Myocardial Injury Complicated by Systolic Dysfunction in a COVID-19-Positive Dog
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials, Methods and Results
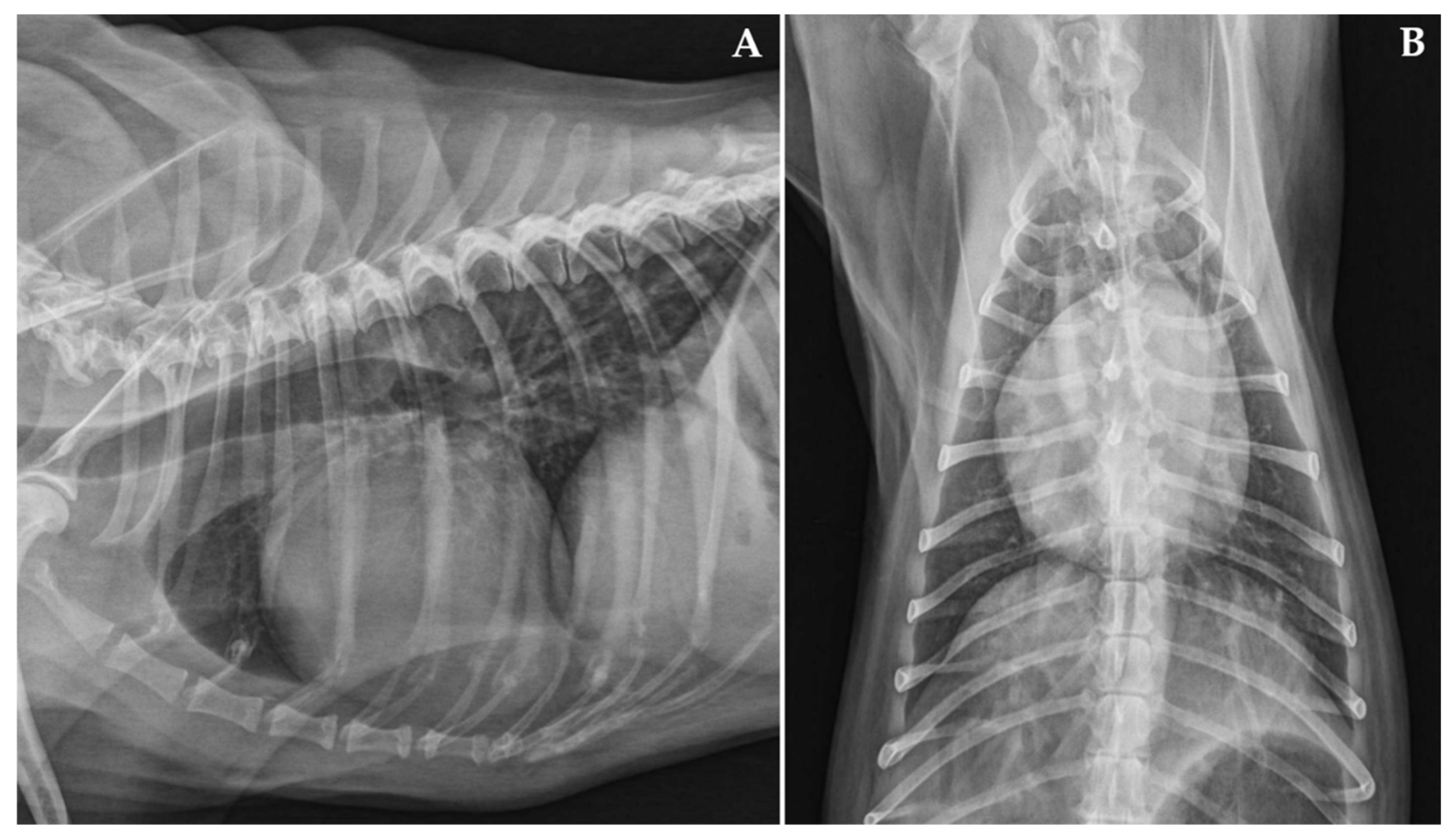
Case Description and Clinical Investigations
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.J.; Barton, L.M.; et al. COVID-19: A multidisciplinary review. Front. Public Health 2020, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Salajegheh Tazerji, S.; Magalhães Duarte, P.; Rahimi, P.; Shahabinejad, F.; Dhakal, S.; Singh Malik, Y.; Shehata, A.A.; Lama Klein, J.; Safdar, M.; Rahman, M.T.; et al. Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: An updated review. J. Transl. Med. 2020, 18, 358. [Google Scholar] [CrossRef] [PubMed]
- Bonilauri, P.; Rugna, G. Animal coronaviruses and SARS-COV-2 in animals, what do we actually know? Life 2021, 11, 123. [Google Scholar] [CrossRef]
- Bonilla-Aldana, D.K.; García-Barco, A.; Jimenez-Diaz, S.D.; Bonilla-Aldana, J.L.; Cardona-Trujillo, M.C.; Muñoz-Lara, F.; Zambrano, L.I.; Salas-Matta, L.A.; Rodriguez-Morales, A.J. SARS-CoV-2 natural infection in animals: A systematic review of studies and case reports and series. Vet. Q. 2021, 41, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.I.; Elia, G.; Grassi, A.; Giordano, A.; Desario, C.; Medardo, M.; Smith, S.L.; Anderson, E.R.; Prince, T.; Patterson, G.T.; et al. Evidence of exposure to SARS-CoV-2 in cats and dogs from households in Italy. Nat. Commun. 2020, 11, 6231. [Google Scholar] [CrossRef] [PubMed]
- Calvet, G.A.; Pereira, S.A.; Ogrzewalska, M.; Pauvolid-Corrêa, A.; Resende, P.C.; Tassinari, W.S.; Costa, A.P.; Keidel, L.O.; da Rocha, A.S.B.; da Silva, M.F.B.; et al. Investigation of SARS-CoV-2 infection in dogs and cats of humans diagnosed with COVID-19 in Rio de Janeiro, Brazil. PLoS ONE 2021, 16, e0250853. [Google Scholar] [CrossRef] [PubMed]
- Dileepan, M.; Di, D.; Huang, Q.; Ahmed, S.; Heinrich, D.; Ly, H.; Liang, Y. Seroprevalence of SARS-CoV-2 (COVID-19) exposure in pet cats and dogs in Minnesota, USA. Virulence 2021, 12, 1597–1609. [Google Scholar] [CrossRef]
