Efficacy and Function of Feathers, Hair, and Glabrous Skin in the Thermoregulation Strategies of Domestic Animals
Abstract
:Simple Summary
Abstract
1. Introduction
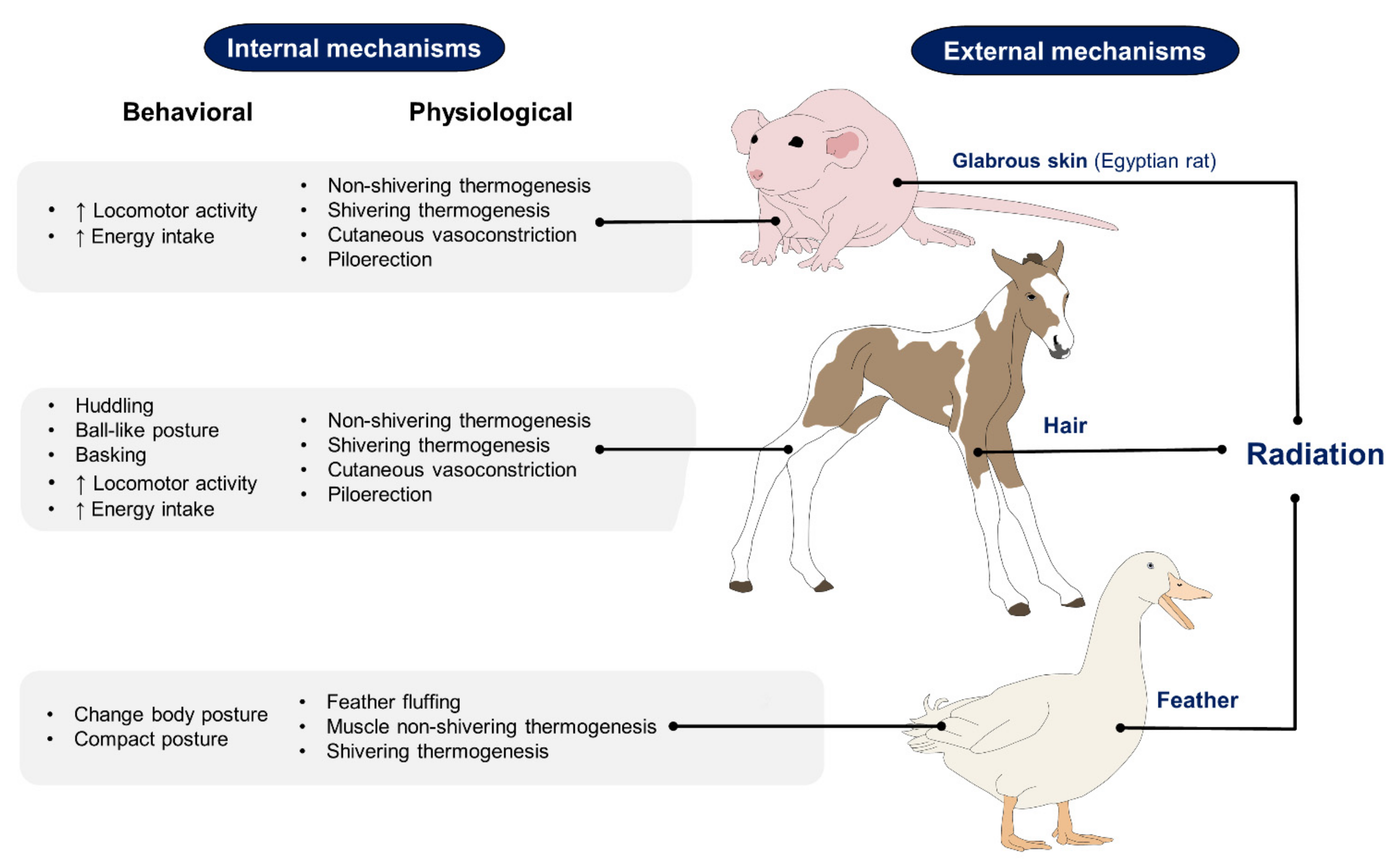
2. Differences in Thermoregulation Strategies between Animals with Feathers, Hair, and Glabrous Skin
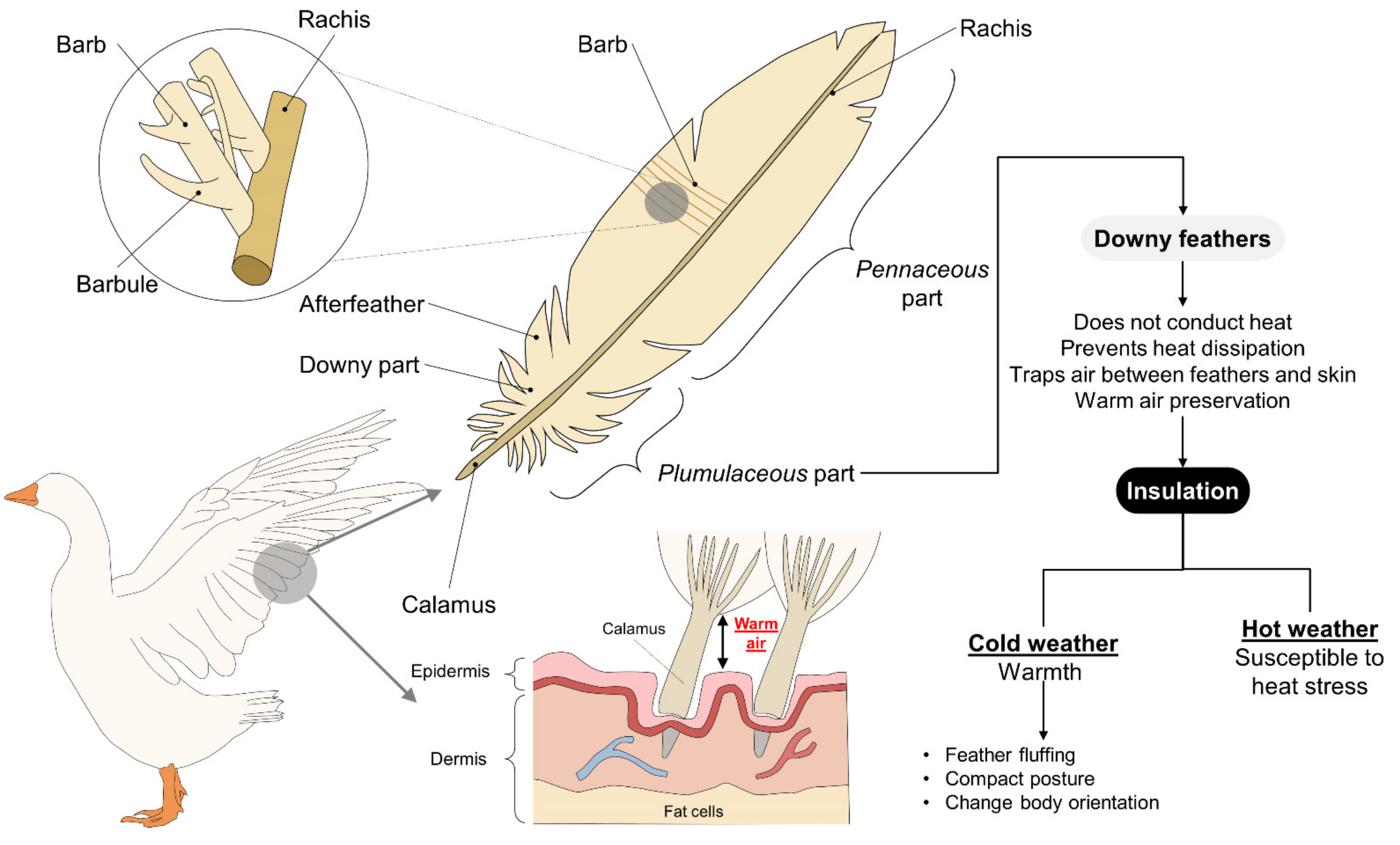
2.1. Birds
2.2. Animals with Hair
2.3. Animals with Glabrous Skin or Fine Hair
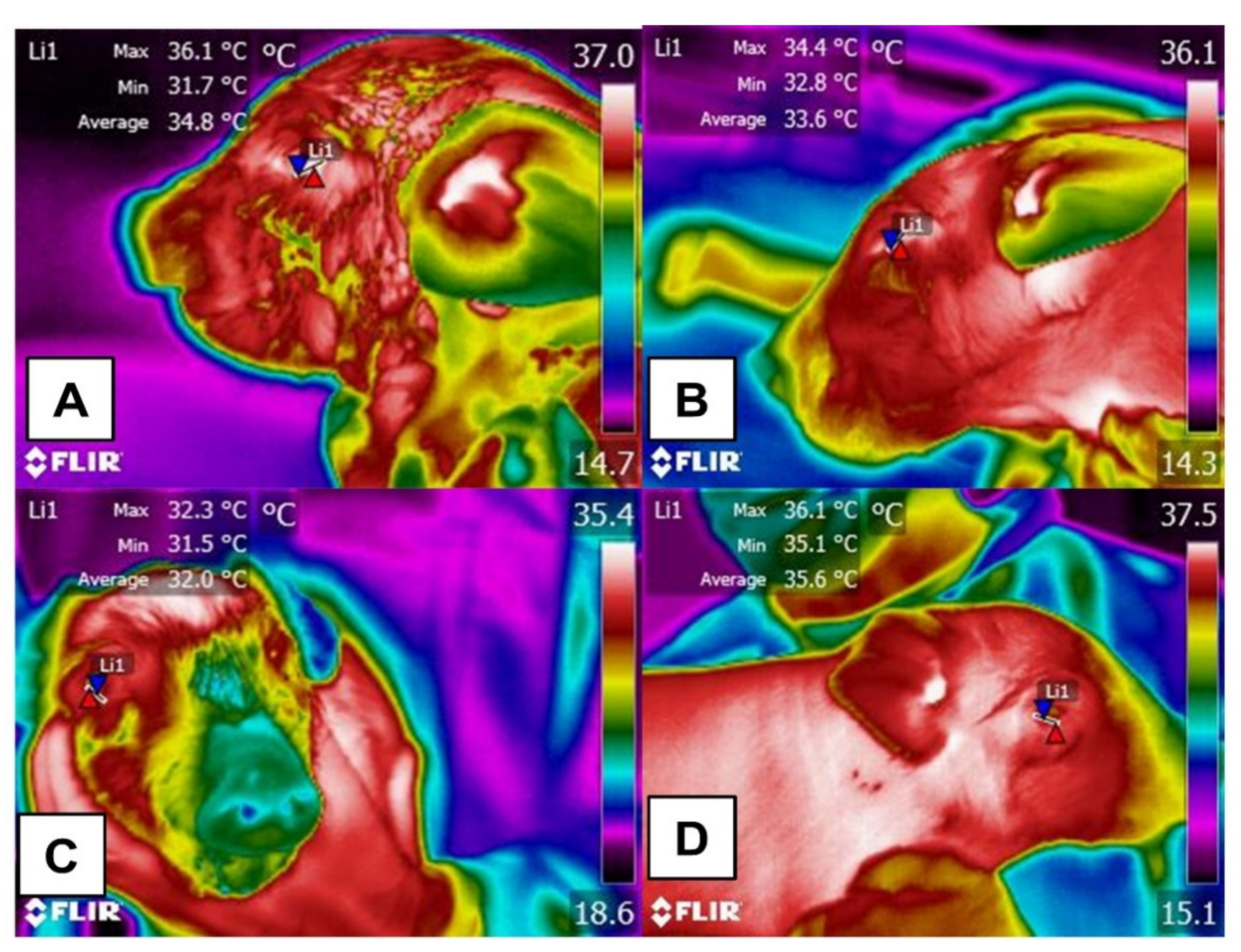
3. Use of IRT as a Minimal Invasive Technique for Detecting Temperature Differences in Animals under Conditions of Heat or Cold Stress
4. Effect of Hair, Feathers, and Glabrous Skin Based on Recent Scientific Findings on the Use of IRT
4.1. Hair
4.2. Feathers
4.3. Glabrous Skin
5. Perspectives on the Use of IRT and Species-Specific Thermal for Animals with Distinct Integumentary Structures
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Fiebig, K.; Jourdan, T.; Kock, M.H.; Merle, R.; Thöne-Reineke, C. Evaluation of infrared thermography for temperature measurement in adult male NMRI nude mice. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 715–724. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Orihuela, A.; Strappini-Asteggiano, A.; Nelly Cajiao-Pachón, M.; Agüera-Buendía, E.; Mora-Medina, P.; Ghezzi, M.; Alonso-Spilsbury, M. Teaching animal welfare in veterinary schools in Latin America. Int. J. Vet. Sci. Med. 2018, 6, 131–140. [Google Scholar] [CrossRef]
- Szekely, M. Thermoregulation energy balance regulatory peptides recent developments. Front. Biosci. 2010, 2, 116. [Google Scholar] [CrossRef] [Green Version]
- Bouchama, A.; Knochel, J.P. Heat stroke. N. Engl. J. Med. 2002, 346, 1978–1988. [Google Scholar] [CrossRef]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Kingma, B. The thermoneutral zone: Implications for metabolic studies. Front. Biosci. 2012, 4, 1975. [Google Scholar] [CrossRef]
- Kingma, B.R.; Frijns, A.J.; Schellen, L.; van Marken Lichtenbelt, W.D. Beyond the classic thermoneutral zone. Temperature 2014, 1, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Schepers, R.J.; Ringkamp, M. Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 2010, 34, 177–184. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Señarís, R.; Ordás, P.; Reimúndez, A.; Viana, F. Mammalian cold TRP channels: Impact on thermoregulation and energy homeostasis. Pflügers Arch. Eur. J. Physiol. 2018, 470, 761–777. [Google Scholar] [CrossRef]
- Kregel, K.C.; Wall, P.T.; Gisolfi, C.V. Peripheral vascular responses to hyperthermia in the rat. J. Appl. Physiol. 1988, 64, 2582–2588. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R37–R46. [Google Scholar] [CrossRef]
- Cheshire, W.P. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. 2016, 196, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Epstein, Y.; Moran, D.S. Thermal comfort and the heat stress indices. Ind. Health 2006, 44, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Mortola, J.P. Social interaction and the thermogenic response of chicken hatchlings. Physiol. Behav. 2021, 232, 113317. [Google Scholar] [CrossRef]
- Robertshaw, D. Mechanisms for the control of respiratory evaporative heat loss in panting animals. J. Appl. Physiol. 2006, 101, 664–668. [Google Scholar] [CrossRef]
- Ratnakaran, A.P.; Sejian, V.; Jose, V.S.; Vaswani, S.; Bagath, M.; Krishnan, G.; Beena, V.; Devi, P.I.; Varma, G.; Bhatta, R. Behavioral responses to livestock adaptation to heat stress challenges. Asian J. Anim. Sci. 2016, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Affolter, V.K.; Moore, P.F. Histologic features of normal canine and feline skin. Clin. Dermatol. 1994, 12, 491–497. [Google Scholar] [CrossRef]
- Mecklenburg, L. An overview on congenital alopecia in domestic animals. Vet. Dermatol. 2006, 17, 393–410. [Google Scholar] [CrossRef]
