Effects of Probiotics on Growth, Survival, Water Quality and Disease Resistance of Red Hybrid Tilapia (Oreochromis spp.) Fingerlings in a Biofloc System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Selected Probiotic
2.2. Pathogen Culture
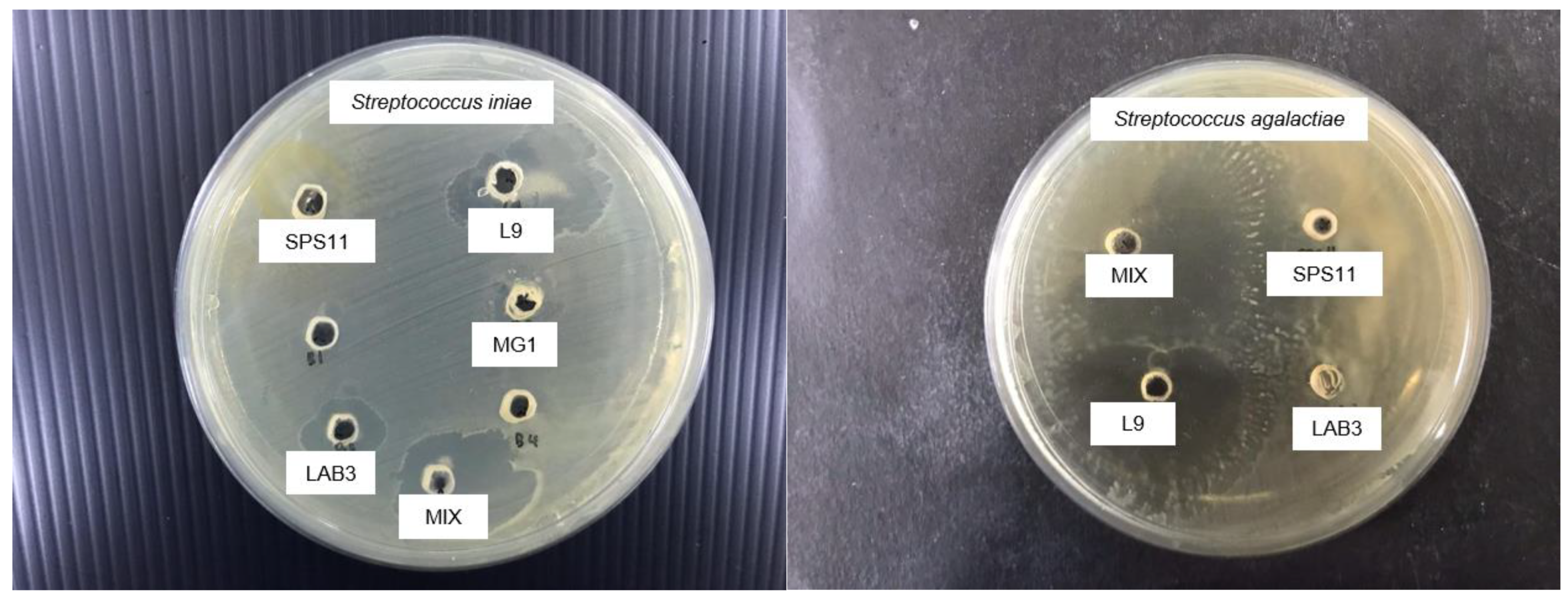
2.3. In Vitro Screening of Probiotics
Well Diffusion Assay
2.4. Addition of Probiotics in a Biofloc Culture System
2.4.1. Red Hybrid Tilapia Fingerlings and Monitoring
2.4.2. Biofloc Preparation, Maintenance, and Experimental Trial
2.4.3. Water Quality Monitoring
2.4.4. Growth Performance
2.5. Challenge Test
2.6. Histopathological Examination
2.7. Statistical Analysis
3. Results
3.1. Probiotic Selection
3.2. Growth and S3.2 Growth and Survival
3.3. Water Quality Parameters
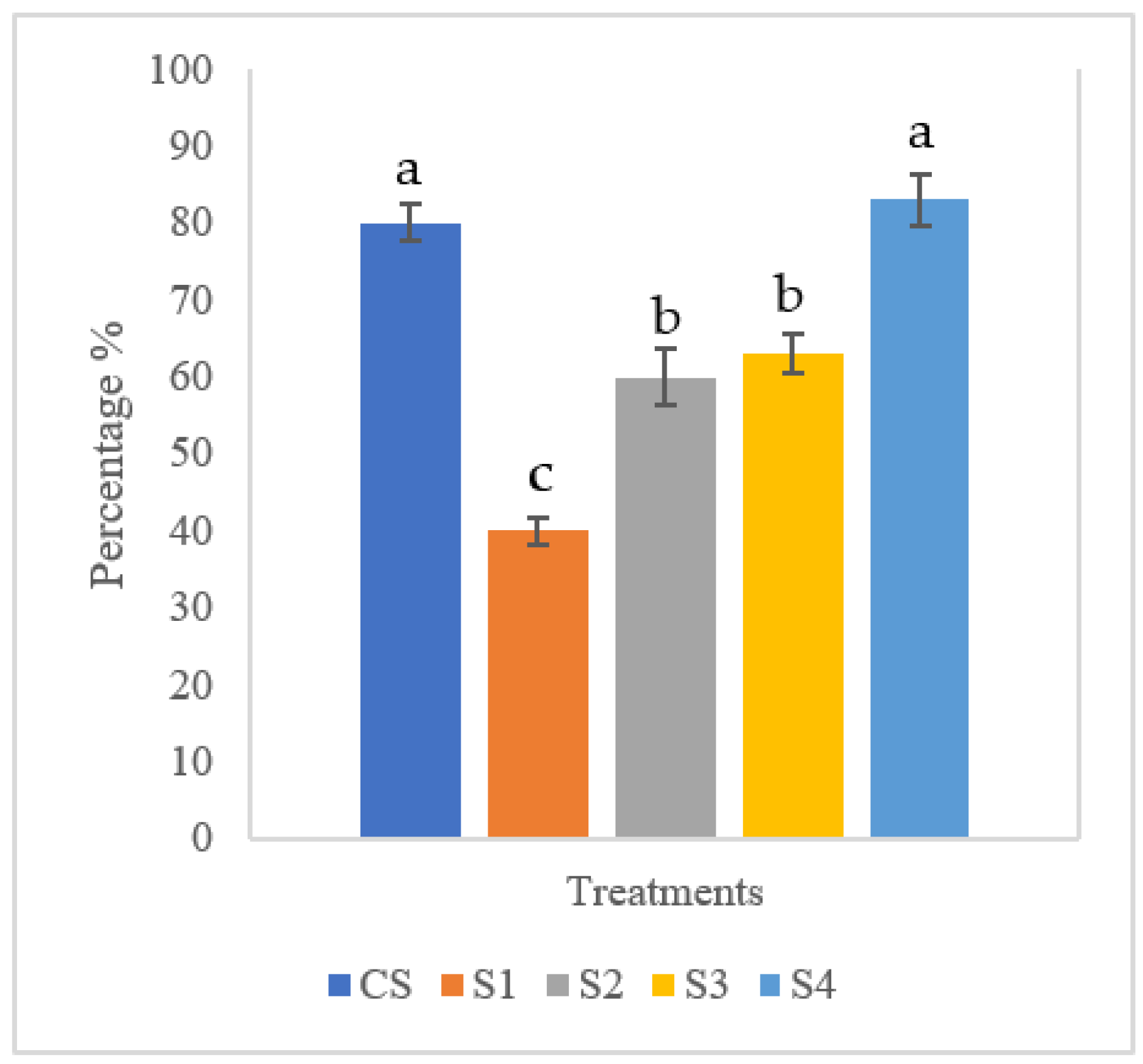
3.4. Challenge Assay of Red Hybrid Tilapia
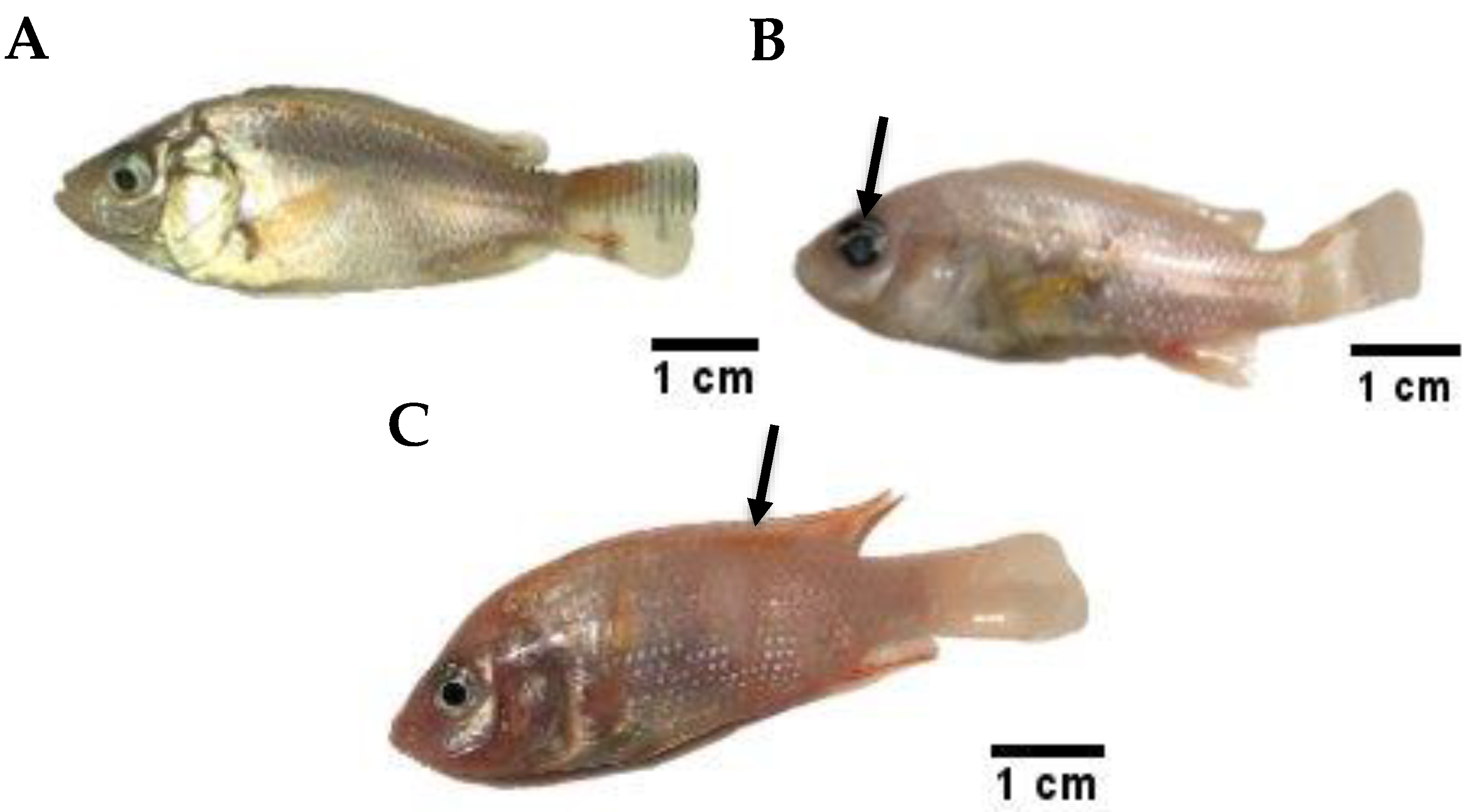
3.5. Clinical Signs and Symptoms
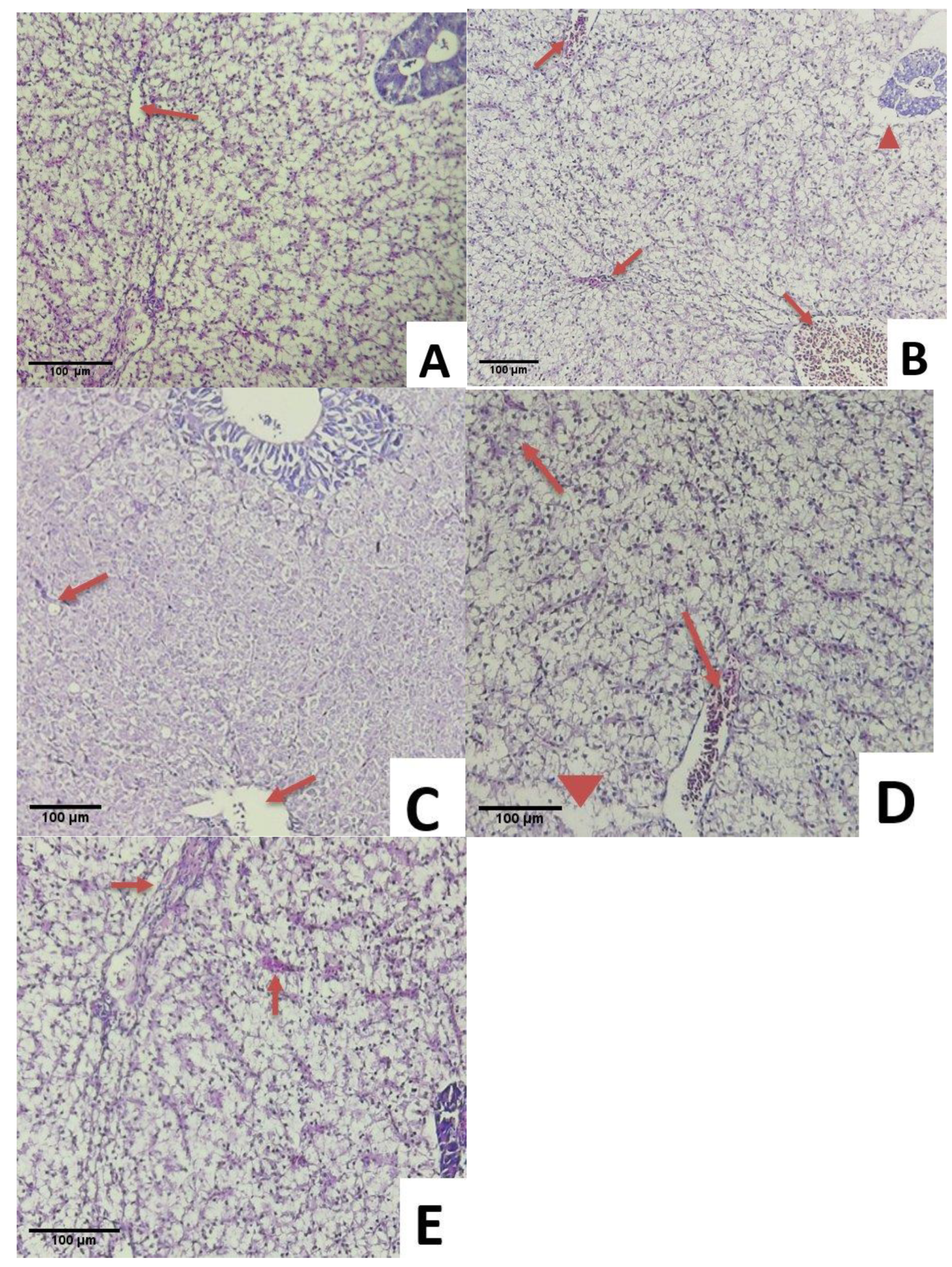
3.6. Histopathological Examination
4. Discussion