- Colitti, B.; Bertolotti, L.; Mannelli, A.; Ferrara, G.; Vercelli, A.; Grassi, A.; Trentin, C.; Paltrinieri, S.; Nogarol, C.; Decaro, N.; et al. Cross-sectional serosurvey of companion animals housed with SARS-CoV-2-infected owners, Italy. Emerg. Infect. Dis. 2021, 27, 1919–1922. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Sereme, Y.; Medkour, H.; Watier-Grillot, S.; Scandola, P.; Ginesta, J.; Andréo, V.; Labarde, C.; Comtet, L.; Pourquier, P.; et al. SARS-CoV-2 antibodies seroprevalence in dogs from France using ELISA and an automated western blotting assay. One Health 2021, 13, 100293. [Google Scholar] [CrossRef]
- Michael, H.T.; Waterhouse, T.; Estrada, M.; Seguin, M.A. Frequency of respiratory pathogens and SARS-CoV-2 in canine and feline samples submitted for respiratory testing in early 2020. J. Small Anim. Pract. 2021, 62, 336–342. [Google Scholar] [CrossRef]
- Perisé-Barrios, A.J.; Tomeo-Martín, B.D.; Gómez-Ochoa, P.; Delgado-Bonet, P.; Plaza, P.; Palau-Concejo, P.; González, J.; Ortiz-Díez, G.; Meléndez-Lazo, A.; Gentil, M.; et al. Humoral responses to SARS-Cov-2 by healthy and sick dogs during the COVID-19 pandemic in Spain. Vet. Res. 2021, 52, 22. [Google Scholar] [CrossRef]
- Smith, S.L.; Anderson, E.R.; Cansado-Utrilla, C.; Prince, T.; Farrell, S.; Brant, B.; Smyth, S.; Noble, P.M.; Pinchbeck, G.L.; Marshall, N.; et al. SARS-CoV-2 neutralising antibodies in dogs and cats in the United Kingdom. Curr. Res. Virol. Sci. 2021, 2, 100011. [Google Scholar] [CrossRef]
- Stevanovic, V.; Vilibic-Cavlek, T.; Tabain, I.; Benvin, I.; Kovac, S.; Hruskar, Z.; Mauric, M.; Milasincic, L.; Antolasic, L.; Skrinjaric, A.; et al. Seroprevalence of SARS-CoV-2 infection among pet animals in Croatia and potential public health impact. Transbound. Emerg. Dis. 2021, 68, 1767–1773. [Google Scholar] [CrossRef]
- Udom, K.; Jairak, W.; Chamsai, E.; Charoenkul, K.; Boonyapisitsopa, S.; Bunpapong, N.; Techakriengkrai, N.; Amonsin, A. Serological survey of antibodies against SARS-CoV-2 in dogs and cats, Thailand. Transbound. Emerg. Dis. 2021. preprint. [Google Scholar] [CrossRef]
- Van Aart, A.E.; Velkers, F.C.; Fischer, E.A.J.; Broens, E.M.; Egberink, H.; Zhao, S.; Engelsma, M.; Hakze-van der Honing, R.W.; Harders, F.; de Rooij, M.M.T.; et al. SARS-CoV-2 infection in cats and dogs in infected mink farms. Transbound. Emerg. Dis. 2021. preprint. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Gao, J.; Huang, K.; Hu, C.; Hui, X.; He, X.; Li, C.; Gong, W.; Lv, C.; et al. A serological survey of severe acute respiratory syndrome coronavirus 2 in dogs in Wuhan. Transbound. Emerg. Dis. 2021. preprint. [Google Scholar] [CrossRef]