- Parker, H.G.; Harris, A.; Dreger, D.L.; Davis, B.W.; Ostrander, E.A. The bald and the beautiful: Hairlessness in domestic dog breeds. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20150488. [Google Scholar] [CrossRef]
- Mauldin, E.A.; Peters-Kennedy, J. Integumentary system. In Jubb, Kennedy & Palmer’s Pathology of Domestic Animals; Grant, M.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 509–736. [Google Scholar]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and behavioral mechanisms of thermoregulation in mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Precht, H.; Christophersen, J.; Hensel, H.; Larcher, W. Heat exchange with the environment. In Temperature and Life, 2nd ed.; Precht, H., Christophersen, J., Hensel, H., Larcher, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1973; pp. 545–564. [Google Scholar]
- Hillman, R.; Gilbert, R. Reproductive diseases. In Rebhun’s Diseases of Dairy Cattle; Divers, T.J., Peek, S.F., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2008; pp. 395–446. [Google Scholar]
- Gordon, C.J.; Becker, P.; Ali, J.S. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol. Behav. 1998, 65, 255–262. [Google Scholar] [CrossRef]
- Yáñez-Pizaña, A.; de la Cruz-Cruz, L.A.; Tarazona-Morales, A.; Roldan-Santiago, P.; Ballesteros-Rodea, G.; Pineda-Reyes, R.; Orozco-Gregorio, H. Physiological and behavioral changes of water buffalo in hot and cold systems: Review. J. Buffalo Sci. 2020, 9, 110–120. [Google Scholar] [CrossRef]
- Lalonde, S.; Beaulac, K.; Crowe, T.G.; Schwean-Lardner, K. The effects of simulated transportation conditions on the core body and extremity temperature, blood physiology, and behavior of white-strain layer pullets. Poult. Sci. 2021, 100, 697–706. [Google Scholar] [CrossRef]
- Nowack, J.; Giroud, S.; Arnold, W.; Ruf, T. Muscle non-shivering thermogenesis and its role in the evolution of endothermy. Front. Physiol. 2017, 8, 889. [Google Scholar] [CrossRef] [Green Version]
- Tattersall, G.J.; Andrade, D.V.; Abe, A.S. Heat exchange from the toucan bill reveals a controllable vascular thermal radiator. Science 2009, 325, 468–470. [Google Scholar] [CrossRef] [Green Version]
- Arfuso, F.; Fazio, F.; Rizzo, M.; Marafioti, S.; Zanghì, E.; Piccione, G. Factors affecting the hematological parameters in different goat breeds from Italy. Ann. Anim. Sci. 2016, 16, 743–757. [Google Scholar] [CrossRef] [Green Version]
- Jerem, P.; Jenni-Eiermann, S.; Herborn, K.; McKeegan, D.; McCafferty, D.J.; Nager, R.G. Eye region surface temperature reflects both energy reserves and circulating glucocorticoids in a wild bird. Sci. Rep. 2018, 8, 1907. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Pereira, M.F.A.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Ávalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical applications and factors involved in validating thermal windows in large rumiants to assess health and productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef]
- Allen, J.; Howell, K. Microvascular imaging: Techniques and opportunities for clinical physiological measurements. Physiol. Meas. 2014, 35, R91–R141. [Google Scholar] [CrossRef]
- Rizzo, M.; Arfuso, F.; Alberghina, D.; Giudice, E.; Gianesella, M.; Piccione, G. Monitoring changes in body surface temperature associated with treadmill exercise in dogs by use of infrared methodology. J. Therm. Biol. 2017, 69, 64–68. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5. [Google Scholar] [CrossRef] [Green Version]
- Hill, R.A.; Weingrill, T.; Barrett, L.; Henzi, S.P. Indices of environmental temperatures for primates in open habitats. Primates 2004, 45, 7–13. [Google Scholar] [CrossRef]
- Milbergue, M.S.; Blier, P.U.; Vézina, F. Large muscles are beneficial but not required for improving thermogenic capacity in small birds. Sci. Rep. 2018, 8, 14009. [Google Scholar] [CrossRef]
