Simple Summary
Enteric methane (CH4) from the anaerobic fermentation of feed carbohydrates in ruminant livestock accounts for 3 to 5% of global greenhouse gas emissions. Among the different CH4 mitigating approaches evaluated to decrease enteric CH4 emissions from ruminants, the feed additive 3-nitrooxypropanol is effective with a mean reduction in CH4 of 30%, depending on animal type, diet and dose. 3-nitrooxypropanol is chemically synthesized and studies show low safety risk with no detrimental effects to animals and humans. 3-nitrooxypropanol was recently approved by regulatory authorities for use in Brazil and Chile and has received a favorable opinion from the scientific panel of the European Food Safety Authority, with approvals in various jurisdictions expected in the near future. As a substantial body of research on 3-nitrooxypropanol is now available, this review offers a timely analysis of the opportunities and challenges of using 3-nitrooxypropanol to mitigate enteric CH4 emissions in ruminant livestock.
Abstract
Methane (CH4) from enteric fermentation accounts for 3 to 5% of global anthropogenic greenhouse gas emissions, which contribute to climate change. Cost-effective strategies are needed to reduce feed energy losses as enteric CH4 while improving ruminant production efficiency. Mitigation strategies need to be environmentally friendly, easily adopted by producers and accepted by consumers. However, few sustainable CH4 mitigation approaches are available. Recent studies show that the chemically synthesized CH4 inhibitor 3-nitrooxypropanol is one of the most effective approaches for enteric CH4 abatement. 3-nitrooxypropanol specifically targets the methyl-coenzyme M reductase and inhibits the final catalytic step in methanogenesis in rumen archaea. Providing 3-nitrooxypropanol to dairy and beef cattle in research studies has consistently decreased enteric CH4 production by 30% on average, with reductions as high as 82% in some cases. Efficacy is positively related to 3-NOP dose and negatively affected by neutral detergent fiber concentration of the diet, with greater responses in dairy compared with beef cattle when compared at the same dose. This review collates the current literature on 3-nitrooxypropanol and examines the overall findings of meta-analyses and individual studies to provide a synthesis of science-based information on the use of 3-nitrooxypropanol for CH4 abatement. The intent is to help guide commercial adoption at the farm level in the future. There is a significant body of peer-reviewed scientific literature to indicate that 3-nitrooxypropanol is effective and safe when incorporated into total mixed rations, but further research is required to fully understand the long-term effects and the interactions with other CH4 mitigating compounds.
1. Introduction
Methane (CH4), a flow gas, is a potent greenhouse gas with a global warming potential 82 times stronger per unit mass than carbon dioxide (CO2) on a 20-year timescale and 28 times more powerful on a 100-year time scale [1]. CH4 emissions from enteric fermentation of plant biomass in the ruminant digestive system generated by methanogenic archaea not only contribute to climate change, but also represent a loss of 2 to 12% of gross energy intake and a potential reduction in feed efficiency [2]. Enteric CH4 from ruminant livestock escapes into the atmosphere mainly through eructation, and contributes 3 to 5% of the global greenhouse gas emissions [3]. The world’s increasing demand for animal-sourced protein products will undoubtedly cause enteric CH4 emissions to increase [4] unless mitigation is adopted. According to Rogelj et al. (2018) [5], CH4 emissions from agricultural production need to be reduced by 24 to 47% by 2050 relative to 2010 to meet the 1.5 °C target of the Paris Agreement [6]. Over 100 countries (including 9 of the world’s top 20 CH4-emitting countries) recently signed a pledge to reduce global CH4 emissions by at least 30% relative to 2020 levels by 2030 [7]. CH4 has an estimated lifetime of 12 yr in the atmosphere [8], hence decreasing global CH4 emissions can limit global climate warming in a short timeframe.
Given the global emphasis on CH4 reduction, numerous mitigation strategies have been studied. These include dietary formulation [9], animal breeding [10], vaccines [11], bromoform-containing seaweeds [12], chemical inhibitors [13], and others. Despite research efforts, few technologies are commercially available that can safely, consistently, and substantially reduce enteric CH4 from ruminant livestock. Diet formulation typically results in only moderate reductions in CH4 (<20%), breeding for low-CH4 emitting animals may bring moderate reductions but requires a long term approach, vaccines against methanogens are at a developmental stage, and given that bromoform is a potential carcinogen the safety risks associated with Asparagopsis sp. seaweeds [12] may limit their extensive use in animal diets. Numerous chemical CH4 inhibitors have been evaluated over the years, and while some have been shown to be highly effective, achieving large reductions in CH4 emissions (>30%), their commercial use has been limited mainly due to safety concerns. One notable exception is 3-nitrooxypropanol (3-NOP), which has been shown in the past decade to be highly effective in decreasing CH4 production while posing minimal safety risk. 3-NOP binds to the CH4-producing enzyme methyl-coenzyme M reductase (MCR), thereby inhibiting the formation of CH4 without negative influence on non-methanogenic bacteria or the animal itself [14,15].
Feed additives that persistently lower CH4 emissions must not have toxic effects for animals, humans and the environment. To be adopted by producers, they need to be easy to use and preferably low cost. An increase in animal productivity would help offset the additional cost of the feed additive and improve profitability [9]. 3-Nitrooxypropanol has been evaluated in approximately 28 in vivo and 7 in vitro ruminant studies and several recent meta-analyses have examined this substantial body of information to examine overall efficacy when 3-NOP is used for enteric CH4 mitigation [16,17,18,19,20,21]. 3-NOP could provide a feasible strategy for CH4 mitigation if it is accepted by consumers and approved by regulatory authorities. 3-NOP recently received a favorable opinion from the scientific panel of the European Food Safety Authority for safety and efficacy in dairy cows. It was recently approved in Brazil and Chile, and regulatory approvals in other jurisdictions are expected in the future. Thus, with impending on-farm use of 3-NOP, there is a need to critically examine the body of information available to enable farmers and technical advisors to make informed decisions. This review provides a comprehensive analysis of the published results and discusses the challenges and opportunities for using 3-NOP to reduce enteric CH4 emissions from ruminant livestock.