- Klaus, J.; Zini, E.; Hartmann, K.; Egberink, H.; Kipar, A.; Bergmann, M.; Palizzotto, C.; Zhao, S.; Rossi, F.; Franco, V.; et al. SARS-CoV-2 infection in dogs and cats from Southern Germany and Northern Italy during the first wave of the COVID-19 pandemic. Viruses 2021, 13, 1453. [Google Scholar] [CrossRef]
- Decaro, N.; Grassi, A.; Lorusso, E.; Patterson, E.I.; Lorusso, A.; Desario, C.; Anderson, E.R.; Vasinioti, V.; Wastika, C.E.; Hughes, G.L.; et al. Long-term persistence of neutralizing SARS-CoV-2 antibodies in pets. Transbound. Emerg. Dis. 2021. preprint. [Google Scholar] [CrossRef]
- Ferasin, L.; Fritz, M.; Ferasin, H.; Becquart, P.; Legros, V.; Leroy, E.M. Myocarditis in naturally infected pets with the British variant of COVID-19. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Carpenter, A.; Ghai, R.R.; Gary, J.; Ritter, J.M.; Carvallo, F.R.; Diel, D.G.; Martins, M.; Murphy, J.; Schroeder, B.; Brightbill, K.; et al. Determining the role of natural SARS-CoV-2 infection in the death of domestic pets: 10 cases (2020–2021). J. Am. Vet. Med. Assoc. 2021, 259, 1032–1039. [Google Scholar] [CrossRef]
- Ferasin, L.; Fritz, M.; Ferasin, H.; Becquart, P.; Corbet, S.; Ar Gouilh, M.; Legros, V.; Leroy, E.M. Infection with SARS-CoV-2 variant B.1.1.7 detected in a group of dogs and cats with suspected myocarditis. Vet. Rec. 2021, 189, e944. [Google Scholar] [CrossRef]
- Giustino, G.; Pinney, S.P.; Lala, A.; Reddy, V.Y.; Johnston-Cox, H.A.; Mechanick, J.I.; Halperin, J.L.; Fuster, V. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J. Am. Coll. Cardiol. 2020, 76, 2011–2023. [Google Scholar] [CrossRef]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef]
- Abou Hassan, O.K.; Sheng, C.C.; Wang, T.K.M.; Cremer, P.C. SARS-CoV-2 myocarditis: Insights into incidence, prognosis, and therapeutic implications. Curr. Cardiol. Rep. 2021, 23, 129. [Google Scholar] [CrossRef]
- Lamb, C.R.; Wikeley, H.; Boswood, A.; Pfeiffer, D.U. Use of breed-specific ranges for the vertebral heart scale as an aid to the radiographic diagnosis of cardiac disease in dogs. Vet. Rec. 2001, 148, 707–711. [Google Scholar] [CrossRef]
- Langhorn, R.; Willesen, J.L. Cardiac troponins in dogs and cats. J. Vet. Intern. Med. 2016, 30, 36–50. [Google Scholar] [CrossRef]
