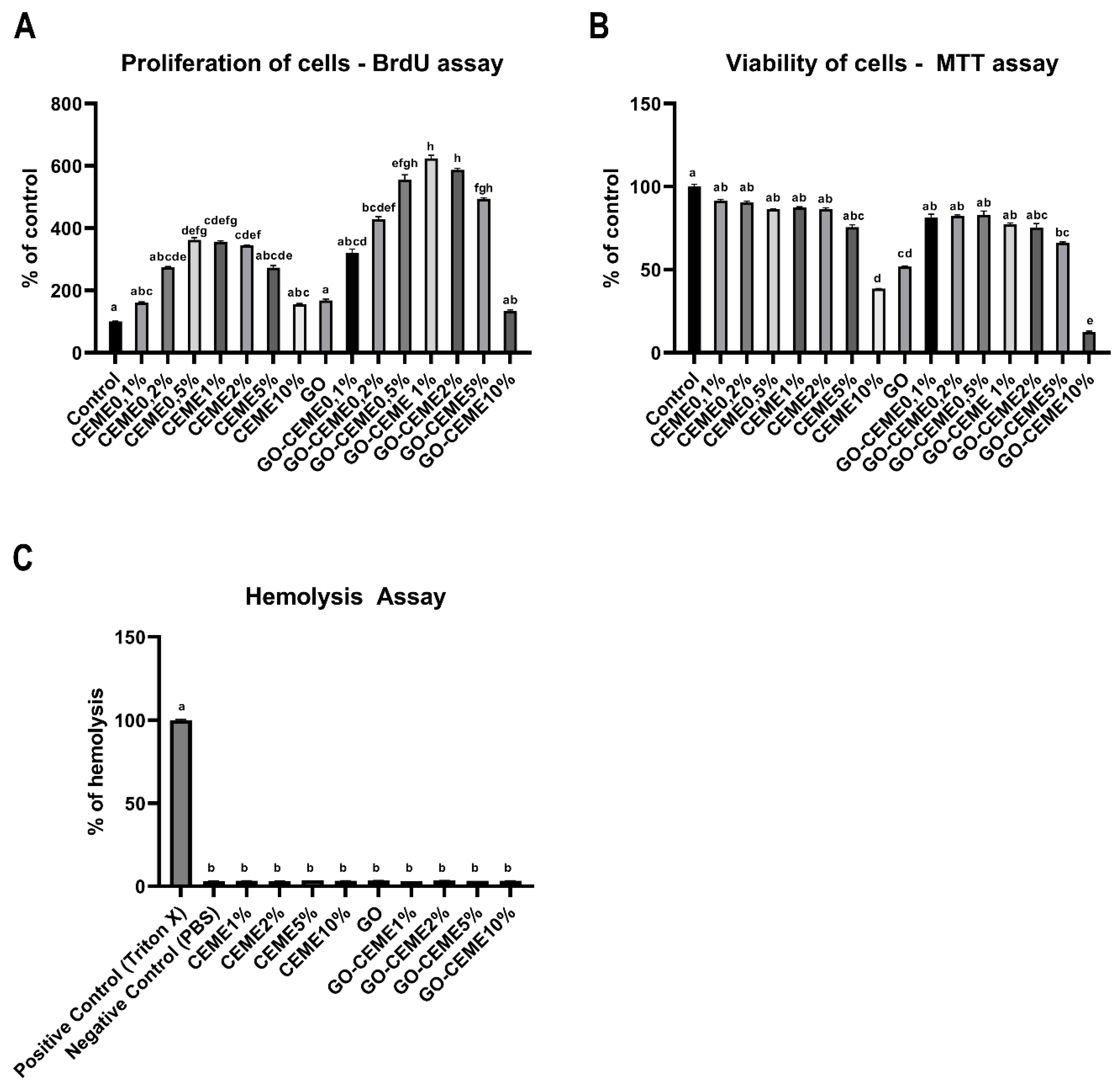
3.2. In Vitro Study: Proliferation of Cells
3.2.1. In Vitro Study: Viability of Cells
3.2.2. Hemolysis Assay
3.3. In Ovo Study: Chicken Embryo Growth and Development
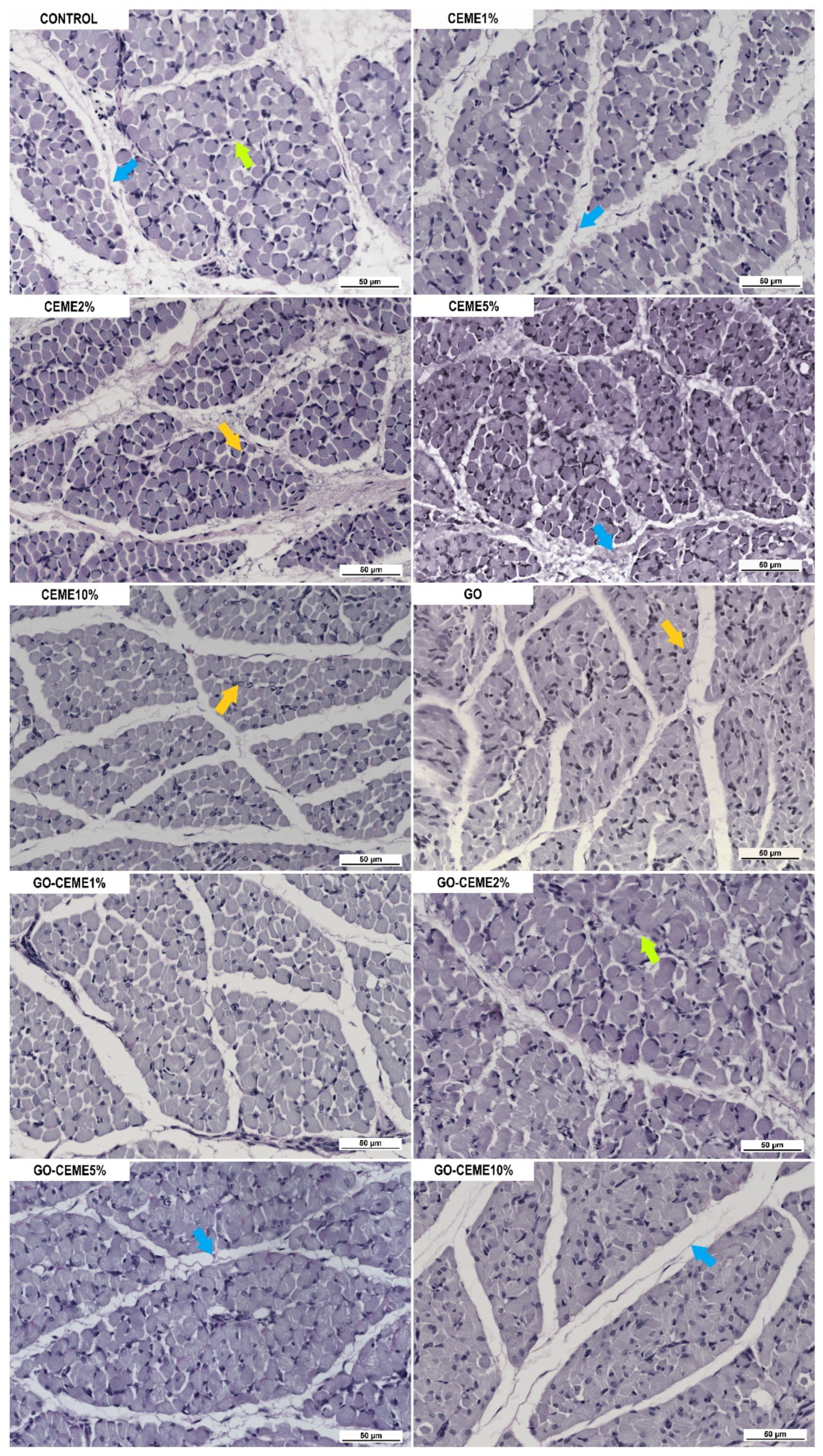
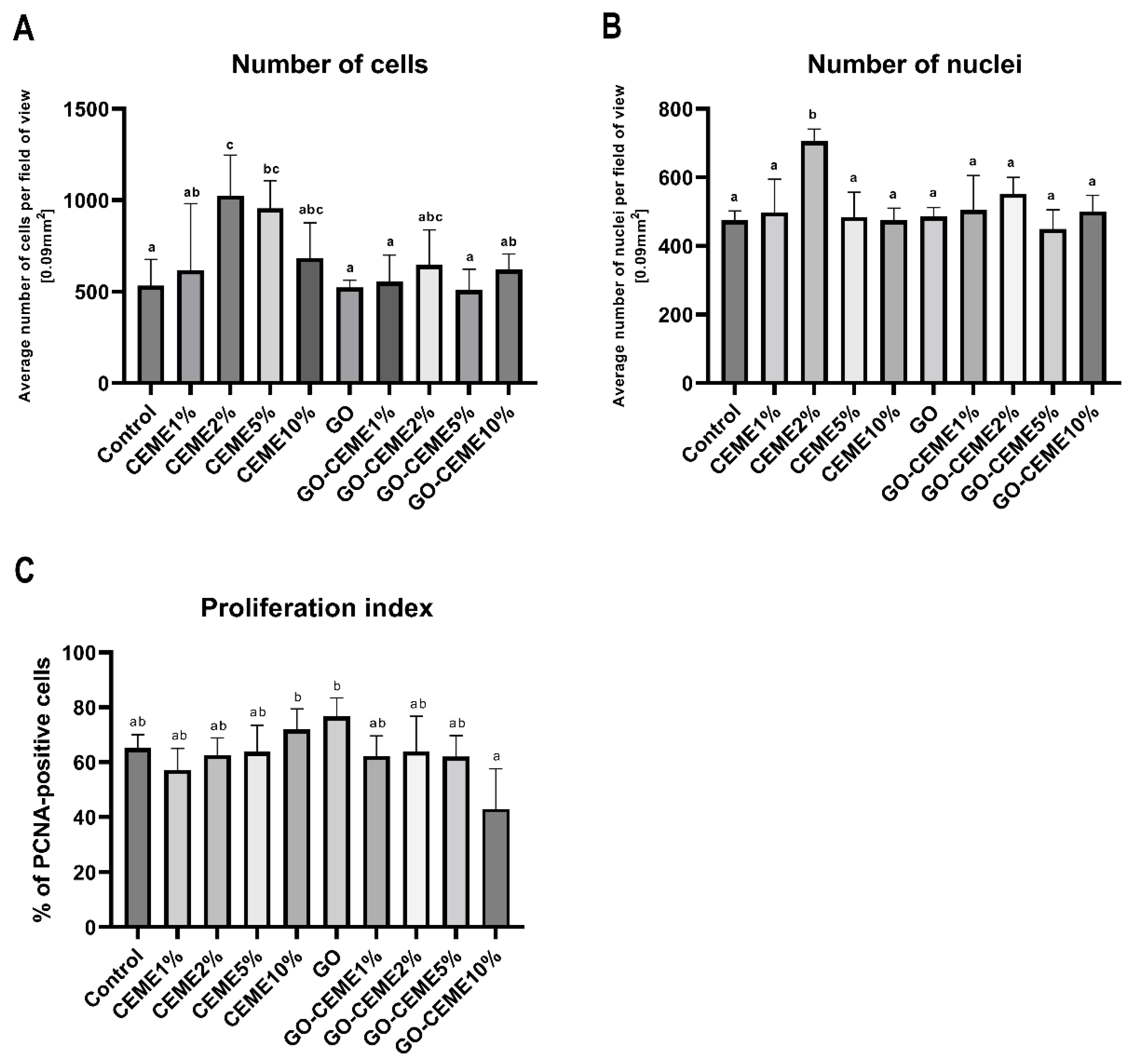
3.4. In Ovo Study: Morphological and Immunohistochemical Evaluation of Muscle Structure
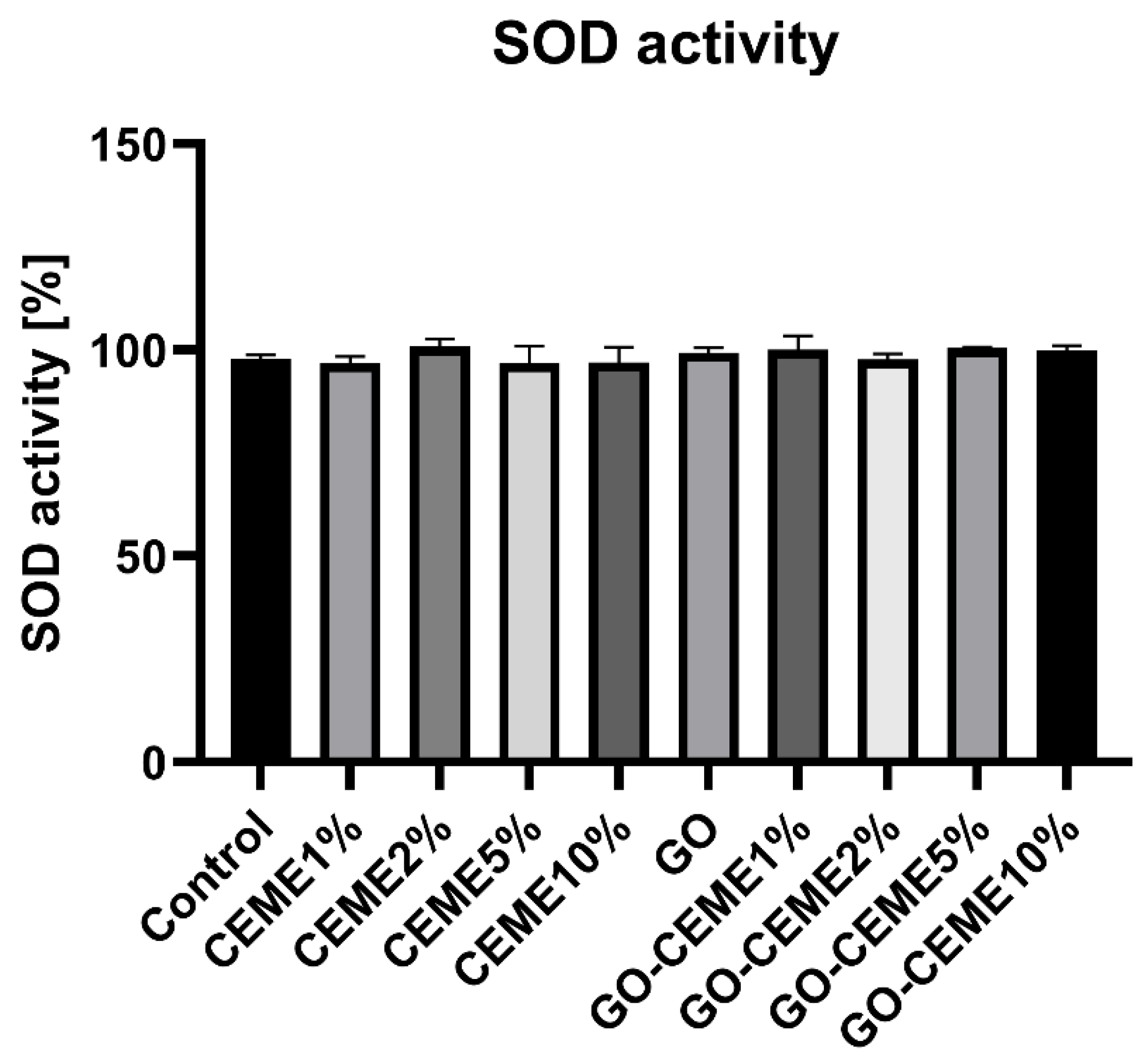
3.5. SOD Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheuermann, G.N.; Bilgili, S.F.; Tuzun, S.; Mulvaney, D.R. Comparison of chicken genotypes: Myofiber number in pectoralis muscle and myostatin ontogeny. Poult. Sci. 2004, 83, 1404–1412. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, Y.; Chen, Y.; Yang, N.; Wang, X.J.; Zhu, D. Systematic identification of genes involved in divergent skeletal muscle growth rates of broiler and layer chickens. BMC Genomics 2009, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Meloche, K.J.; Dozier, W.A.; Brandebourg, T.D.; Starkey, J.D. Skeletal muscle growth characteristics and myogenic stem cell activity in broiler chickens affected by wooden breast. Poult. Sci. 2018, 97, 4401–4414. [Google Scholar] [CrossRef]
- Sihvo, H.K.; Immonen, K.; Puolanne, E. Myodegeneration With Fibrosis and Regeneration in the Pectoralis Major Muscle of Broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef]
- Shafey, T.M.; Mahmoud, A.H.; Alsobayel, A.A.; Abouheif, M.A. Effects of in ovo administration of amino acids on hatchability and performance of meat chickens. S. Afr. J. Anim. Sci. 2014, 44, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, M.K.; Sawosz, E.; Chwalibog, A.; Ostaszewska, T.; Kamaszewski, M.; Grodzik, M.; Skomiał, J. Nano-nutrition of chicken embryos. Effect of gold and taurine nanoparticles on muscle development. J. Anim. Feed Sci. 2010, 19, 277–285. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Kang, D.; Siddiqui, S.H.; Park, J.; Tian, W.; Park, B.; Shim, K. Effects of In Ovo Supplementation with Nanonutrition (L-Arginine Conjugated with Ag NPs) on Muscle Growth, Immune Response and Heat Shock Proteins at Different Chicken Embryonic Development Stages. Animals 2020, 10, 564. [Google Scholar] [CrossRef] [Green Version]