4.1. Probiotic Selection
4.2. Growth Performance
4.3. Water Quality
4.4. Bacterial Challenge and Histopathology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nandlal, S.; Pickering, T. Tilapia fish farming in Pacific Island countries. In Tilapia Hatchery Operation Secretariat of the Pacific Community and Marine Studies Program; The University of the South Pacific: Noumea, New Caledonia, 2004; Volume 1, pp. 1–32. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018. Aquac. Prod. 2018, 1, 20–25. [Google Scholar]
- DOF. Annual Fisheries Statistics 2019; Department of Fisheries, Malaysia, Ministry of Agriculture and Agro-based Industries: Putrajaya, Malaysia, 2020.
- Orachunwong, C.; Thammasart, S.; Lohawatanakul, C. Recent developments in tilapia feeds. In Tilapia: Production, Marketing and Technical Developments. In Proceedings of the Tilapia 2001 International Technical and Trade Conference on Tilapia, Infofish, Kuala Lumpur, Malaysia, 28–30 May 2001; Subasinghe, S., Singh, T., Eds.; pp. 113–122. [Google Scholar]
- Buchanan, J.T.; Stannard, J.A.; Lauth, X.; Ostland, V.E.; Powell, H.C.; Westerman, M.E.; Nizet, V. Streptococcus iniae phosphoglucomutase is a virulence factor and a target for vaccine development. Infect. Immun. 2005, 73, 6935–6944. [Google Scholar] [CrossRef] [Green Version]
- Shelby, R.A.; Shoemaker, C.A.; Evans, J.J.; Klesius, P.H. Development of an Indirect ELISA to Detect Humoral Response to Streptococcus iniae Infection of Nile Tilapia, Oreochromis niloticus. J. Appl. Aquac. 2001, 11, 35–44. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Klesius, P.H.; Plumb, J.A. Killing of Edwardsiella ictaluri by macrophages from channel catfish immune and susceptible to enteric septicemia of catfish. Vet. Immunol. Immunopathol. 1997, 58, 181–190. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Asheg, A.; Eissa, A.E. Streptococcus dysgalactiae: An emerging pathogen of fishes and mammals. Int. J. Vet. Sci. Med. 