- Romito, G.; Cipone, M. Deep and huge transient negative T waves in dogs with myocardial injury. J. Vet. Cardiol. 2021, 36, 131–140. [Google Scholar] [CrossRef]
- Fabbi, M.; De Giuli, L.; Tranquillo, M.; Bragoni, R.; Casiraghi, M.; Genchi, C. Prevalence of Bartonella henselae in Italian stray cats: Evaluation of serology to assess the risk of transmission of Bartonella to humans. J. Clin. Microbiol. 2004, 42, 264–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijkers, G.; Murk, J.L.; Wintermans, B.; van Looy, B.; van den Berge, M.; Veenemans, J.; Stohr, J.; Reusken, C.; van der Pol, P.; Reimerink, J. Differences in antibody kinetics and functionality between severe and mild severe acute respiratory syndrome coronavirus 2 infections. J. Infect. Dis. 2020, 222, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveilliance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada, A.H.; Maisenbacher, H.W., III. Dilated cardiomyopathy in dogs. In Kirk’s Current Veterinary Therapy, XV ed.; Bonagura, J.D., Twedt, D.C., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2014; pp. 795–800. [Google Scholar]
- Stern, J.A.; Meurs, K.M. Myocardial diseases: Canine. In Textbook of Veterinary Internal Medicine, 8th ed.; Ettinger, S.J., Feldman, E.C., Côté, E., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 1269–1277. [Google Scholar]
- Magagnoli, I.; Romito, G.; Troia, R.; Murgia, E.; Giunti, M. Reversible myocardial dysfunction in a dog after resuscitation from cardiopulmonary arrest. J. Vet. Cardiol. 2021, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schober, K.E. Myocarditis. In Kirk’s Current Veterinary Therapy, XIV ed.; Bonagura, J.D., Twedt, D.C., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2009; pp. 804–808. [Google Scholar]
- Lakhdhir, S.; Viall, A.; Alloway, E.; Keene, B.; Baumgartner, K.; Ward, J. Clinical presentation, cardiovascular findings, etiology, and outcome of myocarditis in dogs: 64 cases with presumptive antemortem diagnosis (26 confirmed postmortem) and 137 cases with postmortem diagnosis only (2004–2017). J. Vet. Cardiol. 2020, 30, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-Torres, F. Canine and feline vector-borne diseases in Italy: Current situation and perspectives. Parasit. Vectors 2010, 3, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Decaro, N.; Vaccari, G.; Lorusso, A.; Lorusso, E.; De Sabato, L.; Patterson, E.I.; Di Bartolo, I.; Hughes, G.L.; Teodori, L.; Desario, C.; et al. Possible human-to-dog transmission of SARS-CoV-2, Italy, 2020. Emerg. Infect. Dis. 2021, 27, 1981–1984. [Google Scholar] [CrossRef]