2. 3-Nitrooxypropanol, Mode of Action and Safety
The compound 3-NOP was first chemically synthesised by Ogawa et al. (1990) [22], and a patent was granted for the use of 3-NOP as a CH4 mitigant [23]. It has low molecular weight (121.09 g/mol) and is a small molecule with dual chemical functional groups: a primary alcohol and an organic nitrate ester [24]. The nitrogen (N) atom is indirectly attached to the carbon (C) backbone via a C–O–N bond (chemical structure shown in Figure 1). As a structural analogue of methyl-coenzyme M, 3-NOP specifically targets the nickel enzyme MCR [15].
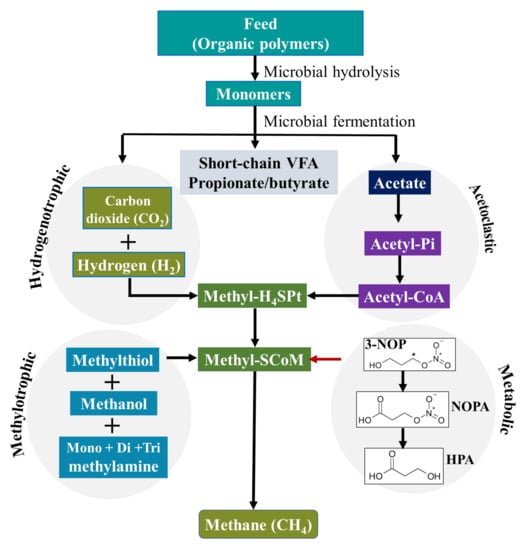
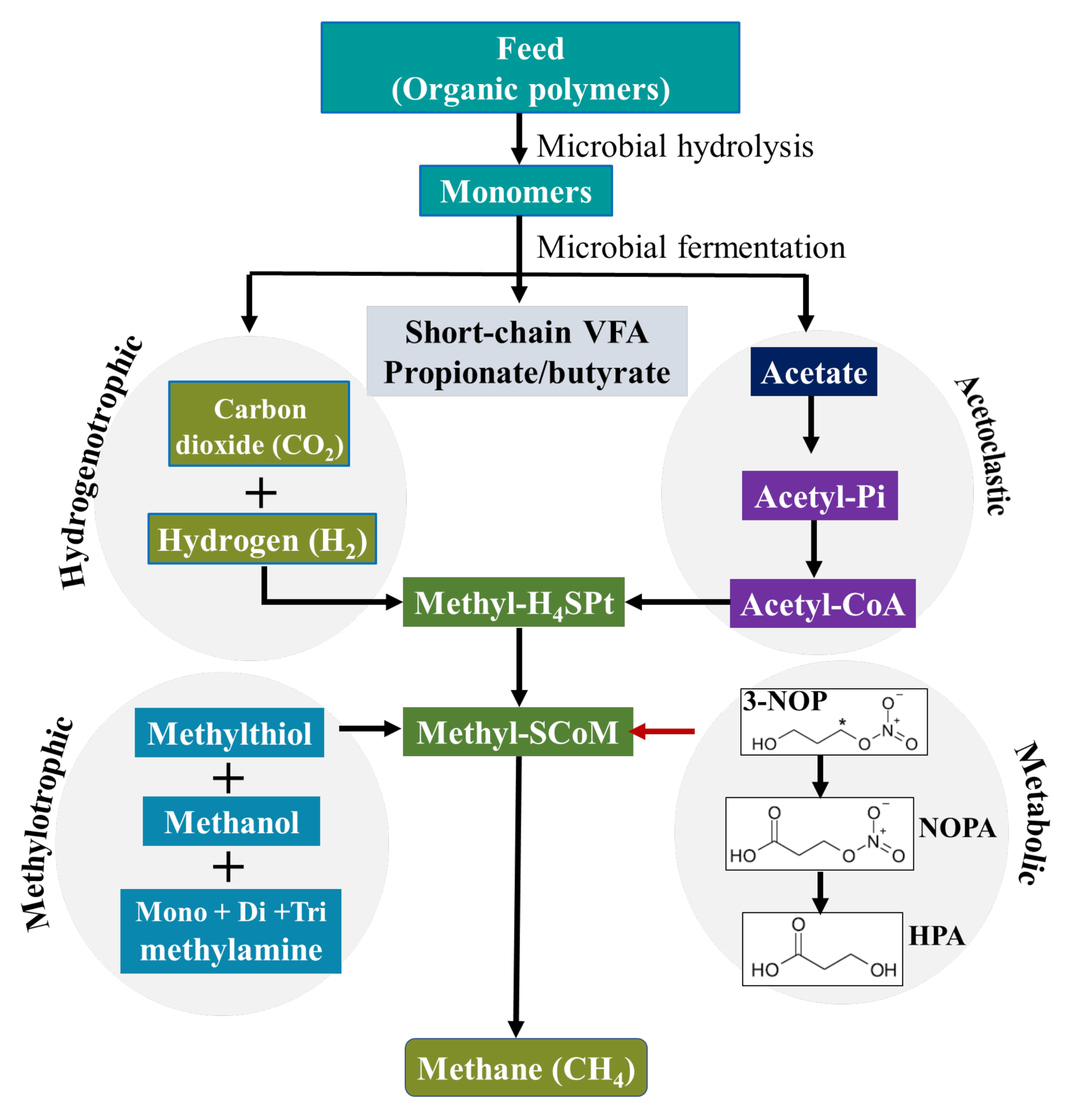
Figure 1.
The main CH4 formation pathway in the rumen of ruminants and its inhibition by 3-NOP [28,29]. (3-NOP = 3-nitrooxypropanol; NOPA = 3-nitrooxypropionic acid; HPA = 3-hydroxypropionic acid).