- George, J.C. Thermogenesis in the avian body and the role of the pineal in thermoregulation. Prog. Clin. Biol. Res. 1982, 92, 217–231. [Google Scholar]
- Graveley, J.M.F.; Burgio, K.R.; Rubega, M. Using a thermal camera to measure heat loss through bird feather coats. J. Vis. Exp. 2020, 160, 1–16. [Google Scholar] [CrossRef]
- D’alba, L.; Carlsen, T.H.; Ásgeirsson, Á.; Shawkey, M.D.; Jónsson, J.E. Contributions of feather microstructure to eider down insulation properties. J. Avian Biol. 2017, 48, 1150–1157. [Google Scholar] [CrossRef]
- Pollock, H.S.; Brawn, J.D.; Agin, T.J.; Cheviron, Z.A. Differences between temperate and tropical birds in seasonal acclimatization of thermoregulatory traits. J. Avian Biol. 2019, 50, jav.02067. [Google Scholar] [CrossRef]
- Irving, L. Birds of Anaktuvuk Pass, Kubuk, and Old Crow; United States National Museum: Washington, DC, USA, 1960; pp. 1–409. [Google Scholar]
- Dove, C.J.; Rijke, A.M.; Wang, X.; Andrews, L.S. Infrared analysis of contour feathers. J. Therm. Biol. 2007, 32, 42–46. [Google Scholar] [CrossRef]
- Barve, S.; Ramesh, V.; Dotterer, T.M.; Dove, C.J. Elevation and body size drive convergent variation in thermo-insulative feather structure of Himalayan birds. Ecography 2021, 44, 680–689. [Google Scholar] [CrossRef]
- Pap, P.L.; Osváth, G.; Daubner, T.; Nord, A.; Vincze, O. Down feather morphology reflects adaptation to habitat and thermal conditions across the avian phylogeny. Evolution 2020, 74, 2365–2376. [Google Scholar] [CrossRef]
- Pap, P.L.; Vincze, O.; Wekerle, B.; Daubner, T.; Vágási, C.I.; Nudds, R.L.; Dyke, G.J.; Osváth, G. A phylogenetic comparative analysis reveals correlations between body feather structure and habitat. Funct. Ecol. 2017, 31, 1241–1251. [Google Scholar] [CrossRef] [Green Version]
- Malheiros, R.D.; Moraes, V.M.B.; Bruno, L.D.G.; Malheiros, E.B.; Furlan, R.L.; Macari, M. Environmental temperature and cloacal and surface temperatures of broiler chicks in first week post-hatch. J. Appl. Poult. Res. 2000, 9, 111–117. [Google Scholar] [CrossRef]
- Shinder, D.; Rusal, M.; Tanny, J.; Druyan, S.; Yahav, S. Thermoregulatory responses of chicks (Gallus domesticus) to low ambient temperatures at an early age. Poult. Sci. 2007, 86, 2200–2209. [Google Scholar] [CrossRef]
- Ng, C.S.; Li, W.-H. Genetic and molecular basis of feather diversity in birds. Genome Biol. Evol. 2018, 10, 2572–2586. [Google Scholar] [CrossRef]
- Alibardi, L. Cell structure of barb ridges in down feathers and juvenile wing feathers of the developing chick embryo: Barb ridge modification in relation to feather evolution. Ann. Anat. 2006, 188, 303–318. [Google Scholar] [CrossRef]
- Cândido, M.G.L.; Tinôco, I.F.F.; Albino, L.F.T.; Freitas, L.C.S.R.; Santos, T.C.; Cecon, P.R.; Gates, R.S. Effects of heat stress on pullet cloacal and body temperature. Poult. Sci. 2020, 99, 2469–2477. [Google Scholar] [CrossRef]
- Peguri, A.; Coon, C. Effect of feather coverage and temperature on layer performance. Poult. Sci. 1993, 72, 1318–1329. [Google Scholar] [CrossRef]
- Nawaz, A.H.; Amoah, K.; Leng, Q.Y.; Zheng, J.H.; Zhang, W.L.; Zhang, L. Poultry response to heat stress: Its physiological, metabolic, and genetic implications on meat production and quality including strategies to improve broiler production in a warming world. Front. Vet. Sci. 2021, 8, 699081. [Google Scholar] [CrossRef]
- Koskenpato, K.; Ahola, K.; Karstinen, T.; Karell, P. Is the denser contour feather structure in pale grey than in pheomelanic brown tawny owls Strix aluco an adaptation to cold environments? J. Avian Biol. 2016, 47, 1–6. [Google Scholar] [CrossRef]
- Stuart-Fox, D.; Newton, E.; Clusella-Trullas, S. Thermal consequences of colour and near-infrared reflectance. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160345. [Google Scholar] [CrossRef]
