Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects
Abstract
Simple Summary
Abstract
1. Introduction
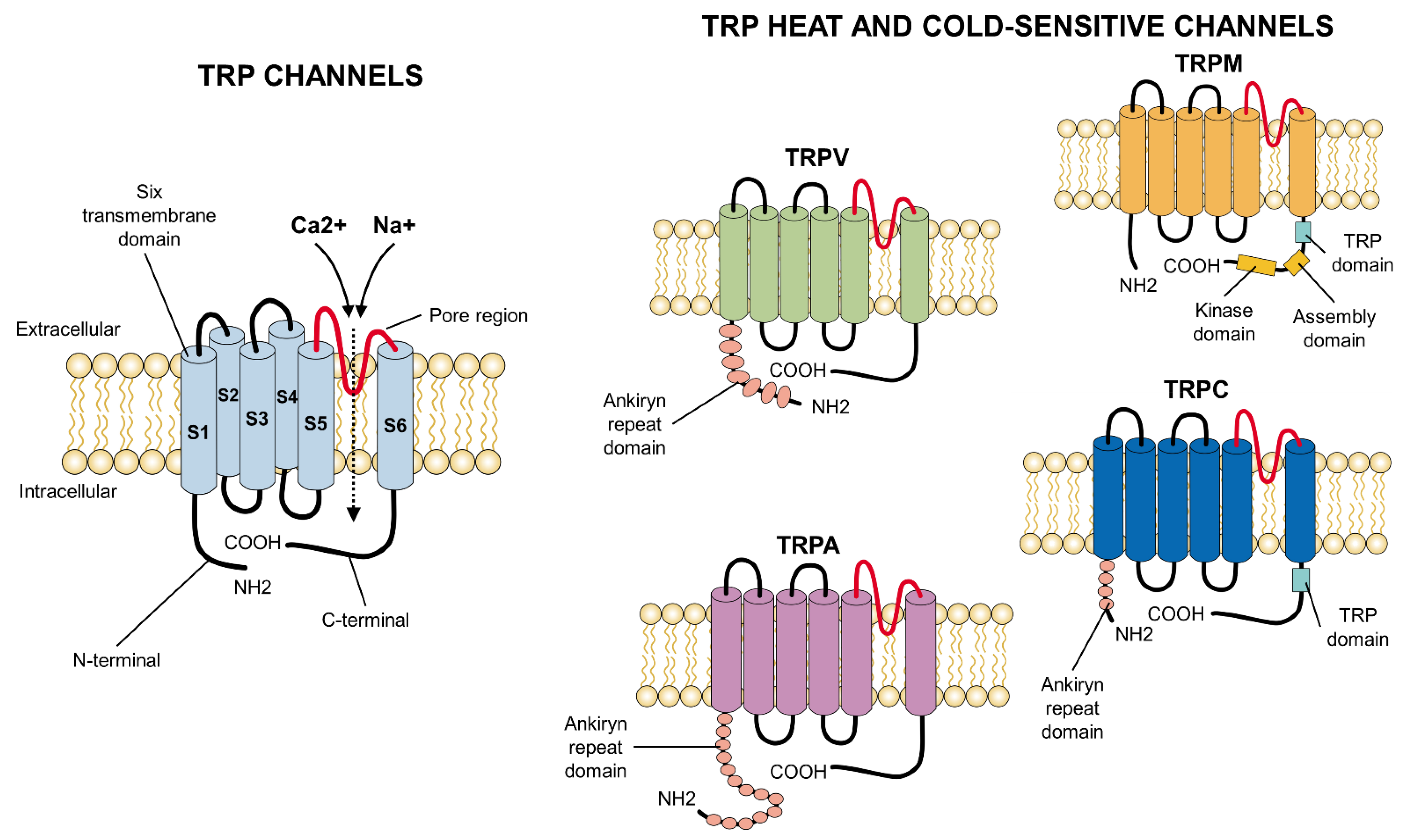
2. Classification of the Ionic TRP Channels
2.1. TRP Families and Subfamilies
2.1.1. Classification of TRP
2.1.2. TRPV (Vanilloid)
2.1.3. TRPM (Melastatin-Related)
2.1.4. TRPA (Ankyrin)
2.1.5. TRPN (No Mechanoreceptor Potential-C)
2.1.6. TRPP (Polycystin)
2.1.7. TRPML (Mucolipin)
2.1.8. TRPVL (Vanilloid-like)
2.1.9. TRPC (Canonical)
2.1.10. TRPS (Soromelastatin)
2.1.11. TRPY (Fungus-Specific TRP Channel)
2.2. Temperature-Sensitive TRP
3. Structure
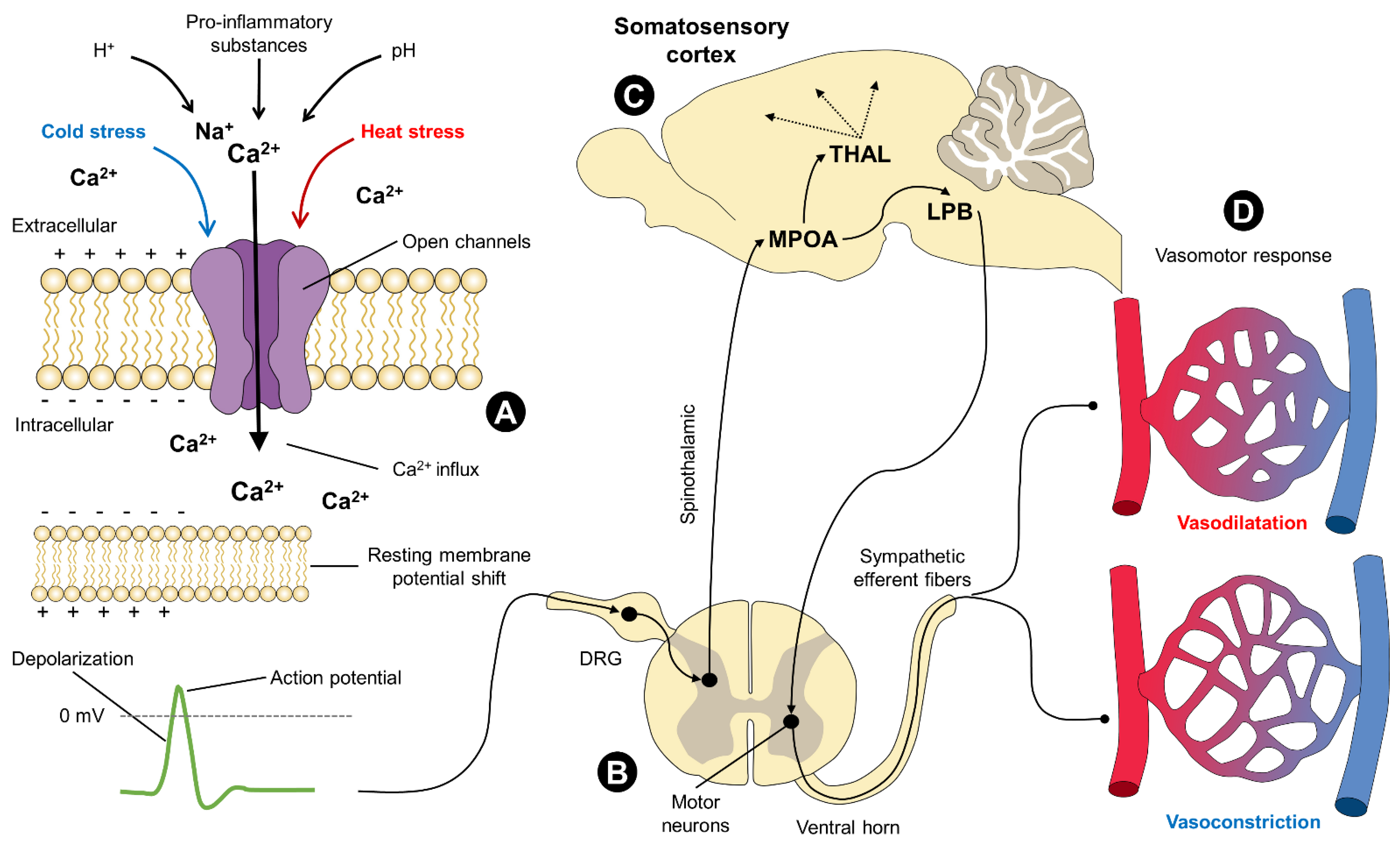
4. Neurophysiology
4.1. The Opening/Closing Mechanism of TRP Channels
4.1.1. Membrane Voltage
4.1.2. Membrane Phospholipids
4.1.3. Phosphorylation
4.1.4. Ligands
5. Heat-Sensitive TRP
5.1. Harmful Heat
5.1.1. TRPV1
5.1.2. TRPV2
5.2. Harmless Heat
5.2.1. TRPV3
5.2.2. TRPV4
5.2.3. TRPM2, TRPM4, and TRPM5
6. Cold-Sensitive TRP
6.1. Harmful Cold
TRPA1
6.2. Harmless Cold
6.2.1. TRPM8
6.2.2. TRPC5
7. Uses of TRP in Disease Treatment
8. Areas of Opportunity
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; José, N.; Casas-Alvarado, A.; Gómez, J.; Mora-Medina, P. Thermal homeostasis in the newborn puppy: Behavioral and physiological responses. J. Anim. Behav. Biometeorol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Nord, A.; Nilsson, J.F.; Sandell, M.I.; Nilsson, J.-Å. Patterns and dynamics of rest-phase hypothermia in wild and captive blue tits during winter. J. Comp. Physiol. B 2009, 179, 737–745. [Google Scholar] [CrossRef]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and behavioral mechanisms of thermoregulation in mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Gonçalves, C.; de Mira, A.; Martínez-Burnes, J.; Gómez, J.; Hernández-Ávalos, I.; Casas, A.; Domínguez, A.; José, N.; Bertoni, A.; et al. Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals 2021, 11, 3472. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Lecona-Butrón, H.; Martínez-Burnes, J.; Mora-Medina, P.; Gómez-Prado, J.; Orihuela, A. Infrared thermal imaging associated with pain in laboratory animals. Exp. Anim. 2021, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1207–R1228. [Google Scholar] [CrossRef] [PubMed]
- Picón-Jaimes, Y.A.; Orozco-Chinome, J.E.; Molina-Franky, J.; Franky-Rojas, M.P. Control central de la temperatura corporal y sus alteraciones: Fiebre, hipertermia e hipotermia. MedUNAB 2020, 23, 118–130. [Google Scholar] [CrossRef][Green Version]
- Kingma, B. The thermoneutral zone: Implications for metabolic studies. Front. Biosci. 2012, 4, 1975–1985. [Google Scholar] [CrossRef]
- Roland, L.; Drillich, M.; Klein-Jöbstl, D.; Iwersen, M. Invited review: Influence of climatic conditions on the development, performance, and health of calves. J. Dairy Sci. 2016, 99, 2438–2452. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Hao, S.; Zhang, M.; Zhang, X.; Wang, D. Aquaporins, evaporative water loss and thermoregulation in heat-acclimated Mongolian gerbils (Meriones unguiculatus). J. Therm. Biol. 2020, 91, 102641. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Miranda-Cortés, A.; Casas-Alvarado, A.; Mora-Medina, P.; Boscato, L.; Hernández-Ávalos, I. Neurobiology and modulation of stress—Induced hyperthermia and fever in animals. Abanico Vet. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Lizarralde, E.; Gutiérrez, A.; Martínez, M. Alteraciones de la termorregulación. Emergencias 2000, 12, 192–207. [Google Scholar]
- Vialard, F.; Olivier, M. Thermoneutrality and immunity: How does cold stress affect disease? Front. Immunol. 2020, 11, 588387. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Wang, D.; Titto, C.G.; Gómez-Prado, J.; Carvajal-De la Fuente, V.; Ghezzi, M.; Boscato-Funes, L.; Barrios-García, H.; Torres-Bernal, F.; Casas-Alvarado, A.; et al. Pathophysiology of fever and application of infrared thermography (IRT) in the detection of sick domestic animals: Recent advances. Animals 2021, 11, 2316. [Google Scholar] [CrossRef]