- Givisiez, P.E.N.; Moreira Filho, A.L.B.; Santos, M.R.B.; Oliveira, H.B.; Ferket, P.R.; Oliveira, C.J.B.; Malheiros, R.D. Chicken embryo development: Metabolic and morphological basis for in ovo feeding technology. Poult. Sci. 2020, 99, 6774–6782. [Google Scholar] [CrossRef]
- Bałaban, J.; Wierzbicki, M.; Zielińska, M.; Szczepaniak, J.; Sosnowska, M.; Daniluk, K.; Cysewski, D.; Koczoń, P.; Chwalibog, A.; Sawosz, E. Effects of graphene oxide nanofilm and chicken embryo muscle extract on muscle progenitor cell differentiation and contraction. Molecules 2020, 25, 1991. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.T.; Liu, J.H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Bitounis, D.; Raveendran-Nair, R.; Servant, A.; Van den Bossche, J.; Kostarelos, K. Purified Graphene Oxide Dispersions Lack In Vitro Cytotoxicity and In Vivo Pathogenicity. Adv. Healthc. Mater. 2013, 2, 433–441. [Google Scholar] [CrossRef]
- Kurantowicz, N.; Strojny, B.; Sawosz, E.; Jaworski, S.; Kutwin, M.; Grodzik, M.; Wierzbicki, M.; Lipińska, L.; Mitura, K.; Chwalibog, A. Biodistribution of a High Dose of Diamond, Graphite, and Graphene Oxide Nanoparticles After Multiple Intraperitoneal Injections in Rats. Nanoscale Res. Lett. 2015, 10, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, O.N.; Fernando, K.A.S.; Wang, B.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.P.; Bunker, C.E. Graphene oxide: A nonspecific enhancer of cellular growth. Am. Chem. Soc. Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef]
- Górska, Z.; Górska, M.Z.; Hotowy, A.; Wierzbicki, M.; Bałaban, J.; Sosnowska, M.; Jaworski, S.; Strojny, B.; Chwalibog, A.; Sawosz, E. Graphene oxide nanofilm and the addition of l-glutamine can promote development of embryonic muscle cells. J. Nanobiotechnology 2020, 18, 1–17. [Google Scholar]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Jastrzębska, A.M.; Kurtycz, P.; Olszyna, A.R. Recent advances in graphene family materials toxicity investigations. J. Nanoparticle Res. 2012, 14, 1320. [Google Scholar] [CrossRef] [Green Version]