2013, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.Y.; Chen, D.F.; Huang, L.Y.; Lian, H.; Wang, J.; Xiao, D.; Lai, W.M. Isolation and characterization of Streptococcus agalactiae from Nile Tilapia Oreochromis niloticus in China. Afr. J. Microbiol. Res. 2013, 7, 317–323. [Google Scholar]
- Zhu, J.; Li, C.; Ao, Q.; Tan, Y.; Luo, Y.; Guo, Y.; Gan, X. Trancriptomic profiling revealed the signatures of acute immune response in tilapia (Oreochromis niloticus) following Streptococcus iniae challenge. Fish Shellfish. Immunol. 2015, 46, 346–353. [Google Scholar] [CrossRef]
- Alsaid, M.; Abuseliana, A.F.; Daud, H.H.; Mustapha, N.M.; Bejo, S.K.; Abdelhadi, Y.M.; Hamdan, R.H. Haematological, biochemical and clinical signs changes following experimental infection of Streptococcus agalactiae in red hybrid tilapia (Oreochromis sp). Aquac. Indones. 2014, 15, 289–295. [Google Scholar] [CrossRef] [Green Version]
- WHO Health and nutritional properties of probiotics in food including powder milk with lactic acid bacteria. Med. Rep. 2001, 12, 328–330.