- Verduzco-Mendoza, A.; Bueno-Nava, A.; Wang, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Casas, A.; Domínguez, A.; Mota-Rojas, D. Experimental applications and factors involved in validating thermal windows using infrared thermography to assess the health and thermostability of laboratory animals. Animals 2021, 11, 3448. [Google Scholar] [CrossRef]
- Ferreira, G.; Raddatz, N.; Lorenzo, Y. Biophysical and molecular features of thermosensitive TRP channels involved in sensory transduction. In TRP Channels in Sensory Transduction; Madrid, R., Bacigalupo, J., Eds.; Springer: London, UK, 2015; pp. 1–39. [Google Scholar]
- González-Alonso, J. Human thermoregulation and the cardiovascular system. Exp. Physiol. 2012, 97, 340–346. [Google Scholar] [CrossRef]
- Lamas, J.A.; Rueda-Ruzafa, L.; Herrera-Pérez, S. Ion channels and thermosensitivity: TRP, TREK, or both? Int. J. Mol. Sci. 2019, 20, 2371. [Google Scholar] [CrossRef]
- Oaklander, A.; Siegel, S. Cutaneous innervation: Form and function. J. Am. Acad. Dermatol. 2005, 53, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Mathie, A.; Peters, J. Ion channels. Br. J. Pharmacol. 2005, 144, 73–94. [Google Scholar]
- Wen, J.; Tingbei, B.O.; Zhao, Z.; Wang, D. Role of transient receptor potential vanilloid-1 in behavioral thermoregulation of the Mongolian gerbil Meriones unguiculatus. Integr. Zool. 2021, 0, 1–11. [Google Scholar] [CrossRef]
- Clapham, D. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Clapham, D.; Runnels, L.; Strübing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Bo, T.; Zhang, X.; Wang, Z.; Wang, D. Thermo-TRPs and gut microbiota are involved in thermogenesis and energy metabolism during low temperature exposure of obese mice. J. Exp. Biol. 2020, 223, jeb218974. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Napolitano, F.; Strappini, A.; Orihuela, A.; Ghezzi, M.D.; Hernández-Ávalos, I.; Mora-Medina, P.; Whittaker, A.L. Pain at the slaughterhouse in ruminants with a focus on the neurobiology of sensitisation. Animals 2021, 11, 1085. [Google Scholar] [CrossRef]
- Lendez, P.A.; Martinez Cuesta, L.; Nieto Farias, M.V.; Vater, A.A.; Ghezzi, M.D.; Mota-Rojas, D.; Dolcini, G.L.; Ceriani, M.C. Alterations in TNF-α and its receptors expression in cows undergoing heat stress. Vet. Immunol. Immunopathol. 2021, 235, 110232. [Google Scholar] [CrossRef]
- Flores-Peinado, S.; Mota-Rojas, D.; Guerrero-Legarreta, I.; Mora-Medina, P.; Cruz-Monterrosa, R.; Gómez-Prado, J.; Guadalupe Hernández, M.; Cruz-Playas, J.; Martínez-Burnes, J. Physiological responses of pigs to preslaughter handling: Infrared and thermal imaging applications. Int. J. Vet. Sci. Med. 2020, 8, 71–84. [Google Scholar] [CrossRef]
- Schepers, R.J.; Ringkamp, M. Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 2010, 34, 177–184. [Google Scholar] [CrossRef]
- Hardie, R.C. A brief history of TRP: Commentary and personal perspective. Pflügers Arch.-Eur. J. Physiol. 2011, 461, 493–498. [Google Scholar] [CrossRef]
- Montell, C.; Rubin, G.M. Molecular characterization of the Drosophila TRP locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Vangeel, L.; Voets, T. Transient receptor potential channels and calcium signaling. Cold Spring Harb. Perspect. Biol. 2019, 11, a035048. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Wu, X.L.; Zhang, G.Y.; Ma, X.; He, D.X. Functional food development: Insights from TRP channels. J. Funct. Foods 2019, 56, 384–394. [Google Scholar] [CrossRef]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. In Membrane Protein Complexes: Structure and Function; Harris, J., Boekema, E., Eds.; Springer: Singapore, 2018; pp. 141–165. [Google Scholar]