- Henderson, C.E.; Huchet, M.; Changeux, J.P. Denervation increases a neurite-promoting activity in extracts of skeletal muscle. Nature 1983, 302, 609–611. [Google Scholar] [CrossRef]
- Yin, Q.; Johnson, J.; Prevette, D.; Oppenheim, R. Cell death of spinal motoneurons in the chick embryo following deafferentation: Rescue effects of tissue extracts, soluble proteins, and neurotrophic agents. J. Neurosci. 1994, 14, 7629–7640. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.; Vaca, K.; McManaman, J.; Appel, S. Selective effects of skeletal muscle extract fractions on motoneuron development in vitro. J. Neurosci. 1986, 6, 439–447. [Google Scholar] [CrossRef]
- Pajtler, K.; Bohrer, A.; Maurer, J.; Schorle, H.; Schramm, A.; Eggert, A.; Schulte, J.H. Production of chick embryo extract for the cultivation of murine neural crest stem cells. J. Vis. Exp. 2010, 1, 45–47. [Google Scholar] [CrossRef] [Green Version]
- Christman, S.A.; Kong, B.W.; Landry, M.M.; Foster, D.N. Chicken embryo extract mitigates growth and morphological changes in a spontaneously immortalized chicken embryo fibroblast cell line. Poult. Sci. 2005, 84, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Strojny, B.; Kurantowicz, N.; Sawosz, E.; Grodzik, M.; Jaworski, S.; Kutwin, M.; Wierzbicki, M.; Hotowy, A.; Lipińska, L.; Chwalibog, A. Long term influence of carbon nanoparticles on health and liver status in rats. PLoS ONE 2015, 10, e0144821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, A.; Sarkar, J.; Ghosh, M.; Bhattacharya, A.; Mukherjee, A.; Chattopadhyay, D.; Acharya, K. Green conversion of graphene oxide to graphene nanosheets and its biosafety study. PLoS ONE 2017, 12, e0171607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández Rosas, J.J.; Ramírez Gutiérrez, R.E.; Escobedo-Morales, A.; Chigo Anota, E. First principles calculations of the electronic and chemical properties of graphene, graphane, and graphene oxide. J. Mol. Model. 