- Bicudo, J.E.P.W.; Vianna, C.R.; Chaui-Berlinck, J.G. Thermogenesis in birds. Biosci. Rep. 2001, 21, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Turnpenny, J.; Wathes, C.; Clark, J.; McArthur, A. Thermal balance of livestock. Agric. For. Meteorol. 2000, 101, 29–52. [Google Scholar] [CrossRef]
- Fortin, D.; Larochelle, J.; Gauthier, G. The effect of wind, radiation and body orientation on the thermal environment of Greater Snow goose goslings. J. Therm. Biol. 2000, 25, 227–238. [Google Scholar] [CrossRef]
- Teulier, L.; Rouanet, J.-L.; Letexier, D.; Romestaing, C.; Belouze, M.; Rey, B.; Duchamp, C.; Roussel, D. Cold-acclimation-induced non-shivering thermogenesis in birds is associated with upregulation of avian UCP but not with innate uncoupling or altered ATP efficiency. J. Exp. Biol. 2010, 213, 2476–2482. [Google Scholar] [CrossRef] [Green Version]
- McKechnie, A.E.; Lovegrove, B.G. Avian facultative hypothermic responses: A Review. Condor 2002, 104, 705–724. [Google Scholar] [CrossRef]
- Sears, M.W.; Angilletta, M.J. Costs and benefits of thermoregulation revisited: Both the heterogeneity and spatial structure of temperature drive energetic costs. Am. Nat. 2015, 185, E94–E102. [Google Scholar] [CrossRef] [Green Version]
- Tattersall, G.J.; Arnaout, B.; Symonds, M.R.E. The evolution of the avian bill as a thermoregulatory organ. Biol. Rev. 2017, 92, 1630–1656. [Google Scholar] [CrossRef]
- LaBarbera, K.; Marsh, K.J.; Hayes, K.R.R.; Hammond, T.T. Context-dependent effects of relative temperature extremes on bill morphology in a songbird. R. Soc. Open Sci. 2020, 7, 192203. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, T.C.M.; Walsberg, G.E.; DeNardo, D.F. Cloacal evaporation: An important and previously undescribed mechanism for avian thermoregulation. J. Exp. Biol. 2007, 210, 741–749. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.; Kaplan-Stein, S.; Adolph, S.; Peralta, J.M. Assessment of non-contact infrared thermometer measurement sites in birds. J. Appl. Anim. Welf. Sci. 2020, 23, 131–139. [Google Scholar] [CrossRef]
- Murphrey, M.B.; Agarwal, S.; Zito, P.M. Anatomy, Hair; StatPearls Publishing: Treasure Island, FL, USA, 2019; p. 1. [Google Scholar]
- Welle, M.M.; Wiener, D.J. The hair follicle. Toxicol. Pathol. 2016, 44, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Marai, I.F.M.; Haeeb, A.A.M. Buffalo’s biological functions as affected by heat stress—A review. Livest. Sci. 2010, 127, 89–109. [Google Scholar] [CrossRef]
- Goncalves, A.A.; Rosetto, G.A.; Tavares, R.F.S.; Rodrigues, S.J.A.; Neves, M.D.; Castro, G.T.; Ribeiro, T.H.; Velasco, G.S.; Baia, S.E.; do Socorro, D.S.S.; et al. Scrotal thermoregulation and sequential sperm abnormalities in buffalo bulls (Bubalus bubalis) under short-term heat stress. J. Therm. Biol. 2021, 96, 102842. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Wang, D.; Titto, C.G.; Gómez-Prado, J.; Fuente, V.C.; Ghezzi, M.; Boscato-Funes, L.; Barrios-García, H.; Torres-Bernal, F.; Casas-Alvarado, A.; et al. Pathophysiology of fever and application of infrared thermography (IRT) in the detection of sick domestic animals: Recent advances. Animals 2021, 11, 2316. [Google Scholar] [CrossRef]
- Khongdee, T.; Sripoon, S.; Vajrabukka, C. The effects of high temperature and wallow on physiological responses of swamp buffaloes (Bubalus bubalis) during winter season in Thailand. J. Therm. Biol. 2011, 36, 417–421. [Google Scholar] [CrossRef]
- McCafferty, D.J.; Pandraud, G.; Gilles, J.; Fabra-Puchol, M.; Henry, P.-Y. Animal thermoregulation: A review of insulation, physiology and behaviour relevant to temperature control in buildings. Bioinspir. Biomim. 2017, 13, 011001. [Google Scholar] [CrossRef] [Green Version]