- Clapham, D.E.; Montell, C.; Schultz, G.; Julius, D. International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: Transient receptor potential channels. Pharmacol. Rev. 2003, 55, 591–596. [Google Scholar] [CrossRef]
- Li, H. TRP channel classification. In Transient Receptor Potential Canonical Channel and Brain Diseases; Wang, Y., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 1–8. [Google Scholar]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Szallasi, A. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Eder, P.; Chang, B.; Molkentin, J.D. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 2010, 107, 7000–7005. [Google Scholar] [CrossRef]
- Reiser, J.; Polu, K.R.; Möller, C.C.; Kenlan, P.; Altintas, M.M.; Wei, C.; Faul, C.; Herbert, S.; Villegas, I.; Avila-Casado, C.; et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005, 37, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ko, J.; Hong, C.; So, I. Structure–function relationship and physiological roles of transient receptor potential canonical (TRPC) 4 and 5 channels. Cells 2019, 9, 73. [Google Scholar] [CrossRef]
- Baylie, R.L.; Brayden, J.E. TRPV channels and vascular function. Acta Physiol. 2011, 203, 99–116. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Pereira, A.M.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Ávalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical applications and factors involved in validating thermal windows used in infrared thermography in cattle and river buffalo to assess health and productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, B.A. Structure-function analysis of TRPV channels. Naunyn. Schmiedebergs. Arch. Pharmacol. 2005, 371, 285–294. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Siemens, J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temp. Multidiscip. Biomed. J. 2015, 2, 178–187. [Google Scholar] [CrossRef]
- Harteneck, C. Function and pharmacology of TRPM cation channels. Naunyn-Schmiedebergs. Arch. Pharmacol. 2005, 371, 307–314. [Google Scholar] [CrossRef]
- Koivisto, A.; Chapman, H.; Jalava, N.; Korjamo, T.; Saarnilehto, M.; Lindstedt, K.; Pertovaara, A. TRPA1: A transducer and amplifier of pain and inflammation. Basic Clin. Pharmacol. Toxicol. 2014, 114, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kindt, K.S.; Viswanath, V.; Macpherson, L.; Quast, K.; Hu, H.; Patapoutian, A.; Schafer, W.R. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat. Neurosci. 2007, 10, 568–577. [Google Scholar] [CrossRef]
- Kowalski, C.W.; Ragozzino, F.J.; Lindberg, J.E.M.; Peterson, B.; Lugo, J.M.; McLaughlin, R.J.; Peters, J.H. Cannabidiol activation of vagal afferent neurons requires TRPA1. J. Neurophysiol. 2020, 124, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, Z.; Sternberg, P.W.; Shawn Xu, X.Z. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 2006, 440, 684–687. [Google Scholar] [CrossRef]
- Xiao, R.; Xu, X.Z.S. Function and regulation of TRP family channels in C. elegans. Pflügers Arch.-Eur. J. Physiol. 2009, 458, 851–860. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, W.; He, Y.; Gorczyca, D.; Xiang, Y.; Cheng, L.E.; Meltzer, S.; Jan, L.Y.; Jan, Y.N. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 2013, 493, 221–225. [Google Scholar] [CrossRef]
- Semmo, M.; Köttgen, M.; Hofherr, A. The TRPP subfamily and polycystin-1 proteins. In Mammalian Transient Receptor Potential (TRP) Cation Channels; Nilius, B., Flockerzi, V., Eds.; Springer: Cham, Switzerland, 2014; pp. 675–711. [Google Scholar]
- Peng, G.; Shi, X.; Kadowaki, T. Evolution of TRP channels inferred by their classification in diverse animal species. Mol. Phylogenet. Evol. 2015, 84, 145–157. [Google Scholar] [CrossRef]
- Srivastava, M.; Simakov, O.; Chapman, J.; Fahey, B.; Gauthier, M.E.; Mitros, T.; Richards, G.S.; Conaco, C.; Dacre, M.; Hellsten, U.; et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 2010, 466, 720–726. [Google Scholar] [CrossRef]