2011, 17, 1133–1139. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. Am. Chem. Soc. Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, L.; Shi, X.; Wang, X.; Yang, Y.; Yang, K.; Liu, T.; Yang, G.; Liu, Z. Surface coating-dependent cytotoxicity and degradation of graphene derivatives: Towards the design of non-toxic, degradable nano-graphene. Small 2014, 10, 1544–1554. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved In Vitro and In Vivo Biocompatibility of Graphene Oxide through Surface Modification: Poly(Acrylic Acid)-Functionalization is Superior to PEGylation. Am. Chem. Soc. Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Gosika, M.; Velachi, V.; Cordeiro, M.N.D.S.; Maiti, P.K. Covalent Functionalization of Graphene with PAMAM Dendrimer and Its Implications on Graphene’s Dispersion and Cytotoxicity. Am. Chem. Soc. Appl. Polym. Mater. 2020, 2, 3587–3600. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Sadanandan, A.R.; Ashokan, A.; Chandran, P.; Girish, C.M.; Menon, D.; Nair, S.V.; Rao, C.N.R.; Koyakutty, M. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small 2012, 8, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.B.; da Silva, M.V.; de Morais Ribeiro, L.N. The chicken embryo as an in vivo experimental model for drug testing: Advantages and limitations. Lab Anim. 2021, 50, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Beedie, S.L.; Mahony, C.; Walker, H.M.; Chau, C.H.; Figg, W.D.; Vargesson, N. Shared mechanism of teratogenicity of anti-angiogenic drugs identified in the chicken embryo model. Sci. Rep. 2016, 6, 30038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi, A.; Sharifi, I.; Tavakkoli, H.; Derakhshanfar, A.; Keyhani, R.; Salari, Z.; Mosallanejad, S.S.; Bamorovat, M. Embryonic toxico-pathological effects of meglumine antimoniate