- Henwood, M.; Lake, D.; Allen, F.; Sange, M. Myocarditis in SARS-CoV-2 negative patients with suspected preceding infection. BMJ Case Rep. 2021, 14, e239513. [Google Scholar] [CrossRef]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Chia, W.N.; Zhu, F.; Ong, S.W.X.; Young, B.E.; Fong, S.W.; Le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef]
- Knies, A.; Ladage, D.; Braun, R.J.; Kimpel, J.; Schneider, M. Persistence of humoral response upon SARS-CoV-2 infection. Rev. Med. Virol. 2021, e2272. [Google Scholar] [CrossRef]
- Sonnleitner, S.T.; Prelog, M.; Jansen, B.; Rodgarkia-Dara, C.; Gietl, S.; Schönegger, C.M.; Koblmüller, S.; Sturmbauer, C.; Posch, W.; Almanzar, G.; et al. Maintenance of neutralizing antibodies over ten months in convalescent SARS-CoV-2 afflicted patients. Transbound. Emerg. Dis. 2021. preprint. [Google Scholar] [CrossRef]
- Dugdale, C.M.; Anahtar, M.N.; Chiosi, J.J.; Lazarus, J.E.; McCluskey, S.M.; Ciaranello, A.L.; Gogakos, T.; Little, B.P.; Branda, J.A.; Shenoy, E.S.; et al. Clinical, laboratory, and radiologic characteristics of patients with initial false-negative SARS-CoV-2 nucleic acid amplification test results. Open Forum Infect. Dis. 2020, 8, ofaa559. [Google Scholar] [CrossRef] [PubMed]
- Kanji, J.N.; Zelyas, N.; MacDonald, C.; Pabbaraju, K.; Khan, M.N.; Prasad, A.; Hu, J.; Diggle, M.; Berenger, B.M.; Tipples, G. False negative rate of COVID-19 PCR testing: A discordant testing analysis. Virol. J. 2021, 18, 13. [Google Scholar] [CrossRef]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; van der Wal, A.C.; Aubry, M.C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef]
- Halushka, M.K.; Vander Heide, R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021, 50, 107300. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Chapman, A.R.; Adamson, P.D.; Mills, N.L. Assessment and classification of patients with myocardial injury and infarction in clinical practice. Heart 2017, 103, 10–18. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Baritussio, A.; Basso, C.; Marcolongo, R. Clinically suspected and biopsy-proven myocarditis temporally associated with SARS-CoV-2 infection. Annu. Rev. Med. 2021. preprint. [Google Scholar] [CrossRef]