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging (2021). alternative approach. Symbiosis 2019, 77, 99–113. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mounes, H.A.; Shady, S.H.; Ahmed, K.M. Effects of yucca, Yucca schidigera, extract and/or yeast, Saccharomyces cerevisiae, as water additives on growth, biochemical, and antioxidants/oxidant biomarkers of Nile tilapia, Oreochromis niloticus. Aquaculture 2021, 533, 736122. [Google Scholar] [CrossRef]
- Hassaan, M.S.; El-Sayed, A.M.I.; Mohammady, E.Y.; Zaki, M.A.; Elkhyat, M.M.; Jarmołowicz, S.; El-Haroun, E.R. Eubiotic effect of a dietary potassium diformate (KDF) and probiotic (Lactobacillus acidophilus) on growth, hemato-biochemical indices, antioxidant status and intestinal functional topography of cultured Nile tilapia Oreochromis niloticus fed diet free fishmeal. Aquaculture 2021, 533, 736147. [Google Scholar]
- El-Kady, A.A.; Magouz, F.I.; Mahmoud, S.A.; Abdel-Rahim, M.M. The effects of some commercial probiotics as water additive on water quality, fish performance, blood biochemical parameters, expression of growth and immune-related genes, and histology of Nile tilapia (Oreochromis niloticus). Aquaculture 2022, 546, 737249. [Google Scholar] [CrossRef]
- Ekasari, J.; Rivandi, D.R.; Firdausi, A.P.; Surawidjaja, E.H.; Zairin, M., Jr.; Bossier, P.; De Schryver, P. Biofloc technology positively affects Nile tilapia (Oreochromis niloticus) larvae performance. Aquaculture 2015, 441, 72–77. [Google Scholar] [CrossRef]
- Kim, S.K.; Pang, Z.; Seo, H.C.; Cho, Y.R.; Samocha, T.; Jang, I.K. Effect of bioflocs on growth and immune activity of Pacific white shrimp, Litopenaeus vannamei postlarvae. Aquac. Res. 2014, 45, 362–371. [Google Scholar] [CrossRef]
- Ray, A.J.; Lotz, J.M. Comparing a chemoautotrophic-based biofloc system and three heterotrophic-based systems receiving different carbohydrate sources. Aquac. Eng. 2014, 63, 54–61. [Google Scholar] [CrossRef]
- Zabidi, A.; Rosland, N.A.; Yaminudin, J.; Karim, M. In Vitro Assessment of Bacterial Strains Associated with Microalgae as Potential Probiotics. Pertanika J. Trop. Agric. Sci. 2021, 44, 205–220. [Google Scholar] [CrossRef]
- Azrin, N.A.R.; Yuzine, E.; Ina-Salwany, M.Y.; Murni, K. The efficacy of potential probiont Bacillus amyloliquefaciens strain L11 in protecting Artemia nauplii and blue crab juveniles against Vibrio harveyi infection. J. Pure Appl. Microbiol. 2019, 13, 923–931. [Google Scholar] [CrossRef]
- Masduki, F.; Min, C.C.; Karim, M. Characterization of Enterococcus hirae Isolated from the Intestine of Seabass (Lates Calcarifer) as a New Potential Probiotic against Pathogenic Vibrios. Curr. Microbiol. 2020, 77, 3962–3968. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book; World Aquaculture Society: Baton Rouge, LA, USA, 2009; p. 182. [Google Scholar]
- Federation, W.E.; APH Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 1998; Volume 21, p. 1378. [Google Scholar]
- Avnimelech, Y.; Kochba, M. Evaluation of nitrogen uptake and excretion by tilapia in bio floc tanks, using 15N tracing. Aquaculture 2009, 287, 163–168. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Biofloc Production Systems for Aquaculture; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2013; Volume 4503, pp. 1–11. [Google Scholar]
- Sahu, M.K.; Swarnakumar, N.S.; Sivakumar, K.; Thangaradjou, T.; Kannan, L. Probiotics in aquaculture: Importance and future perspectives. Indian J. Microbiol. 2008, 48, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Rafiee, G.; Tavabe, K.R.; Abdel-Latif, H.M.; Dawood, M.A. The enrichment of diet with beneficial bacteria (single-or multi-strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 539, 736640. [Google Scholar] [CrossRef]