Due to its molecular structure, 3-NOP is highly soluble and rapidly metabolized in the rumen to very low concentrations of nitrate, nitrite and 1,3-propanediol. Duin et al. (2016) [15] reported that 3-NOP is hydrolyzed in rumen fluid to 1,3-propandiol, a compound of low toxicity, which is further transformed into 3-hydroxypropionic acid (HPA) [25]. Thiel et al. (2019) [24] demonstrated that 3-NOP is first oxidized to 3-nitrooxypropionic acid (NOPA), which is then hydrolyzed to HPA and inorganic nitrate. In ruminants, NOPA is a plasma metabolite and HPA is a compound of naturally occurring intermediary metabolism. HPA is further used by mammalian cells as substrate for synthesis of acetyl-CoA and propanoyl-CoA. The latter serves as substrate for gluconeogenesis and is beneficial for lactating ruminants because propanoyl-CoA is a prominent carbon source [24].
The molecular shape of 3-NOP is similar to that of methyl-coenzyme M, a co-factor involved in methyl transfer during methanogenesis. Duin et al. (2016) [15] showed that 3-NOP specifically binds into MCR and inactivates the enzyme by temporarily oxidizing the nickel ion from oxidation state (+1) to (+2) in the active site, leading to an inhibition of methanogenesis. MCR is a nickel enzyme in which the nickel is bound in a tetrapyrrole derivative named cofactor F430 [26]. This nickel-containing cofactor has to be in the Ni(I) oxidation state for the enzyme to be active to catalyze the CH4-forming step in rumen fermentation. The moderate oxidation potential of 3-NOP makes it inactivate MCR at micromolar concentrations. Duin et al. (2016) [15] showed that 3-NOP preferably targets the active site of MCR in a pose that places its reducible nitrate group in electron transfer distance to Ni(I). Thus, the inhibition of CH4 formation during the last step of the methanogenesis pathway in rumen methanogenic archaea is achieved (Figure 1).
Residues in milk and meat are minute or non-existent and the safety risks of 3-NOP are seemingly low [24,27]. It is reported that 3-NOP and its metabolites pose no mutagenic and genotoxic potential [27]. Although neither 14C-3-NOP nor 14C-NOPA were found in milk [24], further studies over a range of animals and diets are required to confirm the absence of 3-NOP residues in manure, meat or milk to address food safety concerns.
3. Effects on Rumen Fermentation and Methanogenesis
Plant material consumed by ruminants is degraded in the anaerobic environment of the rumen by bacteria, protozoa, and fungi predominantly yielding volatile fatty acids (VFA), CO2, NH3, and CH4 with hydrogen (H2) as intermediate [30]. The VFA (mainly acetate, propionate, butyrate) are metabolized and absorbed as the primary source of energy for ruminant animals, whereas CH4 is formed by methanogenic archaea from CO2 and H2. Hence, enteric CH4 is a by-product of the normal fermentation process of feed in the rumen and hindgut of ruminant livestock and it is the main H2 sink in the rumen. Methanogenesis is a pathway to generate energy for methanogenic archaea [16], whereby MCR, a unique enzyme found in archaea, catalyzes methyl-coenzyme M and coenzyme B to CH4 during the last step of methanogenesis [15].
There are various ways in which 3-NOP affects fermentation and methanogenesis. As a structural analogue of methyl coenzyme M, 3-NOP acts as a competitive inhibitor that selectively binds to and targets the active site of MCR [15], as discussed previously. As a result of inhibiting CH4 formation using 3-NOP, the fermentation pathways are shifted towards alternative H2 sinks such as propionic acid production [16,31]. Most studies consistently report increased propionate proportions at the expense of acetate proportions in rumen fluid with feeding of 3-NOP [14,32,33]. A recent meta-analysis [19] showed that increasing levels of 3-NOP supplementation in dairy diets linearly decreased proportion of acetate and increased that of valerate. In the same meta-analysis but using a beef cattle database, the total VFA concentration and the proportion of acetate were significantly decreased with increasing 3-NOP supplementation, whereas other individual VFA increased [19]. A change in the end-products of rumen fermentation when feeding 3-NOP can have important consequences for animal metabolism. Acetate is metabolized by peripheral tissues and other organs of the portal-drained viscera and completely oxidized to CO2 entering the Krebs cycle to supply energy or used for milk fatty acid synthesis in ruminants, with low proportion absorbed in the rumen epithelium for the formation of ketone bodies [34]. Propionate is metabolized by the liver, which may enter the Krebs cycle to be totally oxidized to CO2 or to produce lactate, pyruvate, and alanine and then entering the gluconeogenesis pathway to synthesize glucose or glycogen, and may also be used as a source of carbon skeleton for new cell synthesis [35]. Ruminal butyrate proportion also tends to increase with 3-NOP supplementation of diets, with butyrate absorbed through the rumen wall and mostly metabolized by rumen epithelial cells as an energy source or converted into β-hydroxybutyrate [36,37].
H2 is used as a substrate by methanogenic archaea to generate energy and this process is decreased in the presence of 3-NOP. Inhibiting methanogenesis can cause dissolved H2 to accumulate in the rumen, and if not totally incorporated into other H2 sinks (e.g., formate, propionate, valerate, caproate, ethanol, lactate, microbial protein and fatty acid synthesis), the H2 gas is expelled from the rumen [38,39] representing a loss of energy. Thus, gaseous H2 emissions can increase in animals receiving 3-NOP [14,36,38].
3-NOP has been shown to have limited effects on the growth characteristics of rumen protozoa and bacteria when tested in vivo and in vitro [37,40,41], but populations of methanogenic archaea were decreased [16]. 3-NOP has also been shown to inhibit abundance of hydrogenotrophic methanogens in some studies [42]. Abundances of methanogens (5.6-fold), Methanomassiliicoccaceae family (4-fold), and Methanobrevibacter (5.6-fold) in rumen pellet samples were decreased with 3-NOP addition compared with the control [43]. Pitta et al. (2021) [42] reported differential responses among methanogens in dairy cows receiving 60 mg 3-NOP/kg DM; Methanobrevibacter was reduced at week 4, Methanobrevibacter ruminantium was reduced from week 8, and Methanosphaera was reduced at weeks 8 and 12. Dosing 200 mg 3-NOP/DM to beef cattle significantly decreased abundances of Methanobrevibacter, Methanomicrobium, and Methanomethylophilus in both rumen fluid and digesta [44]. In addition, the effect of 3-NOP on methanogens depends upon the diet, as Zhang et al. (2020) reported 3-NOP decreased the abundance of Methanobrevibacter in cattle fed barley silage, but not when fed grass hay [45].