using a chick embryo model. PLoS ONE 2018, 13, e0196424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawosz, E.; Jaworski, S.; Kutwin, M.; Hotowy, A.; Wierzbicki, M.; Grodzik, M.; Kurantowicz, N.; Strojny, B.; Lipińska, L.; Chwalibog, A. Toxicity of pristine graphene in experiments in a chicken embryo model. Int. J. Nanomed. 2014, 9, 3913–3922. [Google Scholar]
- Samak, D.H.; El-Sayed, Y.S.; Shaheen, H.M.; El-Far, A.H.; Abd El-Hack, M.E.; Noreldin, A.E.; El-Naggar, K.; Abdelnour, S.A.; Saied, E.M.; El-Seedi, H.R.; et al. Developmental toxicity of carbon nanoparticles during embryogenesis in chicken. Environ. Sci. Pollut. Res. 2020, 27, 19058–19072. [Google Scholar] [CrossRef]
- Szmidt, M.; Sawosz, E.; Urba, K.; Jaworski, S. Toxicity of different forms of graphene in a chicken embryo model. Environ. Sci. Pollut. Res. 2016, 23, 19940–19948. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Gao, T.; Zhao, M.M.; Lv, P.A.; Zhang, L.; Li, J.L.; Jiang, Y.; Gao, F.; Zhou, G.H. In ovo feeding of L-arginine alters energy metabolism in post-hatch broilers. Poult. Sci. 2017, 97, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Qaid, M.M.; Al-Garadi, M.A. Protein and amino acid metabolism in poultry during and after heat stress: A review. Animals 2021, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Uni, Z.; Yadgary, L.; Yair, R. Nutritional limitations during poultry embryonic development. J. Appl. Poult. Res. 2012, 21, 175–184. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Aghayee, S.; Fereydooni, Y.; Talebi, A. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J. Mater. Chem. 2012, 22, 13773–13781. [Google Scholar] [CrossRef]
- Langer, R.; Sydlik, S.A.; Jhunjhunwala, S.; Webber, M.J.; Anderson, D.G. In Vivo Compatibility of Graphene Oxide with Differing Oxidation States. Am. Chem. Soc. Nano 2015, 9, 3866–3874. [Google Scholar]