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila Mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef]
- Wes, P.D.; Chevesich, J.; Jeromin, A.; Rosenberg, C.; Stetten, G.; Montell, C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. USA 1995, 92, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Buniel, M.; Wisnoskey, B.; Glazebrook, P.A.; Schilling, W.P.; Kunze, D.L. Distribution of TRPC channels in a visceral sensory pathway. Novartis Found. Symp. 2004, 258, 236–243. [Google Scholar]
- Sadler, K.E.; Moehring, F.; Shiers, S.I.; Laskowski, L.J.; Mikesell, A.R.; Plautz, Z.R.; Brezinski, A.N.; Mecca, C.M.; Dussor, G.; Price, T.J.; et al. Transient receptor potential canonical 5 mediates inflammatory mechanical and spontaneous pain in mice. Sci. Transl. Med. 2021, 13, eabd7702. [Google Scholar] [CrossRef]
- Wei, H.; Sagalajev, B.; Yüzer, M.A.; Koivisto, A.; Pertovaara, A. Regulation of neuropathic pain behavior by amygdaloid TRPC4/C5 channels. Neurosci. Lett. 2015, 608, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.L.; Matera, D.; Doerner, J.F.; Zheng, N.; Del Camino, D.; Mishra, S.; Bian, H.; Zeveleva, S.; Zhen, X.; Blair, N.T.; et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc. Natl. Acad. Sci. USA 2019, 116, 10156–10161. [Google Scholar] [CrossRef] [PubMed]
- Himmel, N.J.; Gray, T.R.; Cox, D.N.; Ruvinsky, I. Phylogenetics identifies two eumetazoan TRPM clades and an eighth TRP family, TRP soromelastatin (TRPS). Mol. Biol. Evol. 2020, 37, 2034–2044. [Google Scholar] [CrossRef]
- Cai, X.; Clapham, D.E. Ancestral Ca2+ signaling machinery in early animal and fungal evolution. Mol. Biol. Evol. 2012, 29, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Moiseenkova-Bell, V.; Wensel, T.G. Functional and structural studies of TRP channels heterologously expressed in budding yeast. Adv. Exp. Med. Biol. 2011, 704, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Wang, H.; Belkacemi, A.; Jung, M.; Bertl, A.; Schlenstedt, G.; Flockerzi, V.; Beck, A. Identification of inhibitory Ca2+ binding sites in the upper vestibule of the yeast vacuolar TRP channel. iScience 2019, 11, 1–12. [Google Scholar] [CrossRef]
- Jänig, W. Peripheral thermoreceptors in innocuous temperature detection. In Handbook of Clinical Neurology; Romavosky, A., Ed.; Elsevier: London, UK, 2018; Volume 156, pp. 47–56. [Google Scholar]
- Benham, C.; Gunthorpe, M.; Davis, J. TRPV channels as temperature sensors. Cell Calcium 2003, 33, 479–487. [Google Scholar] [CrossRef]
- Xu, H.; Ramsey, I.; Kotecha, S.; Moran, M.; Chong, J.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Vriens, J.; Suh, S.; Benham, C.; Droogmans, G.; Nilius, B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J. Biol. Chem. 2002, 277, 47044–47051. [Google Scholar] [CrossRef]
- Güler, A.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- McKemy, D. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol. Pain 2005, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Galindo, R.; Díaz, I.J.; Vargas, L. Los canales TRP y su participación en la termotransducción. Rev. La Univ. Ind. Santander. Salud 2008, 40, 110–119. [Google Scholar]
- Fernández-Carvajal, A.; Fernández-Ballester, G.; Devesa, I.; González-Ros, J.M.; Ferrer-Montiel, A. New strategies to develop novel pain therapies: Addressing thermoreceptors from different points of view. Pharmaceuticals 2011, 5, 16–48. [Google Scholar] [CrossRef]
- Zhao, Y.; McVeigh, B.M.; Moiseenkova-Bell, V.Y. Structural pharmacology of TRP channels. J. Mol. Biol. 2021, 433, 166914. [Google Scholar] [CrossRef]
- Liu, C.; Montell, C. Forcing open TRP channels: Mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 2015, 460, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Montell, C. Drosophila TRP channels. Pflügers Arch.-Eur. J. Physiol. 2005, 451, 19–28. [Google Scholar] [CrossRef]