- Cena, K.; Clark, J.A.; Spotila, J.R. Thermoregulation. In Biology of the Integument; Bereiter-Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin, Germany, 1986; pp. 517–534. [Google Scholar]
- Jørgensen, M.G.H.; Mejdell, C.M.; Bøe, K.E. Effects of hair coat characteristics on radiant surface temperature in horses. J. Therm. Biol. 2020, 87, 102474. [Google Scholar] [CrossRef]
- Osthaus, B.; Proops, L.; Long, S.; Bell, N.; Hayday, K.; Burden, F. Hair coat properties of donkeys, mules and horses in a temperate climate. Equine Vet. J. 2018, 50, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Webb, D.R.; Porter, W.P.; McClure, P.A. Development of insulation in juvenile rodents: Functional compromise in insulation. Funct. Ecol. 1990, 4, 251. [Google Scholar] [CrossRef]
- Soppela, P.; Nieminen, M.; Saarela, S.; Hissa, R. The influence of ambient temperature on metabolism and body temperature of newborn and growing reindeer calves (Rangifer tarandus tarandus L.). Comp. Biochem. Physiol. Part. A Physiol. 1986, 83, 371–386. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K. Scaling: Why Is Animal Size So Important; Cambridge University Press: Cambridge, UK, 1995; pp. 1–245. [Google Scholar]
- Pearson, L.E.; Weitzner, E.L.; Burns, J.M.; Hammill, M.O.; Liwanag, H.E.M. From ice to ocean: Changes in the thermal function of harp seal pelt with ontogeny. J. Comp. Physiol. B 2019, 189, 501–511. [Google Scholar] [CrossRef]
- Gmuca, N.V.; Pearson, L.E.; Burns, J.M.; Liwanag, H.E.M. The fat and the furriest: Morphological changes in harp seal fur with ontogeny. Physiol. Biochem. Zool. 2015, 88, 158–166. [Google Scholar] [CrossRef]
- Dawson, T.J.; Webster, K.N.; Maloney, S.K. The fur of mammals in exposed environments; Do crypsis and thermal needs necessarily conflict? The polar bear and marsupial koala compared. J. Comp. Physiol. B 2014, 184, 273–284. [Google Scholar] [CrossRef]
- Chaplin, G.; Jablonski, N.G.; Sussman, R.W.; Kelley, E.A. The role of piloerection in primate thermoregulation. Folia Primatol. 2014, 85, 1–17. [Google Scholar] [CrossRef]
- Wolf, B.O.; Walsberg, G.E. The role of the plumage in heat transfer processes of birds. Am. Zool. 2000, 40, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Wacker, C.B.; McAllan, B.M.; Körtner, G.; Geiser, F. The functional requirements of mammalian hair: A compromise between crypsis and thermoregulation? Sci. Nat. 2016, 103, 53. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Mecklenburg, L. Congenital alopecia. In Hair Loss Disorders in Domestic Animals; Mecklenburg, L., Linek, M., Tobin, D.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 93–94. [Google Scholar]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; José, N.; Casas-Alvarado, A.; Gómez, J.; Mora-Medina, P. Thermal homeostasis in the newborn puppy: Behavioral and physiological responses. J. Anim. Behav. Biometeorol. 2021, 9, 1–25. [Google Scholar] [CrossRef]
- Le Dividich, J.; Noblet, J. Colostrum intake and thermoregulation in the neonatal pig in relation to environmental temperature. Neonatology 1981, 40, 167–174. [Google Scholar] [CrossRef]
- Le Dividich, J.; Noblet, J. Thermoregulation and energy metabolism in the neonatal pig. Ann. Rech. Vet. 1983, 14, 375–381. [Google Scholar]
- Satterfield, M.C. Brown adipose tissue growth and development: Significance and nutritional regulation. Front. Biosci. 2011, 16, 1589. [Google Scholar] [CrossRef] [Green Version]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Lin, J.; Cao, C.; Tao, C.; Ye, R.; Dong, M.; Zheng, Q.; Wang, C.; Jiang, X.; Qin, G.; Yan, C.; et al. Cold adaptation in pigs depends on UCP3 in beige adipocytes. J. Mol. Cell Biol. 2017, 9, 364–375. [Google Scholar] [CrossRef]