Most studies showed no effect of 3-NOP on ammonia N concentration, except when a high level of 3-NOP was used [16,37,46]. In the meta-analysis of Jayanegara et al. (2018) [16], addition of 3-NOP increased rumen pH (pH = 0.56 (±0.13) × 3-NOP (g/kg DMI) + 6.40 (±0.05) (R2 = 0.69, n = 14, p < 0.01)), although Haisan et al. (2017) [41] and Lopes et al. (2016) [46] reported no effects of 3-NOP on ruminal pH. An increase in rumen pH may be related to the observed increase in feeding frequency of animals consuming 3-NOP compared to control [47]. It may also be related to decreased DMI, decreased total VFA concentration and increased butyrate molar percentage and uptake from the rumen [48].
4. Mitigation of Enteric CH4 Using 3-Nitrooxypropanol
4.1. Method of Providing 3-Nitrooxypropanol to Animals
Use of 3-NOP for CH4 mitigation has been evaluated in animals in confinement, with no published research with grazing animals. Various methods of providing 3-NOP to ruminant livestock have been used: 3-NOP delivered directly into the rumen at feeding time [33], top dressed onto feed in a manger [40], mixed into a total mixed ration (TMR) [49], incorporated into a concentrate pellet [50], and added to the roughage component [50]. 3-NOP was shown to be effective when added to the TMR or a component of the ration, but the mitigation effect when dosing it into the rumen was transitory indicating the product may rapidly leave the rumen in the liquid outflow. Incorporating 3-NOP into a ration or a component of the ration (concentrate, forage), appears to lead to a more continuous presence in the rumen as animals consume their feed throughout the day [14]. Several studies have also shown that once 3-NOP is removed from the diet, its effect on CH4 is negated within several days [49,51].
4.2. Efficacy and Uncertanty
Inclusion of 3-NOP in ruminant diets decreases enteric CH4 emissions in a dose–response manner [16,17,19]. In the meta-analysis of Dijkstra et al. (2018) [17] from 11 studies, the average 3-NOP dose used in beef cattle was 144 mg/kg of DM, ranging from 50 to 345 mg/kg of DM; in dairy cattle, the average dose was 81 mg/kg of DM, ranging from 27 to 135 mg/kg of DM. An intermediate 3-NOP dose (111.2 mg/kg DM) was evaluated in a sheep study [52].
Several meta-analyses report that increasing dosage level of 3-NOP linearly decreased enteric CH4 emissions (Table 1). When enteric CH4, expressed relative to digested organic matter (DOM) or DMI, was regressed against dietary 3-NOP dose (mg/kg of DM), the R2 was relatively high [16]. In addition, Romero-Perez et al. (2014) [40] reported a linear effect of 3-NOP dose (47, 144 and 305 mg/kg DM) on total CH4 emissions (g/d) per animal. Vyas et al. (2016) [53] also reported a linear effect of 3-NOP dose between 100 and 200 mg/kg DM on CH4 yield (g/kg DMI, maximum decrease of 45%) in feedlot cattle. In mid- to late- lactation dairy cows, Hristov et al. (2015) [14] observed a linear effect of 3-NOP dose from 40 to 80 mg/kg DM on enteric CH4 emission (g/d). Melgar et al. (2020) evaluated 6 levels of inclusion of 3-NOP (40, 60, 80, 100, 150, and 200 mg/kg of feed DM) in dairy cows and observed a linear effect of 3-NOP dose (with maximum mitigation effect at 150 mg/kg but with no statistical difference among 100, 150, and 200 mg/kg). In contrast, no linear response to 3-NOP concentration was observed in beef cattle by Alemu et al. (2021) [38,54], for reasons that are not clear.

Table 1.
Linear relationships between enteric CH4 and dose of 3-NOP (g/kg DM [16], mg/kg DM [17,19]) in ruminant diets.
When examined across published studies, the efficacy of 3-NOP in decreasing CH4 emissions was greater in dairy cattle (R2 = 0.92) compared with beef cattle (R2 = 0.80) [17,19], when compared at the same dose. Based on the meta-analysis by Kim et al. (2020) [19], dosing 100 mg 3-NOP/kg DMI would be predicted to decrease enteric CH4 emissions in dairy cattle by 36.4% compared with 17.3% in beef cattle. This difference between cattle type is confounded by the types of diets and level of DMI in these studies. According to equations in the meta-analysis by Kim et al. (2020) [19], a dose of 60 to 80 mg 3-NOP/kg DMI for dairy cows and 150 to 200 mg 3-NOP/kg DMI for beef cattle would be expected to decrease enteric CH4 production by 30%. In a meta-analysis, Dijkstra et al. (2018) [17] showed that in addition to 3-NOP dose, type of animal and nutrient composition of the diet explained most of the variability in 3-NOP response. An increased neutral detergent fiber concentration of the diet was shown to negatively affect the anti-methanogenic effect of 3-NOP (10 g/kg DM increase in dietary neutral detergent fiber lowers the efficacy of 3-NOP to decrease CH4 production by 1.64 ± 0.33%) [17]. Therefore, in the same cattle type, the mitigation effect of 3-NOP has been greater in high concentrate diets [40,51,55] and less in high fiber diets [17,38]. For example, several studies using 3-NOP as a feed additive have reported very high reductions in CH4 emissions from feedlot cattle fed grain-based diets (82% in Vyas et al. (2016) [51] and 80% in McGinn et al. (2019) [56]). Other factors causing variability in response to 3-NOP may be related to method used to measure CH4 emissions (chambers, sulfur hexafluoride tracer technique, and Greenfeed system), duration that cattle were fed 3-NOP (short- vs. long-term), and interaction effects when 3-NOP was combined with other mitigation strategies (e.g., monensin [55], unsaturated fatty acids [57], higher concentrate proportion [37], and others; Table 2 and Table 3).