- Menaga, M.; Felix, S.; Charulatha, M.; Gopalakannan, A.; Mohanasundari, C.; Boda, S. In Vivo efficiency of Bacillus sp. isolated from biofloc system on growth, haematological, immunological and antioxidant status of genetically improved farmed tilapia (GIFT). Indian J. Exp. Biol. 2020, 58, 714–721. [Google Scholar]
- Kathia, C.M.; del Carmen, M.D.M.; Aida, H.P.; Jorge, C.M.; Félix, A.G.J.; Amadeo, B.M.J. Effect of two probiotics on bacterial community composition from biofloc system and their impact on survival and growth of tilapia (Oreochromis niloticus). Int. J. Fish. Aquat. Stud. 2018, 6, 525–533. [Google Scholar]
- Krummenauer, D.; Poersch, L.; Romano, L.A.; Lara, G.R.; Encarnação, P.; Wasielesky Jr, W. The effect of probiotics in a Litopenaeus vannamei biofloc culture system infected with Vibrio parahaemolyticus. J. Appl. Aquac. 2014, 26, 370–379. [Google Scholar] [CrossRef]
- Dantas Jr, E.M.; Valle, B.C.S.; Brito, C.M.S.; Calazans, N.K.F.; Peixoto, S.R.M.; Soares, R.B. Partial replacement of fishmeal with biofloc meal in the diet of postlarvae of the Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 2016, 22, 335–342. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Luo, G.; Gao, Q.; Wang, C.; Liu, W.; Sun, D.; Li, L.; Tan, H. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Oreochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofloc system. Aquaculture 2014, 422, 1–7. [Google Scholar] [CrossRef]
- Liñan-Vidriales, M.A.; Peña-Rodríguez, A.; Tovar-Ramírez, D.; Elizondo-González, R.; Barajas-Sandoval, D.R.; Ponce-Gracía, E.I.; José, C.R.-J.; Balcázar, L.; Quiroz-Guzmán, E. Effect of rice bran fermented with Bacillus and Lysinibacillus species on dynamic microbial activity of Pacific white shrimp (Penaeus vannamei). Aquaculture 2021, 531, 735958. [Google Scholar] [CrossRef]
- Lin, Y.S.; Saputra, F.; Chen, Y.C.; Hu, S.Y. Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio). Fish Shellfish. Immunol. 2019, 86, 410–419. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M. Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morphology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquac. Int. 2015, 23, 203–217. [Google Scholar] [CrossRef]
- Hamid, N.H.; Daud, H.M.; Kayansamruaj, P.; Hassim, H.A.; Yusoff MS, M.; Bakar SN, A.; Srisapoome, P. Short-and long-term probiotic effects of Enterococcus hirae isolated from fermented vegetable wastes on the growth, immune responses, and disease resistance of hybrid catfish (Clarias gariepinus × Clarias macrocephalus). Fish Shellfish. Immunol. 2021, 114, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Yang, J.; Li, Y.; Guan, C.; Wu, F. Effect of biofloc technology on growth, digestive enzyme activity, hematology, and immune response of genetically improved farmed tilapia (Oreochromis niloticus). Aquaculture 2015, 448, 135–141. [Google Scholar] [CrossRef]
- Pérez-Fuentes, J.A.; Hernández-Vergara, M.P.; Pérez-Rostro, C.I.; Fogel, I. C: N ratios affect nitrogen removal and production of Nile tilapia Oreochromis niloticus raised in a biofloc system under high density cultivation. Aquaculture 2016, 452, 247–251. [Google Scholar] [CrossRef]