- Renaudeau, D.; Leclercq-Smekens, M.; Herin, M. Differences in skin characteristics in European (Large White) and Caribbean (Creole) growing pigs with reference to thermoregulation. Anim. Res. 2006, 55, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Daly, T.J.M.; Buffenstein, R. Skin morphology and its role in thermoregulation in mole-rats, Heterocephalus glaber and Cryptomys hottentotus. J. Anat. 1998, 193, 495–502. [Google Scholar] [CrossRef]
- Ward, J.; McCafferty, D.J.; Houston, D.C.; Ruxton, G.D. Why do vultures have bald heads? The role of postural adjustment and bare skin areas in thermoregulation. J. Therm. Biol. 2008, 33, 168–173. [Google Scholar] [CrossRef]
- Boldt, L.-H.; Fraszl, W.; Röcker, L.; Schefold, J.C.; Steinach, M.; Noack, T.; Gunga, H.-C. Changes in the haemostatic system after thermoneutral and hyperthermic water immersion. Eur. J. Appl. Physiol. 2008, 102, 547–554. [Google Scholar] [CrossRef]
- Wanner, S.P.; Prímola-Gomes, T.N.; Pires, W.; Guimarães, J.B.; Hudson, A.S.R.; Kunstetter, A.C.; Fonseca, C.G.; Drummond, L.R.; Damasceno, W.C.; Teixeira-Coelho, F. Thermoregulatory responses in exercising rats: Methodological aspects and relevance to human physiology. Temperature 2015, 2, 457–475. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.T.; Lin, S.Z. Cerebral ischemia is the main cause for the onset of heat stroke syndrome in rabbits. Experientia 1992, 48, 225–227. [Google Scholar] [CrossRef]
- Crandall, C.G.; Etzel, R.A.; Farr, D.B. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am. J. Physiol. Heart Circ. Physiol. 1999, 277, 2348–2352. [Google Scholar] [CrossRef] [Green Version]
- Mack, G.W.; Foote, K.M.; Nelson, W.B. Cutaneous vasodilation during local heating: Role of local cutaneous thermosensation. Front. Physiol. 2016, 7, 622. [Google Scholar] [CrossRef] [Green Version]
- Shelton, L.J.; White, C.E.; Felt, S.A. A comparison of non-contact, subcutaneous, and rectal temperatures in captive owl monkeys (Aotus sp.). J. Med. Primatol. 2006, 35, 346–351. [Google Scholar] [CrossRef]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef]
- Pereira, C.B.; Czaplik, M.; Blanik, N.; Rossaint, R.; Blazek, V.; Leonhardt, S. Contact-free monitoring of circulation and perfusion dynamics based on the analysis of thermal imagery. Biomed. Opt. Express 2014, 5, 1075–1089. [Google Scholar] [CrossRef] [Green Version]
- Fortuna, E.L.; Carney, M.M.; Macy, M.; Stanley, R.M.; Younger, J.G.; Bradin, S.A. Accuracy of non-contact infrared thermometry versus rectal thermometry in young children evaluated in the emergency department for fever. J. Emerg. Nurs. 2010, 36, 101–104. [Google Scholar] [CrossRef]
- Ataş Berksoy, E.; Bağ, Ö.; Yazici, S.; Çelik, T. Use of noncontact infrared thermography to measure temperature in children in a triage room. Medicine 2018, 97, e9737. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Cook, N.; Tessaro, S.V.; Deregt, D.; Desroches, G.; Dubeski, P.L.; Tong, A.K.W.; Godson, D.L. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; Gómez, J.; Boscato, L.; Gutiérrez-Pérez, O.; Cruz, V.; et al. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2021, 9, 2101. [Google Scholar] [CrossRef]
- Labeur, L.; Villiers, G.; Small, A.H.; Hinch, G.N.; Schmoelzl, S. Infrared thermal imaging as a method to evaluate heat loss in newborn lambs. Res. Vet. Sci. 2017, 115, 517–522. [Google Scholar] [CrossRef]
- Vicente-Pérez, R.; Avendaño-Reyes, L.; Correa-Calderón, A.; Mellado, M.; Meza-Herrera, C.A.; Montañez-Valdez, O.D.; Macías-Cruz, U. Relationships of body surface thermography with core temperature, birth weight and climatic variables in neonatal lambs born during early spring in an arid region. J. Therm. Biol. 2019, 82, 142–149. [Google Scholar] [CrossRef]
- De Pantoja, M.H.A.; Esteves, S.N.; Jacinto, M.A.C.; Pezzopane, J.R.M.; de Paz, C.C.P.; da Silva, J.A.R.; de Lourenço Junior, J.B.; Brandão, F.Z.; Moura, A.B.B.; Romanello, N.; et al. Thermoregulation of male sheep of indigenous or exotic breeds in a tropical environment. J. Therm. Biol. 2017, 69, 302–310. [Google Scholar] [CrossRef]