Table 2.
Summary of 3-nitrooxypropanol (3-NOP) effects on in vivo fermentation, digestibility, microbes and enteric CH4 production in ruminants.

Table 3.
Summary of 3-nitrooxypropanol (3-NOP) effects on in vitro fermentation, digestibility, microbes and enteric CH4 production in ruminants.
4.3. Effectiveness of 3-Nitrooxypropanol in Long-Term Studies
In 5 long-term experiments (defined as 10-week [50], 12-week [14], 15-week [55], 16-week [49], and 34-week [51] feeding periods), CH4 yield (g/kg DM) was significantly linearly (R2 = 0.91, n = 19, p < 0.01) decreased with increasing level of 3-NOP addition [19]. Thus, it appears that overall, the responses in long-term studies have been generally similar to those observed in short-term studies. Hristov et al. (2015) [14] reported 30% less CH4 (g/d) on average for lactating dairy cows fed 40 to 80 mg 3-NOP/kg DMI over 12 weeks. In 10- [50] and 15-week [60] experiments with dairy cattle, CH4 (g/d) decreased on average over the study by 26 to 28% with 3-NOP (40 to 80 mg/kg feed DMI), and this effect did not diminish over time. Romero-Perez et al. (2015) [49] reported adding 3-NOP to a beef cattle diet for 16 weeks resulted in a sustained reduction in enteric CH4 emissions (59%; 9.16 vs. 22.46 g/kg DMI), with no decline in response when measurements were repeated over time. In a 34-week feeding study by Vyas et al. (2016) [51], supplementation of 3-NOP at 200 mg/kg DM decreased, on average, emission of enteric CH4 (g/d) by 82% in feedlot finishing beef cattle with the effect negated within days once 3-NOP supplementation was discontinued. However, in a beef cattle feedlot study by McGinn et al. (2019) [56], there was a small, constant decline in CH4 emission reduction (from 80% to 60% reduction over 90 d using micrometeorological methods), indicating a possible adaptation of the rumen microbiome. Similarly, in a dairy cattle study by Melgar et al. (2020) [36], the CH4 mitigation effect of 3-NOP decreased over 15 weeks. Alemu et al. (2021) [54] reported a 22% reduction in efficacy of 3-NOP to decrease CH4 yield (g/kg DM) in beef cattle when a low dose (100 mg/kg DMI) was fed for 16 weeks, but no reduction in efficacy occurred over time when higher doses were used (125 to 150 mg /kg DMI). Nonetheless, other studies have shown no decline in the effectiveness of 3-NOP over time [14,38,49]. It is evident that further research is needed to determine whether the response to 3-NOP is maintained over the long-term. Studies with repeated measurements over the feeding period and over multiple years for mature beef cows and over multiple lactations for dairy cows are needed to ensure the mitigation effect of 3-NOP is persistant. This is an important aspect given that the potential for adaptation of the rumen microbiome such that compounds diminish in effectiveness has been shown with other rumen modifying compounds (e.g., ionophores [69], essential oils [70], etc.).
5. Effects of 3-Nitrooxypropanol on Dry Matter Intake, Digestibility and Animal Productivity
5.1. Effects of 3-Nitrooxypropanol on Dry Matter Intake and Digestibility
The effects of 3-NOP on DMI appear to be different among studies and may depend on dose, animal type, diet and the duration of feeding [54]. In the meta-analysis of Kim et al. (2020) [19] from 14 beef cattle studies, DMI tended to decrease (slope = −0.0016, p = 0.06, and R2 = 0.17) as the dose of 3-NOP supplemented increased. However, using a dairy cattle database, Kim et al. (2020) [19] reported that 3-NOP supplementation had no significant linear relationship with DMI. Using a combined beef and dairy cattle database, Kim et al. (2020) [19] reported that increasing the dose of 3-NOP supplementation linearly decreased DMI (slope = −0.0017, R2 = 0.17, p < 0.05). However, in the meta-analysis of Jayanegara et al. (2018) [16] from 12 studies, DMI from ruminants (dairy cows, beef cattle, and sheep) was not linearly decreased with increasing level of 3-NOP addition.
Inconsistent effects of 3-NOP on DMI between beef and dairy cattle studies may be due to the higher doses of 3-NOP used in most beef studies. For example, in dairy cattle studies that used a dose of 40 to 80 mg 3-NOP/kg DM, DMI was not affected [14,31,46]. However, in a beef cattle study with doses of 47, 144 and 305 mg/kg DM, a linear decrease in DMI was reported [40]. Additionally using relatively high doses of 3-NOP (200 mg/kg DM) in beef cattle fed backgrounding diets, Vyas et al. (2016) [51] and Vyas et al. (2018) [55] reported 8% and 7% reductions in DMI, respectively, compared with control animals. Alemu et al. (2021) [54] reported an initial reduction in DMI (kg/d) of 6.1% and 6.4% for feedlot finishing diets fed 100 and 150 mg 3-NOP/kg DM, respectively, but after 56 days of consuming 3-NOP, there was no difference in DMI between treatment and control cattle. This trend may indicate an adaptive response of the cattle over time. The decrease in DMI with the higher doses of 3-NOP typically fed in beef studies might be due in part to palatability effects [54]. In addition, the high starch concentration of beef cattle finishing diets results in a rumen fermentation with greater molar proportion of propionate, compared with dairy cattle. A further increase in molar proportion of propionate with feeding of 3-NOP may augment the hyperphagic effect of absorbed propionate causing DMI to decline [71]. Other factors may be related to chemical composition and particle size of the diet, and silage fermentation products [71].