- Peretto, G.; Villatore, A.; Rizzo, S.; Esposito, A.; De Luca, G.; Palmisano, A.; Vignale, D.; Cappelletti, A.M.; Tresoldi, M.; Campochiaro, C.; et al. The spectrum of COVID-19-associated myocarditis: A patient-tailored multidisciplinary approach. J. Clin. Med. 2021, 10, 1974. [Google Scholar] [CrossRef]

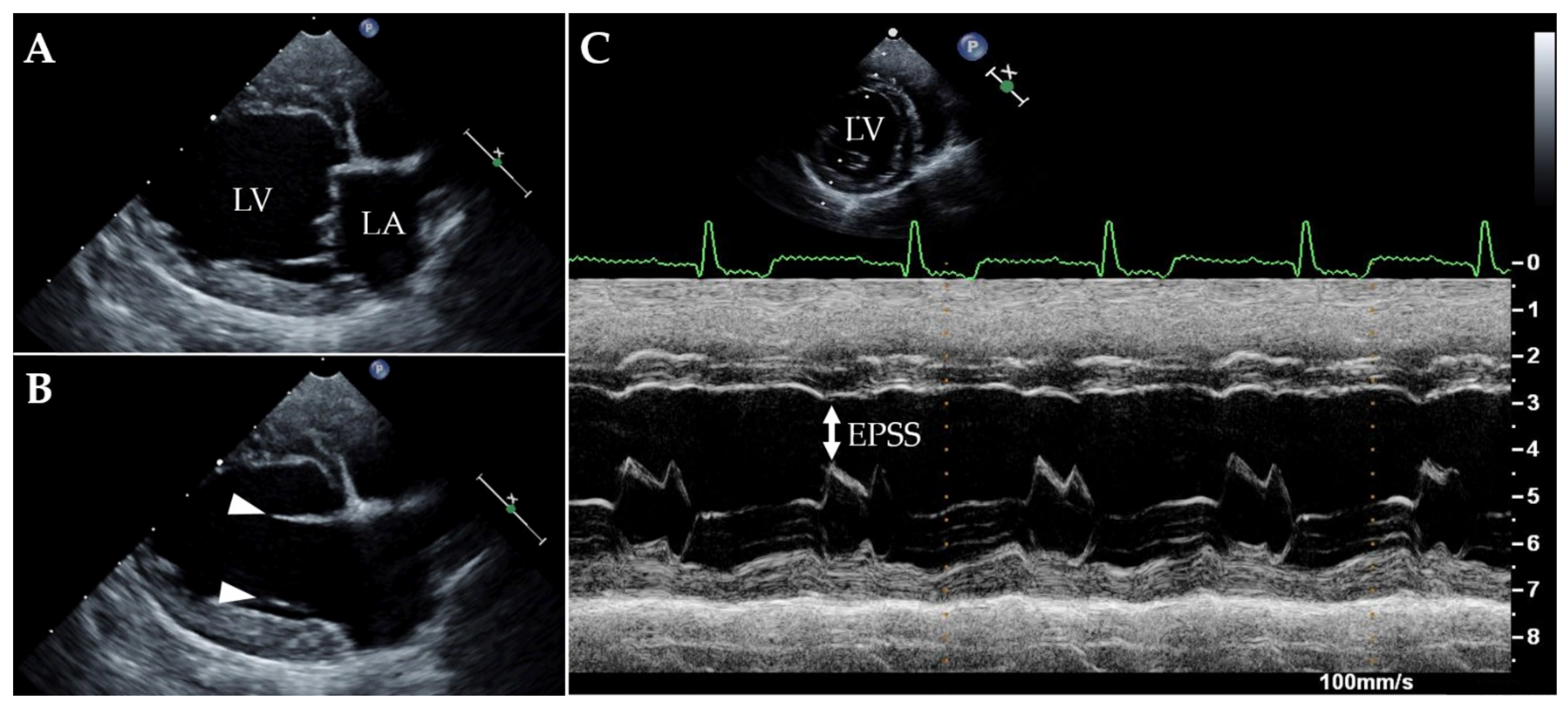
| Parameter | T0 | T1 | Reference Intervals |
|---|---|---|---|
| LA/Ao | 1.2 | 1.15 | <1.6 a |
| LAD (mm) | 30 | 28 | 22.1–33.1 b |
| LVIDDn | 1.95 | 1.9 | 1.27–1.85 c |
| LVIDSn | 1.64 | 1.48 | 0.71–1.26 c |
| EDVI (mL/m2) | 148 | 126 | 49.8–122.4 d |
| ESVI (mL/m2) | 96 | 70 | 13.2–38.0 d |
| SF (%) | 16 | 22 | 30–49 d |
| EF (%) | 35 | 45 | 57.8–82.1 d |
| EPSS (mm) | 12 | 9 | <6.5 e |
| Serological Test | T0 | T1 | T2 |
|---|---|---|---|
| sVNT | 60% | 66% | 60% |
| VNT | 1/20 | 1/10 | 1/10 |
| ELISA | 27.37% | 22.55% | negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romito, G.; Bertaglia, T.; Bertaglia, L.; Decaro, N.; Uva, A.; Rugna, G.; Moreno, A.; Vincifori, G.; Dondi, F.; Diana, A.; et al. Myocardial Injury Complicated by Systolic Dysfunction in a COVID-19-Positive Dog. Animals 2021, 11, 3506. https://doi.org/10.3390/ani11123506
Romito G, Bertaglia T, Bertaglia L, Decaro N, Uva A, Rugna G, Moreno A, Vincifori G, Dondi F, Diana A, et al. Myocardial Injury Complicated by Systolic Dysfunction in a COVID-19-Positive Dog. Animals. 2021; 11(12):3506. https://doi.org/10.3390/ani11123506
Chicago/Turabian StyleRomito, Giovanni, Teresa Bertaglia, Luigi Bertaglia, Nicola Decaro, Annamaria Uva, Gianluca Rugna, Ana Moreno, Giacomo Vincifori, Francesco Dondi, Alessia Diana, and et al. 2021. "Myocardial Injury Complicated by Systolic Dysfunction in a COVID-19-Positive Dog" Animals 11, no. 12: 3506. https://doi.org/10.3390/ani11123506
APA StyleRomito, G., Bertaglia, T., Bertaglia, L., Decaro, N., Uva, A., Rugna, G., Moreno, A., Vincifori, G., Dondi, F., Diana, A., & Cipone, M. (2021). Myocardial Injury Complicated by Systolic Dysfunction in a COVID-19-Positive Dog. Animals, 11(12), 3506. https://doi.org/10.3390/ani11123506