- Pulido-Rodríguez, L.F.; Titto, C.G.; de Bruni, G.A.; Froge, G.A.; Fuloni, M.F.; Payan-Carrera, R.; Henrique, F.L.; de Geraldo, A.C.A.P.M.; Pereira, A.M.F. Effect of solar radiation on thermoregulatory responses of Santa Inês sheep and their crosses with wool and hair Dorper sheep. Small Rumin. Res. 2021, 202, 106470. [Google Scholar] [CrossRef]
- Darcan, N.K.; Cankaya, S.; Karakok, S.G. The Effects of skin pigmentation on physiological factors of thermoregulation and grazing behaviour of dairy goats in a hot and humid climate. Asian-Australas J. Anim. Sci. 2009, 22, 727–731. [Google Scholar] [CrossRef]
- Silva, E.M.N.; Souza, B.B.; Silva, G.A.; Alcântara, M.D.B.; Cunha, M.G.G.; Marques, B.A.A. Avaliação da adaptabilidade de caprinos leiteiros no semiárido brasileiro com auxílio da termografia infravermelha. J. Anim. Behav. Biometeorol. 2014, 2, 95–101. [Google Scholar] [CrossRef]
- Kwon, C.J.; Brundage, C.M. Quantifying body surface temperature differences in canine coat types using infrared thermography. J. Therm. Biol. 2019, 82, 18–22. [Google Scholar] [CrossRef]
- Autio, E.; Heiskanen, M.-L.; Mononen, J. Thermographic evaluation of the lower critical temperature in weanling horses. J. Appl. Anim. Welf. Sci. 2007, 10, 207–216. [Google Scholar] [CrossRef]
- Topalidou, A.; Ali, N.; Sekulic, S.; Downe, S. Thermal imaging applications in neonatal care: A scoping review. BMC Pregnancy Childbirth 2019, 19, 381. [Google Scholar] [CrossRef]
- Lubkowska, A.; Szymański, S.; Chudecka, M. Surface body temperature of full-term healthy newborns immediately after birth—Pilot study. Int. J. Environ. Res. Public Health 2019, 16, 1312. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Mora-Medina, P.; Ogi, A.; Mariti, C.; Olmos-Hernández, A.; Martínez-Burnes, J.; Hernández-Ávalos, I.; Sánchez-Millán, J.; Gazzano, A. Blood biomarker profile alterations in newborn canines: Effect of the mother’s weight. Animals 2021, 11, 2307. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Miranda-Cortés, A.; Casas-Alvarado, A.; Mora-Medina, P.; Boscato-Funes, L.; Hernández-Ávalos, I. Neurobiología y modulación de la hipertermia inducida por estrés agudo y fiebre en los animales. Abanico Vet. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Church, J.; Cook, N.; Schaefer, A. Recent Applications of Infrared Thermography for Animal Welfare and Veterinary Research: Everything from Chicks to Elephants. In Proceedings of the Conference InfraMation, Las Vegas, NV, USA, 9 December 2009. [Google Scholar]
- Cook, N.J.; Schaefer, A.L.; Korver, D.R.; Haley, D.B.; Feddes, J.J.R.; Church, J.S. Minimally-invasive assessments of the behavioural and physiological effects of enriched colony cages on laying hens. Open Agric. J. 2011, 5, 10–18. [Google Scholar]
- Herborn, K.A.; Jerem, P.; Nager, R.G.; McKeegan, D.E.F.; McCafferty, D.J. Surface temperature elevated by chronic and intermittent stress. Physiol. Behav. 2018, 191, 47–55. [Google Scholar] [CrossRef]
- Jerem, P.; Herborn, K.; McCafferty, D.; McKeegan, D.; Nager, R. Thermal imaging to study stress non-invasively in unrestrained birds. J. Vis. Exp. 2015, 2015, 53184. [Google Scholar] [CrossRef] [Green Version]
- Powers, D.R.; Langland, K.M.; Wethington, S.M.; Powers, S.D.; Graham, C.H.; Tobalske, B.W. Hovering in the heat: Effects of environmental temperature on heat regulation in foraging hummingbirds. R. Soc. Open Sci. 2017, 4, 171056. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, W.F.; Clarke, R.H. Using infrared thermography to detect night-roosting birds. J. F. Ornithol. 2019, 90, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Loyau, T.; Zerjal, T.; Rodenburg, T.B.; Fablet, J.; Tixier-Boichard, M.; Pinard-van der Laan, M.H.; Mignon-Grasteau, S. Heritability of body surface temperature in hens estimated by infrared thermography at normal or hot temperatures and genetic correlations with egg and feather quality. Animal 2016, 10, 1594–1601. [Google Scholar] [CrossRef]