In earlier work, it was assumed that inhibiting methanogenesis would decrease diet digestibility. Methanogenesis is the main route of cofactor re-oxidation in the rumen and when inhibiting methanogenesis, elevated H2 concentration can hinder cofactor re-oxidation and thus inhibit fermentation [72]. Reduced co-factors need to be re-oxidized in the rumen for fermentation to continue. However, studies have shown no adverse effects of 3-NOP on diet digestibility in beef cattle [40], early-lactation dairy cows [31], or in specific breeds of cattle (Friesian Holstein, Angus and Segurena breeds) [16]. Additionally, a relatively small increase in apparent total-tract digestibility of several nutrients upon feeding 3-NOP was reported in some studies. These include DM [14,31,41], organic matter [31], crude protein [14,36,57], neutral detergent fiber [31,41], acid detergent fiber [14], gross energy [31] and starch [57]; however, these small improvements in digestibility are not likely to affect animal performance.
5.2. Effects of 3-Nitrooxypropanol on Animal Productivity
Improvements in animal performance when supplementing diets with CH4 would help incentivize producers to adopt such a technology [14,19]. Theoretically, a decrease in CH4 production could provide more metabolizable energy intake for productive purposes, such as milk production or growth if DMI is not proportionally decreased, and the shift in ruminal fermentation end-products are in a form that could be used as energy substrates [73]. In the meta-analysis of Jayanegara et al. (2018) [16], increasing the level of 3-NOP in beef cattle diets significantly improved gain to feed ratio (slope = 0.05, p < 0.01, and R2 = 0.94) and did not show any adverse effects on average daily gain. Using a dairy cattle database, addition of 3-NOP increased milk fat concentration (slope = 1.5, p < 0.05, and R2 = 0.47) and tended to increase milk protein concentration, whereas other lactation performance characteristics were not affected by addition of 3-NOP [16]. In the meta-analysis of Kim et al. (2020) [19], 3-NOP supplementation of dairy diets tended to increase milk fat and milk protein and decrease milk yield, but 3-NOP had no effect on fat corrected milk or milk lactose percentage. Ungerfeld (2018) [18] also reported no relationship between inhibiting methanogenesis and DMI-adjusted energy corrected milk production.
When examining individual beef cattle studies, supplementing 3-NOP to finishing cattle improved gain-to-feed ratio by 3% [55], with no adverse effects on weight gain [61]. In dairy cattle studies, feeding 3-NOP increased milk protein (g/100 g of milk) [31,33] and milk fat content (g/100 g of milk) by up to 8% [31,46]. Improvements is milk quality (milk fat and milk protein) may have resulted from a slight increase in net energy intake for lactation due to the decrease in feed energy lost as CH4 or the shift in fermentation end-products towards increased propionate synthesis. Schilde et al. (2021) [37] reported an energy corrected milk yield (kg/d) reduction of 8.8% with inclusion of 3-NOP (60 mg 3-NOP/kg DM) in high concentrate feed as compared with the control without 3-NOP in cows from parturition until d 120 postpartum. Many studies indicate that feeding 3-NOP to dairy cows did not affect milk yield [14,31,32,33,41,50,60], although there is a lack of large-scale long-term studies.
6. Practical Considerations for Use on Farm
While the extensive body of published literature under controlled research conditions indicates that 3-NOP consistently decreases CH4 production from ruminant livestock by on average 30%, it is important to state that many of these studies are short-term and even the long-term studies have been limited to several months in duration. No published study has examined the effects of feeding 3-NOP over multiple lactations or seasons, which is important information for farmers. 3-NOP has been used in commercial conditions [38,54], but further information on using 3-NOP under a broad range of feeding systems is still needed. Furthermore, the use of 3-NOP on-farm as a feed additive requires regulatory approval, which has been granted thus far in Brazil and Chile.
When using a CH4 mitigation strategy it is important to ensure that emissions elsewhere in the supply chain are not inadvertently increased. Thus, the impact of using 3-NOP for enteric CH4 mitigation on other emissions, such as manure CH4 emissions also need to be considered. Nkemka et al. (2019) [74] showed no residual effects of feeding 3-NOP to beef cattle on manure CH4 emissions when the manure was used in an anaerobic digester. Owens et al. (2020) [75] showed no residual effects of feeding 3-NOP to beef cattle on greenhouse gas (CH4, CO2 and nitrous oxide) emissions from manure decomposition during storage. However, in a laboratory scale study using soils amended with manure from cattle fed 3-NOP, Weber et al. (2021) [76] showed that GHG emissions were dependent on soil texture. For coarse-textured soil (Black Chernozemic), GHG emissions were greater when amended with manure from cattle fed 3-NOP compared with control manure (mainly due to increased nitrous oxide emissions), but this effect was not observed for other soil types or when the manure was first composted. The possible carryover effects of feeding 3-NOP on manure CH4 emissions needs further study.
In addition, the emissions from producing 3-NOP need to be included when evaluating the net impact on total greenhouse gases, even though CO2 emissions from manufacturing 3-NOP are very small in comparison to the decrease in CH4 production. The emission factor for 3-NOP was reported as 47.9 kg CO2e/kg 3-NOP in the study by Alvarez-Hess et al. (2019) [77] and 52 kg CO2e/kg 3-NOP in the study by Kebreab and Feng (2021) [78]. Thus, dosing 60 mg 3-NOP/kg DMI to dairy cattle and 150 mg 3-NOP/kg feed DMI to beef cattle, respectively would represent approximately 3 g CO2e/kg feed DM (equivalent to 0.1 g CH4) in dairy cattle compared with 8 g CO2e/kg feed DM (equivalent to 0.3 g CH4) in beef cattle [78].
At present, use of 3-NOP is limited to confinement non-organic systems using formulated diets, as it needs to be fed as part of the ration. Globally, it is estimated that 37% of enteric CH4 emissions from ruminant livestock production is pasture-based [79], and thus a significant proportion of ruminant farming is currently excluded from the potential for mitigation using 3-NOP in its present form. However, research is ongoing to extend its application under grazing conditions [20]. This may include adding 3-NOP to pasture supplements, use of lick blocks, encapsulation, slow-release ruminal devices, and so forth. At present, little is known of the effectiveness of 3-NOP for grazing ruminants. Another method of using 3-NOP has been to administer it to neonatal animals, a concept referred to as early life programming. The central idea is that the developing microbial community of the newborn ruminant is more malleable than that of the adult ruminant and that its manipulation could have long-lasting effects. In a study by Meale et al. [59], 3-NOP was dosed daily (3 mg 3-NOP/kg BW) to calves from birth until 14 weeks (3 weeks after weaning), and a 12% reduction in CH4 emissions (g/d) was observed for 9 weeks after 3-NOP dosing was discontinued. Furthermore, a reduction in CH4 was noted when measured almost a year later. While early life intervention to decrease CH4 emissions is still at an early stage, it remains a possibility for future application and may have potential especially for grazing ruminants where delivery mechanisms for 3-NOP are limited.