- Dauda, A.B.; Romano, N.; Ebrahimi, M.; Teh, J.C.; Ajadi, A.; Chong, C.M.; Kamarudin, M.S. Influence of carbon/nitrogen ratios on biofloc production and biochemical composition and subsequent effects on the growth, physiological status and disease resistance of African catfish (Clarias gariepinus) cultured in glycerol-based biofloc systems. Aquaculture 2018, 483, 120–130. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Tilapia Culture in Salt Water: Environmental Requirements, Nutritional Implications and Economic Potentials; Eight Symposium on Advances in Nutritional Aquaculture: Nuevo Leon, Mexico, 2006. [Google Scholar]
- Hosain, M.E.; Amin, S.N.; Arshad, A.; Kamarudin, M.S.; Karim, M. Effects of carbon sources on the culture of giant river prawn in biofloc system during nursery phase. Aquac. Rep. 2021, 19, 100607. [Google Scholar] [CrossRef]
- Ridha, M.T.; Hossain, M.A.; Azad, I.S.; Saburova, M. Effects of three carbohydrate sources on water quality, water consumption, bacterial count, growth and muscle quality of Nile tilapia (Oreochromis niloticus) in a biofloc system. Aquac. Res. 2020, 51, 4225–4237. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Danaher, J.J.; Bailey, D.S.; Shultz, R.C. Development of a Biofloc System for the Production of Tilapia. Aquaculture 2008, 277, 138–145. [Google Scholar]
- Mallasen, M.; Valenti, W.C.; Ismael, D. Effects of nitrate concentration on larval development of the giant river prawn, Macrobrachium rosenbergii. J. Appl. Aquac. 2004, 14, 55–69. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Forster, I.; Conquest, L.; Dominy, W.; Kuo, W.C.; David Horgen, F. Determination of microbial community structures of shrimp floc cultures by biomarkers and analysis of floc amino acid profiles. Aquac. Res. 2008, 39, 118–133. [Google Scholar] [CrossRef]
- Ekasari, J.; Crab, R.; Verstraete, W. Primary nutritional content of bio-flocs cultured with different organic carbon sources and salinity. Hayati J. Biosci. 2010, 17, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Amal, M.N.A.; Saad, M.Z.; Zahrah, A.S.; Zulkafli, A.R. Water quality influences the presence of Streptococcus agalactiae in cage cultured red hybrid tilapia, Oreochromis niloticus× Oreochromis mossambicus. Aquac. Res. 2015, 46, 313–323. [Google Scholar] [CrossRef]
- Yiagnisis, M.; Athanassopoulou, F. Bacteria isolated from diseased wild and farmed marine fish in Greece. Recent. Adv. Fish Farms 2011, 27, 61–69. [Google Scholar]
- Hadi, A.A.; Alwan, S.F. Histopathological changes in gills, liver and kidney of fresh water fish, Tilapia zillii, exposed to aluminum. Int. J. Pharm. Life Sci. 2012, 3, 11. [Google Scholar]
- Okwari, O.O.; Ettarh, R.R.; Akpogomeh, B.A.; Eteng, M.U. Gastric anti-secretory and anti-ulcerogenic effects of Dombeya buettneri in rats. J. Ethnopharmacol. 2000, 71, 315–319. [Google Scholar] [CrossRef]
- Suwannasang, A.; Dangwetngam, M.; Issaro, A.; Phromkunthong, W.; Suanyuk, N. Pathological manifestations and immune responses of serotypes Ia and III Streptococcus agalactiae infections in Nile tilapia (Oreochromis niloticus). Songklanakarin J. Sci. Technol. 2014, 36, 499–506. [Google Scholar]
- Abdullah, S.; Omar, N.; Yusoff, S.M.; Obukwho, E.B.; Nwunuji, T.P.; Hanan, L.; Samad, J. Clinicopathological features and immunohistochemical detection of antigens in acute experimental Streptococcus agalactiae infection in Red Hybrid Tilapia (Oreochromis spp.). Springerplus 2013, 2, 286. Available online: http://www.springerplus.com/content/2/1/286 (accessed on 15 June 2021). [CrossRef] [Green Version]
- Woo, P.T.; Buchmann, K. Fish Parasites: Pathobiology and Protection; CABI: Wallingford, Oxfordshire, UK, 2012. [Google Scholar]