- Hsieh, H.S.; Zepp, R.G. Reactivity of graphene oxide with reactive oxygen species (hydroxyl radical, singlet oxygen, and superoxide anion). Environ. Sci. Nano 2019, 6, 3734–3744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ylihärsilä, H.; Kajantie, E.; Osmond, C.; Forsén, T.; Barker, D.J.P.; Eriksson, J.G. Birth size, adult body composition and muscle strength in later life. Int. J. Obes. 2007, 31, 1392–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.W.; McMurtry, J.P.; Coon, C.N. Developmental changes of plasma insulin, glucagon, insulin-like growth factors, thyroid hormones, and glucose concentrations in chick embryos and hatched chicks. Poult. Sci. 2007, 86, 673–683. [Google Scholar] [CrossRef]
- McLaughlin, S.H.; Bulleid, N.J. Thiol-independent interaction of protein disulphide isomerase with type X collagen during intra-cellular folding and assembly. Biochem. J. 1998, 331, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Widmer, C.; Gebauer, J.M.; Brunstein, E.; Rosenbaum, S.; Zaucke, F.; Drögemüller, C. Molecular basis for the action of the collagen-specific chaperone Hsp47 / SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. USA 2012, 109, 13243–13247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, A.; Messéant, J.; Parlakian, A.; Lemaitre, M.; Roy, P.; Delacroix, C.; Lilienbaum, A.; Hovhannisyan, Y.; Furling, D.; Klein, A.; et al. Desmin prevents muscle wasting, exaggerated weakness and fragility, and fatigue in dystrophic mdx mouse. J. Physiol. 2020, 598, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Mado, K.; Chekulayev, V.; Shevchuk, I.; Puurand, M.; Tepp, K.; Kaambre, T. On the role of tubulin, plectin, desmin, and vimentin in the regulation of mitochondrial energy fluxes in muscle cells. Am. J. Physiol. Cell Physiol. 2019, 316, C657–C667. [Google Scholar] [CrossRef] [PubMed]
- Moutal, A.; White, K.A.; Chefdeville, A.; Laufmann, R.N.; Vitiello, P.F.; Feinstein, D.; Weimer, J.M.; Khanna, R. Dysregulation of CRMP2 Post-Translational Modifications Drive Its Pathological Functions. Mol. Neurobiol. 2019, 56, 6736–6755. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Cha, C.; Ye, Y.; Zhang, J.; Li, S.; Wu, F.; Gong, S.; Guo, G. CRMP4 and CRMP2 interact to coordinate cytoskeleton dynamics, regulating growth cone development and axon elongation. Neural Plast. 2015, 2015, 947423. [Google Scholar] [CrossRef] [PubMed]