7. Conclusions
In conclusion, there is overwhelming scientific evidence that incorporation of 3-NOP in the diets of ruminant livestock inhibits enteric CH4 emissions in a dose-dependent manner without negative effects on animal production. Safety risks for animals and humans appear to be minimal. Overall effects on animal productivity are small, albeit positive, with improvements in milk quality (milk fat and milk protein) in some dairy cattle studies and feed conversion efficiency in some beef cattle studies. Multi-year published studies are needed to determine the long-term impacts of using 3-NOP for CH4 mitigation and further research is required to explore practical use of 3-NOP for grazing animals. If approved by regulatory authorities, use of 3-NOP in ruminant diets represents a significant advance in terms of offering livestock producers a practical means of lowering CH4 emissions.
Author Contributions
R.D. and G.Y. contributed equally to the writing of this article. Conceptualization—R.D. and G.Y.; Writing—original draft preparation, R.D., G.Y. and K.A.B.; Writing—review and editing, K.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Agriculture Well Breeds Engineering Major Projects of Shandong Province (Grant No. 2020LZGC014) and High-level Personnel Scientific Research Funds of Qingdao Agricultural University (Grant No. 6631118032).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Edenhofer, O. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, MA, USA, 2015; Volume 3. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Amim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.; Vellinga, T.; Opio, C.; Steinfeld, H. Productivity gains and greenhouse gas emissions intensity in dairy systems. Livest. Sci. 2011, 139, 100–108. [Google Scholar] [CrossRef]
- Reisinger, A.; Clark, H.; Cowie, A.L.; Emmet-Booth, J.; Gonzalez Fischer, C.; Herrero, M.; Howden, M.; Leahy, S. How necessary and feasible are reductions of methane emissions from livestock to support stringent temperature goals? Phil. Trans. R. 2021, A379, 20200452. [Google Scholar] [CrossRef] [PubMed]
- Rogelj, J.; Shindell, D.; Jiang, K.; Fifita, S.; Forster, P.; Ginzburg, V.; Handa, C.; Kheshgi, H.; Kobayashi, S.; Kriegler, E. Mitigation pathways compatible with 1.5 °C in the context of sustainable development. In Global Warming of 1.5 °C; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018; pp. 93–174. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019. [Google Scholar]
- Monaco, A.; Ross, K.; Waskow, D.; Ge, M. How Methane Emissions Contribute to Climate Change. 2021. Available online: https://www.wri.org/insights/methane-gas-emissions-climate-change (accessed on 20 November 2021).
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group14 I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; Arias, P., Bellouin, N., Coppola, E., Jones, R., Krinner, G., Marotzke, J., Naik, V., Palmer, M., Plattner, G.-K., Rogelj, J., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Beauchemin, K.A.; Ungerfeld, E.; Eckard, R.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, s2–s16. [Google Scholar] [CrossRef] [Green Version]
- Breider, I.S.; Wall, E.; Garnsworthy, P.C. Short communication: Heritability of methane production and genetic correlations with milk yield and body weight in Holstein-Friesian dairy cows. J. Dairy Sci. 2019, 102, 7277–7281. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Xue, B.; Peng, Q.; Wang, Z.; Yan, T.; Wang, L. Immunization against rumen methanogenesis by vaccination with a new recombinant protein. PLoS ONE 2015, 10, e0140086. [Google Scholar] [CrossRef] [Green Version]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; de Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 2021, 16, e0247820. [Google Scholar]
- Henderson, G.; Cook, G.; Ronimus, R. Enzyme- and gene-based approaches for developing methanogen-specific compounds to control ruminant methane emissions: A review. Anim. Prod. Sci. 2016, 58, 1017–1026. [Google Scholar] [CrossRef]
- Hristov, A.; Oh, J.; Giallongo, F.; Frederick, T.; Harper, M.; Weeks, H.; Branco, A.; Moate, P.; Deighton, M.; Williams, S.; et al. An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc. Natl. Acad. Sci. USA 2015, 112, 10663–10668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duin, E.; Wagner, T.; Shima, S.; Prakash, D.; Cronin, B.; Yáñez-Ruiz, D.; Duval, S.; Rümbeli, R.; Stemmler, R.; Thauer, R.; et al. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc. Natl. Acad. Sci. USA 2016, 113, 6172–6177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayanegara, A.; Sarwono, K.A.; Kondo, M.; Matsui, H.; Ridla, M.; Laconi, E.B.; Nahrowi. Use of 3-nitrooxypropanol as feed additive for mitigating enteric methane emissions from ruminants: A meta-analysis. Ital. J. Anim. Sci. 2018, 17, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, J.; Bannink, A.; France, J.; Kebreab, E.; van Gastelen, S. Short communication: Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J. Dairy Sci. 2018, 101, 9041–9047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungerfeld, E.M. Inhibition of rumen methanogenesis and ruminant productivity: A meta-analysis. Front. Vet. Sci. 2018, 5, 113. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Baek, Y.; Lee, S.; Seo, J. The effects of dietary supplementation with 3-nitrooxypropanol on enteric methane emissions, rumen fermentation, and production performance in ruminants: A meta-analysis. J. Anim. Sci. Technol. 2020, 62, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Arndt, C.; Hristov, A.; Price, W.; McClelland, S.; Pelaez, A.; Cueva, S.; Oh, J.; Bannink, A.; Bayat, A.; Crompton, L. Strategies to Mitigate Enteric Methane Emissions by Ruminants-a Way to Approach the 2.0 °C Target; preprint 20210085288; CABI: Wallingford, UK, 2021. [Google Scholar] [CrossRef]