- Tzuc, J.T.; Escalante, D.R.; Herrera, R.R.; Cortés, G.G.; Ortiz, M.L.A. Microbiota from Litopenaeus vannamei: Digestive tract microbial community of Pacific white shrimp (Litopenaeus vannamei). SpringerPlus 2014, 3, 280. Available online: http://www.springerplus.com/content/3/1/280 (accessed on 3 July 2021). [CrossRef] [Green Version]


| Code | Group |
|---|---|
| C | Control: Freshwater |
| B1 | Control: Biofloc |
| MG1 | Biofloc with commercial probiotics MG1 |
| MP | Biofloc with MP (Lysinibacillus fusiformis SPS11, Enterococcus hirae LAB3, Bacillus amyloliquefaciens L9) |
| Code | Group |
|---|---|
| CS | Control: Freshwater only |
| S1 | Streptococcus agalactiae 108 CFU mL−1 |
| S2 | Biofloc + Streptococcus agalactiae 108 CFU mL−1 |
| S3 | Biofloc + MG1 + Streptococcus agalactiae 108 CFU mL−1 |
| S4 | Biofloc + MP + Streptococcus agalactiae 108 CFU mL−1 |
| Pathogen | S. agalactiae | S. iniae | |||
|---|---|---|---|---|---|
| Probiotic | 108 CFU mL−1 | 106 CFU mL−1 | 108 CFU mL−1 | 106 CFU mL−1 | |
| SPS11 | 7.6 ± 0.4 a | 5.4 ± 0.4 b | 6.65 ± 0.35 a | 4.7 ± 0.7 b | |
| L9 | 12.6 ± 0.6 a | 8.8 ± 0.2 b | 11.7 ± 0.7 a | 9.4 ± 0.4 b | |
| LAB 3 | 7.75 ± 0.75 a | 5.6 ± 0.4 c | 6.7 ± 0.7 b | 5.7 ± 0.3 c | |
| MP | 10.2 ± 0.2 a | 8.05 ± 0.95 c | 10.75 ± 1 a | 9.3 ± 0.7 b | |
| MG1 | 4.65 ± 0.4 a | 2.25 ± 0.25 c | 3.4 ± 0.6 b | 1.7 ± 0.3 c | |
| Parameters | CS | B1 | MG1 | MP |
|---|---|---|---|---|
| Survival (%) | 91 ± 0.91 a | 84 ± 1.78 a | 89 ± 1.28 a | 90 ± 1.16 a |
| IBW (g) | 0.57 ± 0.05 a | 0.53 ± 0.02 a | 0.4 ± 0.06 a | 0.51 ± 0.05 a |
| FBW (g) | 8.051 ± 1.4 c | 12.53 ± 1 b | 11.56 ± 1.34 b | 15.07 ± 1.30 a |
| SGR (% day−1) | 2.05 ± 0.20 a | 3.07 ± 0.33 b | 2.83 ± 0.30 b | 3.73 ± 0.23 b |
| FCR | 1.39 ± 0.08 a | 0.92 ± 0.10 b | 1.0 ± 0.10 b | 0.76 ± 0.04 b |
| Parameters | CS | B1 | MG1 | MP |
|---|---|---|---|---|
| Temperature (°C) | 27.57 ± 0.5 a | 27.65 ± 0.1 a | 27.48 ± 0.14 a | 27.61± 0.08 a |
| DO (mg L−1) | 3.53 ± 0.35 a | 3.24 ± 0.23 a | 3.45 ± 0.34 a | 3.40 ± 0.35 a |
| pH | 6.32 ± 0.08 a | 6.50 ± 0.01 a | 6.67± 0.03 a | 6.51± 0.02 a |
| NO2-N (mg L−1) | 2 ± 0.14 a | 1.45 ± 0.21 b | 1.32 ± 0.08 b | 0.84 ± 0.07 c |
| NO3-N (mg L−1) | 0.8 ± 0.31 c | 2.20 ± 0.45 b | 2.70 ± 0.3 a | 2.00 ± 0.22 b |
| NH4-N (mg L−1) | 3.8 ± 0.3 a | 2.97 ± 0.17 b | 3.20 ± 0.29 b | 2.60 ± 0.08 c |
| SD (mg L−1) | - | 23.00 ± 2.3 b | 27.30 ± 2.7 b | 31.27 ± 3.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabidi, A.; Yusoff, F.M.; Amin, N.; Yaminudin, N.J.M.; Puvanasundram, P.; Karim, M.M.A. Effects of Probiotics on Growth, Survival, Water Quality and Disease Resistance of Red Hybrid Tilapia (Oreochromis spp.) Fingerlings in a Biofloc System. Animals 2021, 11, 3514. https://doi.org/10.3390/ani11123514
Zabidi A, Yusoff FM, Amin N, Yaminudin NJM, Puvanasundram P, Karim MMA. Effects of Probiotics on Growth, Survival, Water Quality and Disease Resistance of Red Hybrid Tilapia (Oreochromis spp.) Fingerlings in a Biofloc System. Animals. 2021; 11(12):3514. https://doi.org/10.3390/ani11123514
Chicago/Turabian StyleZabidi, Aimi, Fatimah Md Yusoff, Nurul Amin, Nur Jasmin Mohd Yaminudin, Puvaneswari Puvanasundram, and Murni Marlina Abd Karim. 2021. "Effects of Probiotics on Growth, Survival, Water Quality and Disease Resistance of Red Hybrid Tilapia (Oreochromis spp.) Fingerlings in a Biofloc System" Animals 11, no. 12: 3514. https://doi.org/10.3390/ani11123514
APA StyleZabidi, A., Yusoff, F. M., Amin, N., Yaminudin, N. J. M., Puvanasundram, P., & Karim, M. M. A. (2021). Effects of Probiotics on Growth, Survival, Water Quality and Disease Resistance of Red Hybrid Tilapia (Oreochromis spp.) Fingerlings in a Biofloc System. Animals, 11(12), 3514. https://doi.org/10.3390/ani11123514