| Group | Egg Mass [G] | Embryo Body Weight [G] | Heart Weight [G] | Liver Weight [G] |
|---|---|---|---|---|
| Control | 54.1 ± 4.42 | 45.1 ± 3.52 | 0.23 ± 0.014 | 0.63 ± 0.063 |
| CEME1% | 53.7 ± 2.95 | 44.5 ± 2.46 | 0.23 ± 0.043 | 0.67 ± 0.094 |
| CEME2% | 52.6 ± 3.05 | 43.9 ± 2.32 | 0.24 ± 0.071 | 0.61 ± 0.103 |
| CEME5% | 52.9 ± 2.70 | 44.2 ± 2.72 | 0.23 ± 0.032 | 0.62 ± 0.092 |
| CEME10% | 55.6 ± 3.25 | 45.6 ± 3.28 | 0.24 ± 0.024 | 0.59 ± 0.091 |
| GO | 54.8 ± 3.55 | 47.0 ± 5.49 | 0.22 ± 0.023 | 0.63 ± 0.142 |
| GO-CEME1% | 51.9 ± 3.49 | 43.0 ± 2.98 | 0.23 ± 0.028 | 0.64 ± 0.085 |
| GO-CEME2% | 55.8 ± 3.35 | 51.2 ± 2.71 | 0.19 ± 0.072 | 0.57 ± 0.067 |
| GO-CEME5% | 51.2 ± 3.41 | 47.5 ± 3.57 | 0.22 ± 0.027 | 0.65 ± 0.100 |
| GO-CEME10% | 52.9 ± 2.74 | 44.4 ± 1.78 | 0.24 ± 0.035 | 0.63 ± 0.081 |
| Group | Albumins [G/L] | ALT [U/L] | AP [U/L] | AST [U/L] | Total Protein [G/L] | Creatinine [µmol/L] | Urea [Mmol/L] | Globulins [G/L] | LDH [U/L] | Triglyceride [Mmol/L] | Glucose [Mmol/L] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 3.15 ± 0.663 | 3.38 ± 1.230 | 2738 ±320.2 | 214.2 ± 52.80 | 13.3 ± 1.72 | 19.6 ± 12.19 | 3.56 ± 0.861 ab | 10.1 ± 1.07 | 1100 ± 84.2 | 0.71 ± 0.203 | 14.2 ± 0.30 |
| CEME1% | 4.05 ± 1.011 | 2.68 ± 1.681 | 2858 ± 1267.2 | 180.7 ± 62.41 | 15.8 ± 2.18 | 28.5 ± 4.47 | 4.06 ± 0.333 ab | 11.7 ± 1.18 | 1156 ± 343.1 | 1.08 ± 0.372 | 12.4 ± 0.64 |
| CEME2% | 3.01 ± 0.285 | 2.52 ± 0.143 | 2218 ± 176.5 | 171.5 ± 21.07 | 12.1 ± 0.85 | 29.9 ± 1.98 | 3.94 ± 0.534 ab | 9.1 ± 0.57 | 909 ± 76.3 | 0.84 ± 1.345 | 12.3 ± 0.28 |
| CEME5% | 3.87 ± 0.413 | 3.92 ± 1.502 | 2844 ± 521.9 | 214.9 ± 56.96 | 15.5 ± 1.10 | 28.1 ± 6.18 | 4.13 ± 0.645 b | 11.6 ± 0.82 | 1213 ± 279.9 | 1.01 ± 0.231 | 12.4 ± 0.53 |