- Barnett, J.L.; Hemsworth, P.H.; Cronin, G.M.; Jongman, E.C.; Hutson, G.D. A review of the welfare issues for sows and piglets in relation to housing. Aust. J. Agric. Res. 2001, 52, 1–28. [Google Scholar]
- Kammersgaard, T.S.; Pedersen, L.J.; Jorgensen, E. Hypothermia in neonatal piglets: Interactions and causes of individual differences. J. Anim. Sci. 2011, 7, 2073–2085. [Google Scholar] [CrossRef]
- Herpin, P.; Damon, M.; Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 2002, 78, 25–45. [Google Scholar] [CrossRef]
- Schmitt, O.; Reigner, S.; Bailly, J.; Ravon, L.; Billon, Y.; Gress, L.; Bluy, L.; Canario, L.; Gilbert, H.; Bonnet, A.; et al. Thermoregulation at birth differs between piglets from two genetic lines divergent for residual feed intake. Animal 2021, 15, 100069. [Google Scholar] [CrossRef]
- Lowe, G.; Sutherland, M.; Waas, J.; Schaefer, A.; Cox, N.; Stewart, M. Infrared thermography—A non-invasive method of measuring respiration rate in calves. Animals 2019, 9, 535. [Google Scholar] [CrossRef] [Green Version]
- Mota-Rojas, D.; Napolitano, F.; Braghieri, A.; Guerrero-Legarreta, I.; Bertoni, A.; Martínez-Burnes, J.; Cruz-Monterrosa, R.; Gómez, J.; Ramírez-Bribiesca, E.; Barrios-García, H.; et al. Thermal biology in river buffalo in the humid tropics: Neurophysiological and behavioral responses assessed by infrared thermography. J. Anim. Behav. Biometeorol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Habeeb, A.A.; Napolitano, F.; Sarubbi, J.; Ghezzi, M.; Ceriani, M.C.; Cuibus, A.; Martínez-Burnes, J.; Braghieri, A.; Lendez, P.A. Chapter 23. River buffalo, European cattle and indian cattle welfare: Enviromental, physiological and behavioral aspect in response to natural and atificial shade. In El Búfalo de Agua en Latinoamerica, Hallazgos Recientes, 3rd ed.; Napolitano, F., Mota-Rojas, D., Guerrero-Legarreta, I., Orihuela, A., Eds.; BM Editores: Mexico City, Mexico, 2020; pp. 960–1016. [Google Scholar]
- Elias, B.; Starling, M.; Wilson, B.; McGreevy, P. Influences on infrared thermography of the canine eye in relation to the stress and arousal of racing greyhounds. Animals 2021, 11, 103. [Google Scholar] [CrossRef]
- Napolitano, F.; Mota-Rojas, D.; Guerrero-Legarreta, I.; Orihuela, A. The Latin American River Buffalo, Recent Findings, 3rd ed.; BM Editores: Mexico City, Mexico, 2020; pp. 1–1545. Available online: https://www.lifescienceglobal.com/journals/journal-of-buffalo-science/97-abstract/jbs/4550-el-bufalo-de-agua-en-latinoamerica-hallazgos-recientes (accessed on 2 October 2021).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Rojas, D.; Titto, C.G.; de Mira Geraldo, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and Function of Feathers, Hair, and Glabrous Skin in the Thermoregulation Strategies of Domestic Animals. Animals 2021, 11, 3472. https://doi.org/10.3390/ani11123472
Mota-Rojas D, Titto CG, de Mira Geraldo A, Martínez-Burnes J, Gómez J, Hernández-Ávalos I, Casas A, Domínguez A, José N, Bertoni A, et al. Efficacy and Function of Feathers, Hair, and Glabrous Skin in the Thermoregulation Strategies of Domestic Animals. Animals. 2021; 11(12):3472. https://doi.org/10.3390/ani11123472
Chicago/Turabian StyleMota-Rojas, Daniel, Cristiane Gonçalves Titto, Ana de Mira Geraldo, Julio Martínez-Burnes, Jocelyn Gómez, Ismael Hernández-Ávalos, Alejandro Casas, Adriana Domínguez, Nancy José, Aldo Bertoni, and et al. 2021. "Efficacy and Function of Feathers, Hair, and Glabrous Skin in the Thermoregulation Strategies of Domestic Animals" Animals 11, no. 12: 3472. https://doi.org/10.3390/ani11123472