- Izquierdo, C.; Martín-Martínez, M.; Gómez-Monterrey, I.; González-Muñiz, R. TRPM8 channels: Advances in structural studies and pharmacological modulation. Int. J. Mol. Sci. 2021, 22, 8502. [Google Scholar] [CrossRef] [PubMed]
- López-Romero, A.E.; Hernández-Araiza, I.; Torres-Quiroz, F.; Tovar-Y-Romo, L.B.; Islas, L.D.; Rosenbaum, T. TRP ion channels: Proteins with conformational flexibility. Channels 2019, 13, 207–226. [Google Scholar] [CrossRef]
- Singh, A.K.; McGoldrick, L.L.; Sobolevsky, A.I. Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat. Struct. Mol. Biol. 2018, 25, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Benítez-Angeles, M.; Sánchez-Hernández, R.; Morales-Lázaro, S.L.; Hiriart, M.; Morales-Buenrostro, L.E.; Torres-Quiroz, F. TRPV4: A physio and pathophysiologically significant ion channel. Int. J. Mol. Sci. 2020, 21, 3837. [Google Scholar] [CrossRef]
- Minke, B.; Cook, B. TRP channel proteins and signal transduction. Physiol. Rev. 2002, 82, 429–472. [Google Scholar] [CrossRef]
- Hardie, R.C. Regulation of TRP channels via lipid second messengers. Annu. Rev. Physiol. 2003, 65, 735–759. [Google Scholar] [CrossRef]
- Kalia, J.; Swartz, K.J. Exploring structure-function relationships between TRP and Kv channels. Sci. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Ahmmed, G.U.; Vogel, S.M.; Malik, A.B. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 2006, 13, 693–708. [Google Scholar] [CrossRef]
- Vriens, J.; Nilius, B.; Voets, T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 2014, 15, 573–589. [Google Scholar] [CrossRef]
- Mulier, M.; Vriens, J.; Voets, T. TRP channel pores and local calcium signals. Cell Calcium 2017, 66, 19–24. [Google Scholar] [CrossRef]
- Nilius, B.; Prenen, J.; Droogmans, G.; Voets, T.; Vennekens, R.; Freichel, M.; Wissenbach, U.; Flockerzi, V. Voltage dependence of the Ca2+-activated cation channel TRPM4. J. Biol. Chem. 2003, 278, 30813–30820. [Google Scholar] [CrossRef]
- Cao, E. Structural mechanism of transient receptor potential ion channels. J. Gen. Physiol. 2020, 152, e201811998. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Talavera, K.; Owsianik, G.; Prenen, J.; Droogmans, G.; Voets, T. Gating of TRP channels: A voltage connection? J. Physiol. 2005, 567, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Talavera, K.; Owsianik, G.; Nilius, B. Sensing with TRP channels. Nat. Chem. Biol. 2005, 1, 85–92. [Google Scholar] [CrossRef]
- Voets, T.; Owsianik, G.; Janssens, A.; Talavera, K.; Nilius, B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat. Chem. Biol. 2007, 3, 174–182. [Google Scholar] [CrossRef]
- Stein, A.; Ufret-Vincenty, C.; Hua, L.; Santana, L.; Gordon, S. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J. Gen. Physiol. 2006, 128, 509–522. [Google Scholar] [CrossRef]
- Karashima, Y.; Prenen, J.; Meseguer, V.; Owsianik, G.; Voets, T.; Nilius, B. Modulation of the transient receptor potential channel TRPA1 by phosphatidylinositol 4,5-biphosphate manipulators. Pflug. Arch. 2008, 457, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Tang, Z.; Liu, Q.; Patel, K.; Maag, D.; Geng, Y.; Dong, X. Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell 2008, 133, 475–485. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G.; Voets, T. Transient receptor potential channels meet phosphoinositides. EMBO J. 2008, 27, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Nilius, B. Modulation of TRPs by PIPs. J. Physiol. 2007, 582, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Bhave, G.; Hu, H.J.; Glauner, K.S.; Zhu, W.; Wang, H.; Brasier, D.J.; Oxford, G.S.; Gereau IV, R.W. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc. Natl. Acad. Sci. USA 2003, 100, 12480–12485. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Starowicz, K.; Moriello, A.S.; Vivese, M.; Orlando, P.; Di Marzo, V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): Effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp. Cell Res. 2007, 313, 1911–1920. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.R.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Z.; Gu, Q.; Wang, C.; Colton, C.K.; Tang, J.; Kinoshita-Kawada, M.; Lee, L.Y.; Wood, J.D.; Zhu, M.X. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 2004, 279, 35741–35748. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Tan, C.H.; McNaughton, P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nature 2016, 536, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Riera, C.E.; Huising, M.O.; Follett, P.; Leblanc, M.; Halloran, J.; Van Andel, R.; De Magalhaes Filho, C.D.; Merkwirth, C.; Dillin, A. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 2014, 157, 1023–1036. [Google Scholar] [CrossRef]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Gracheva, E.O.; Cordero-Morales, J.F.; González-Carcacía, J.A.; Ingolia, N.T.; Manno, C.; Aranguren, C.I.; Weissman, J.S.; Julius, D. Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature 2011, 476, 88–91. [Google Scholar] [CrossRef]
- Leffler, A.; Linte, R.M.; Nau, C.; Reeh, P.; Babes, A. A high-threshold heat-activated channel in cultured rat dorsal root ganglion neurons resembles TRPV2 and is blocked by gadolinium. Eur. J. Neurosci. 2007, 26, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Wang, Y.; Flores, C.M.; Qin, N. Activation properties of heterologously expressed mammalian TRPV2. J. Biol. Chem. 2007, 282, 15894–15902. [Google Scholar] [CrossRef]
- Park, U.; Vastani, N.; Guan, Y.; Raja, S.N.; Koltzenburg, M.; Caterina, M.J. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. 2011, 31, 11425–11436. [Google Scholar] [CrossRef] [PubMed]
- Desai, B.N.; Clapham, D.E. TRP channels and mice deficient in TRP channels. Pflügers Arch.-Eur. J. Physiol. 2005, 451, 11–18. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, M.X. TRPV3. Handbook of Experimental Pharmacology; Nilius, B., Flockerzi, V., Eds.; Springer: London, UK, 2014; pp. 273–291. [Google Scholar]
- Xiao, R.; Tian, J.; Tang, J.; Zhu, M.X. The TRPV3 mutation associated with the hairless phenotype in rodents is constitutively active. Cell Calcium 2008, 43, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Li, X.; Yu, Y.; Wang, J.; Caterina, M.J. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain 2011, 7, 1744–8069. [Google Scholar] [CrossRef]
- Miyamoto, T.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2011, 2, 1–12. [Google Scholar] [CrossRef]
- Chung, M.-K.; Lee, H.; Caterina, M.J. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J. Biol. Chem. 2003, 278, 32037–32046. [Google Scholar] [CrossRef]
- Ding, X.-L.; Wang, Y.-H.; Ning, L.-P.; Zhang, Y.; Ge, H.-Y.; Jiang, H.; Wang, R.; Yue, S.-W. Involvement of TRPV4-NO-cGMP-PKG pathways in the development of thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav. Brain Res. 2010, 208, 194–201. [Google Scholar] [CrossRef]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar] [CrossRef]
- Vilar, B.; Tan, C.-H.; McNaughton, P.A. Heat detection by the TRPM2 ion channel. Nature 2020, 584, E5–E12. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; Reis, F.D.C.; Heppenstall, P.; Wende, H.; Siemens, J.; De Castro Reis, F.; Heppenstall, P.; et al. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016, 353, 1393–1398. [Google Scholar] [CrossRef]
- Talavera, K.; Yasumatsu, K.; Voets, T.; Droogmans, G.; Shigemura, N.; Ninomiya, Y.; Margolskee, R.F.; Nilius, B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 2005, 438, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef]
- Chen, J.; Kang, D.; Xu, J.; Lake, M.; Hogan, J.O.; Sun, C.; Walter, K.; Yao, B.; Kim, D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat. Commun. 2013, 4, 2501. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.S.; Woolf, C.J.; Corey, D.P. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006, 50, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Karashima, Y.; Talavera, K.; Everaerts, W.; Janssens, A.; Kwan, K.Y.; Vennekens, R.; Nilius, B.; Voets, T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1273–1278. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef]