| CEME10% | 3.42 ± 0.142 | 3.58 ± 2.343 | 2249 ± 309.6 | 202.6 ± 118.14 | 13.3 ± 1.02 | 37.3 ± 18.31 | 4.17 ± 0.912 b | 10.0 ± 0.95 | 1029. ± 542.3 | 1.22 ± 1.212 | 12. ± 1.65 |
| GO | 3.52 ± 0.522 | 4.52 ± 0.786 | 2199 ± 439.4 | 299.4 ± 29.25 | 13.6 ± 1.28 | 30.6 ± 6.12 | 4.19 ± 0.583 b | 10.1 ± 0.88 | 1448 ± 389.0 | 1.02 ± 0.343 | 11.55 ± 1.20 |
| GO-CEME1% | 3.15 ± 0.217 | 3.05 ± 1.345 | 2161 ± 377.5 | 200.3 ± 3.25 | 12.9 ± 0.85 | 23.6 ± 2.33 | 3.31 ± 0.034 ab | 9.8 ± 0.64 | 1189 ± 269.5 | 1.36 ± 0.465 | 14.7 ± 1.27 |
| GO-CEME2% | 3.68 ± 1.131 | 3.68 ± 1.50 | 2629 ± 939.1 | 200.6 ± 51.81 | 15.0 ± 3.67 | 26.1 ± 4.29 | 3.75 ± 0.593 ab | 11.3 ± 2.60 | 1117 ± 187.7 | 0.64 ± 0.161 | 12.0 ± 0.68 |
| GO-CEME5% | 3.9 ± 0.911 | 2.5 ± 1.55 | 2496 ± 403.6 | 190.0 ± 46.74 | 15.0 ± 2.13 | 22.8 ± 3.16 | 3.04 ± 0.200 a | 11.1 ± 1.27 | 1177. ± 289.1 | 1.04 ± 0.384 | 14.5 ± 0.74 |
| GO-CEME10% | 3.13 ± 0.394 | 3.71 ± 1.61 | 2732 ± 707.2 | 170.4 ± 3.92 | 12.7 ± 1.49 | 22.8 ± 2.61 | 3.37 ± 0.445 ab | 9.6 ± 1.21 | 1130 ± 365.6 | 1.04 ± 0.472 | 11.9 ± 1.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bałaban, J.; Zielińska, M.; Wierzbicki, M.; Ostaszewska, T.; Fajkowska, M.; Rzepakowska, M.; Daniluk, K.; Sosnowska, M.; Chwalibog, A.; Sawosz, E. Effect of Muscle Extract and Graphene Oxide on Muscle Structure of Chicken Embryos. Animals 2021, 11, 3467. https://doi.org/10.3390/ani11123467
Bałaban J, Zielińska M, Wierzbicki M, Ostaszewska T, Fajkowska M, Rzepakowska M, Daniluk K, Sosnowska M, Chwalibog A, Sawosz E. Effect of Muscle Extract and Graphene Oxide on Muscle Structure of Chicken Embryos. Animals. 2021; 11(12):3467. https://doi.org/10.3390/ani11123467
Chicago/Turabian StyleBałaban, Jaśmina, Marlena Zielińska, Mateusz Wierzbicki, Teresa Ostaszewska, Magdalena Fajkowska, Małgorzata Rzepakowska, Karolina Daniluk, Malwina Sosnowska, André Chwalibog, and Ewa Sawosz. 2021. "Effect of Muscle Extract and Graphene Oxide on Muscle Structure of Chicken Embryos" Animals 11, no. 12: 3467. https://doi.org/10.3390/ani11123467