- Knowlton, W.M.; Bifolck-Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350. [Google Scholar] [CrossRef]
- Chen, J.; Joshi, S.K.; Didomenico, S.; Perner, R.J.; Mikusa, J.P.; Gauvin, D.M.; Segreti, J.A.; Han, P.; Zhang, X.F.; Niforatos, W.; et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011, 152, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.; Garami, A.; Lehto, S.G.; Pakai, E.; Tekus, V.; Pohoczky, K.; Youngblood, B.D.; Wang, W.; Kort, M.E.; Kym, P.R.; et al. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J. Neurosci. 2014, 34, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Nakatsuka, K.; Takahashi, K.; Fukuta, N.; Imagawa, T.; Ohta, T.; Tominaga, M. Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J. Biol. Chem. 2012, 287, 30743–30754. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Rossato, M.; Granzotto, M.; Macchi, V.; Porzionato, A.; Petrelli, L.; Calcagno, A.; Vencato, J.; De Stefani, D.; Silvestrin, V.; Rizzuto, R.; et al. Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol. Cell. Endocrinol. 2014, 383, 137–146. [Google Scholar] [CrossRef]
- Ma, S.; Yu, H.; Zhao, Z.; Luo, Z.; Chen, J.; Ni, Y.; Jin, R.; Ma, L.; Wang, P.; Zhu, Z.; et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J. Mol. Cell Biol. 2012, 4, 88–96. [Google Scholar] [CrossRef]
- Tajino, K.; Matsumura, K.; Kosada, K.; Shibakusa, T.; Inoue, K.; Fushiki, T.; Hosokawa, H.; Kobayashi, S. Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2128–R2135. [Google Scholar] [CrossRef]
- Nilius, B. TRP channels in disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2007, 1772, 805–812. [Google Scholar] [CrossRef]
- Yang, D.; Kim, J. Emerging role of transient receptor potential (TRP) channels in cancer progression. BMB Rep. 2020, 53, 125–132. [Google Scholar] [CrossRef]
- Hoshi, Y.; Okabe, K.; Shibasaki, K.; Funatsu, T.; Matsuki, N.; Ikegaya, Y.; Koyama, R. Ischemic brain injury leads to brain edema via hyperthermia-induced TRPV4 activation. J. Neurosci. 2018, 38, 5700–5709. [Google Scholar] [CrossRef]
- Mustafa, S.; Ismael, H.N. Ethanol potentiates heat response in the carotid artery via TRPV1. Life Sci. 2017, 188, 83–86. [Google Scholar] [CrossRef]
- Tan, C.H.; McNaughton, P.A. TRPM2 and warmth sensation. Pflug. Arch. 2018, 470, 787–798. [Google Scholar] [CrossRef]
- Di, A.; Gao, X.P.; Qian, F.; Kawamura, T.; Han, J.; Hecquet, C.; Ye, R.D.; Vogel, S.M.; Malik, A.B. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nat. Immunol. 2012, 13, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, S.; Radin, J.N.; Chatuvedi, R.; Piazuelo, M.B.; Horvarth, D.J.; Cortado, H.; Gu, Y.; Dixon, B.; Gu, C.; Lange, I.; et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017, 10, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef] [PubMed]
- Broad, L.; Mogg, A.; Eberle, E.; Tolley, M.; Li, D.; Knopp, K. TRPV3 in drug development. Pharmaceuticals 2016, 9, 55. [Google Scholar] [CrossRef]
- Joshi, N.K.; Maharaj, N.; Thomas, A. The TRPV3 receptor as a pain target: A therapeutic promise or just some more new biology? Open Drug Discov. J. 2010, 2, 89–97. [Google Scholar] [CrossRef]
- Hernández-Avalos, I.; Valverde, A.; Ibancovichi-Camarillo, J.A.; Sánchez-Aparicio, P.; Recillas-Morales, S.; Osorio-Avalos, J.; Rodríguez-Velázquez, D.; Miranda-Cortés, A.E. Clinical evaluation of postoperative analgesia, cardiorespiratory parameters and changes in liver and renal function tests of paracetamol compared to meloxicam and carprofen in dogs undergoing ovariohysterectomy. PLoS ONE 2020, 15, e0223697. [Google Scholar] [CrossRef]
- Domínguez-Oliva, A.; Casas-Alvarado, A.; Miranda-Cortés, A.E.; Hernández-Ávalos, I. Clinical pharmacology of tramadol and tapentadol, and their therapeutic efficacy in different models of acute and chronic pain in dogs and cats. J. Adv. Vet. Anim. Res. 2021, 8, 404–422. [Google Scholar] [CrossRef]
- Hernandez-Ávalos, I.; Mota-Rojas, D.; Mora-Medina, P.; Martínez-Burnes, J.; Casas Alvarado, A.; Verduzco-Mendoza, A.; Lezama-García, K.; Olmos-Hernandez, A. Review of different methods used for clinical recognition and assessment of pain in dogs and cats. Int. J. Vet. Sci. Med. 2019, 7, 43–54. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Li, B.; Xu, L.; Kamau, P.M.; Zheng, J.; Yang, F.; Yang, S.; Lai, R. Molecular basis for heat desensitization of TRPV1 ion channels. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Gram, D.X.; Holst, J.J.; Szallasi, A. TRPV1: A potential therapeutic target in type 2 diabetes and comorbidities? Trends Mol. Med. 2017, 23, 1002–1013. [Google Scholar] [CrossRef]
- Iadarola, M.J.; Sapio, M.R.; Raithel, S.J.; Mannes, A.J.; Brown, D.C. Long-term pain relief in canine osteoarthritis by a single intra-articular injection of resiniferatoxin, a potent TRPV1 agonist. Pain 2018, 159, 2105–2114. [Google Scholar] [CrossRef]
- Pomeraniec, I.J.; Mannes, A.; Iadarola, M.; Williams, T.; Heiss, J.D. Intrathecal resiniferatoxin for medication-refractory pain in advanced cancer. Clin. Neurosurg. 2020, 67, E201–E203. [Google Scholar] [CrossRef]
- Vriens, J.; Voets, T. Sensing the heat with TRPM3. Pflug. Arch.-Eur. J. Physiol. 2018, 470, 799–807. [Google Scholar] [CrossRef]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Yang, Y.D.; Lee, J.; Lee, B.; Kim, T.; Jang, Y.; Back, S.K.; Na, H.S.; Harfe, B.D.; Wang, F.; et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 2012, 15, 1015–1021. [Google Scholar] [CrossRef]
- Dulin, N.O. Calcium-activated chloride channel ANO1/TMEM16A: Regulation of expression and signaling. Front. Physiol. 2020, 11, 1428. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Shibasaki, K.; Furue, H.; Uta, D.; Tominaga, M. Physiological significances of TRP–ANO1 interaction. Pain Res. 2018, 33, 1–9. [Google Scholar] [CrossRef]
- Malanga, G.A.; Yan, N.; Stark, J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad. Med. 2015, 127, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Freiwald, J.; Magni, A.; Fanlo-Mazas, P.; Paulino, E.; Sequeira de Medeiros, L.; Moretti, B.; Schleip, R.; Solarino, G. A role for superficial heat therapy in the management of non-specific, mild-to-moderate low back pain in current clinical practice: A narrative review. Life 2021, 11, 780. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lezama-García, K.; Mota-Rojas, D.; Pereira, A.M.F.; Martínez-Burnes, J.; Ghezzi, M.; Domínguez, A.; Gómez, J.; de Mira Geraldo, A.; Lendez, P.; Hernández-Ávalos, I.; et al. Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects. Animals 2022, 12, 106. https://doi.org/10.3390/ani12010106
Lezama-García K, Mota-Rojas D, Pereira AMF, Martínez-Burnes J, Ghezzi M, Domínguez A, Gómez J, de Mira Geraldo A, Lendez P, Hernández-Ávalos I, et al. Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects. Animals. 2022; 12(1):106. https://doi.org/10.3390/ani12010106
Chicago/Turabian StyleLezama-García, Karina, Daniel Mota-Rojas, Alfredo M. F. Pereira, Julio Martínez-Burnes, Marcelo Ghezzi, Adriana Domínguez, Jocelyn Gómez, Ana de Mira Geraldo, Pamela Lendez, Ismael Hernández-Ávalos, and et al. 2022. "Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects" Animals 12, no. 1: 106. https://doi.org/10.3390/ani12010106
APA StyleLezama-García, K., Mota-Rojas, D., Pereira, A. M. F., Martínez-Burnes, J., Ghezzi, M., Domínguez, A., Gómez, J., de Mira Geraldo, A., Lendez, P., Hernández-Ávalos, I., Falcón, I., Olmos-Hernández, A., & Wang, D. (2022). Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects. Animals, 12(1), 106. https://doi.org/10.3390/ani12010106