- Almeida, A.K.; Hegarty, R.S.; Cowie, A. Meta-analysis quantifying the potential of dietary additives and rumen modifiers for methane mitigation in ruminant production systems. Anim. Nutr. 2021, 7, 1219–1230. [Google Scholar] [CrossRef]
- Ogawa, T.; Nakazato, A.; Sato, M.; Hatayama, K. Synthesis of 2-and 3-nitrooxypropanol by chemoselective reduction of methyl 2-and 3-nitrooxypropionoate. Synthesis 1990, 1990, 459–460. [Google Scholar] [CrossRef]
- Duval, S.; Kindermann, M. Use of Nitrooxy Organic Molecules in Feed for Reducing Enteric Methane Emissions in Ruminants, and/or to Improve Ruminant Performance. International Patent WO2012084629A1, 28 April 2012. [Google Scholar]
- Thiel, A.; Rümbeli, R.; Mair, P.; Yeman, H.; Beilstein, P. 3-NOP: ADME studies in rats and ruminating animals. Food Chem. Toxicol. 2019, 125, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Gingell, R.; Kirkpatrick, J.B.; Steup, D.R. Subchronic toxicity study of 1, 3-propanediol administered orally to rats. Int. J. Toxicol. 2000, 19, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Thiel, A.; Schoenmakers, A.; Verbaan, I.; Chenal, E.; Etheve, S.; Beilstein, P. 3-NOP: Mutagenicity and genotoxicity assessment. Food Chem. Toxicol. 2019, 123, 566–573. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Waldrip, H.M.; Parker, D.; Todd, R.W.; Brauer, D. Dietary mitigation of enteric methane emissions from ruminants: A review of plant tannin mitigation options. Anim. Nutr. 2020, 6, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Honan, M.; Feng, X.; Tricarico, J.M.; Kebreab, E. Feed additives as a strategic approach to reduce enteric methane production in cattle: Modes of action, effectiveness and safety. Anim. Prod. Sci. 2021, 1, 15. [Google Scholar] [CrossRef]
- Wolin, M.J. Fermentation in the rumen and human large intestine. Science 1981, 213, 1463–1468. [Google Scholar] [CrossRef]
- van Gastelen, S.; Dijkstra, J.; Binnendijk, G.; Duval, S.; Heck, J.; Kindermann, M.; Zandstra, T.; Bannink, A. 3-Nitrooxypropanol decreases methane emissions and increases hydrogen emissions of early lactation dairy cows, with associated changes in nutrient digestibility and energy metabolism. J. Dairy Sci. 2020, 103, 8074–8093. [Google Scholar] [CrossRef] [PubMed]
- Haisan, J.; Sun, Y.; Guan, L.; Beauchemin, K.; Iwaasa, A.; Duval, S.; Barreda, D.; Oba, M. The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation. J. Dairy Sci. 2014, 97, 3110–3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, C.K.; Humphries, D.J.; Kirton, P.; Kindermann, M.; Duval, S.; Steinberg, W. Effects of 3-nitrooxypropanol on methane emission, digestion, and energy and nitrogen balance of lactating dairy cows. J. Dairy Sci. 2014, 97, 3777–3789. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rémond, D.; Ortigues, I.; Jouany, J.-P. Energy substrates for the rumen epithelium. Proc. Nutr. Soc. 2007, 54, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Melgar, A.; Harper, M.T.; Oh, J.; Giallongo, F.; Young, M.E.; Ott, T.L.; Duval, S.; Hristov, A.N. Effects of 3-nitrooxypropanol on rumen fermentation, lactational performance, and resumption of ovarian cyclicity in dairy cows. J. Dairy Sci. 2020, 103, 410–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilde, M.; von Soosten, D.; Hüther, L.; Meyer, U.; Zeyner, A.; Dänicke, S. Effects of 3-nitrooxypropanol and varying concentrate feed proportions in the ration on methane emission, rumen fermentation and performance of periparturient dairy cows. Arch. Anim. Nutr. 2021, 75, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Alemu, A.W.; Pekrul, L.K.; Shreck, A.L.; Booker, C.W.; McGinn, S.M.; Kindermann, M.; Beauchemin, K.A. 3-Nitrooxypropanol decreased enteric methane production from growing beef cattle in a commercial feedlot: Implications for sustainable beef cattle production. Front. Anim. Sci. 2021, 2, 641590. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. A theoretical comparison between two ruminal electron sinks. Front. Microbiol. 2013, 4, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Perez, A.; Okine, E.; McGinn, S.; Guan, L.; Oba, M.; Duval, S.; Kindermann, M.; Beauchemin, K. The potential of 3-nitrooxypropanol to lower enteric methane emissions from beef cattle. J. Amim. Sci. 2014, 92, 4682–4693. [Google Scholar]
- Haisan, J.; Sun, Y.; Guan, L.; Beauchemin, K.A.; Iwaasa, A.; Duval, S.; Kindermann, M.; Barreda, D.R.; Oba, M. The effects of feeding 3-nitrooxypropanol at two doses on milk production, rumen fermentation, plasma metabolites, nutrient digestibility, and methane emissions in lactating Holstein cows. Anim. Prod. Sci. 2017, 57, 282–289. [Google Scholar] [CrossRef]
- Pitta, D.; Melgar, A.; Hristov, A.; Indugu, N.; Narayan, K.; Pappalardo, C.; Hennessy, M.; Vecchiarelli, B.; Kaplan-Shabtai, V.; Kindermann, M.; et al. Temporal changes in total and metabolically active ruminal methanogens in dairy cows supplemented with 3-nitrooxypropanol. J. Dairy Sci. 2021, 104, 8721–8735. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, G.; Duval, S.; Kindermann, M.; Schirra, H.J.; Denman, S.E.; McSweeney, C.S. 3-NOP vs. halogenated compound: Methane production, ruminal fermentation and microbial community response in forage fed cattle. Front. Microbiol. 2018, 9, 1582. [Google Scholar] [CrossRef] [PubMed]
- Gruninger, R.J.; Zhang, X.M.; Smith, M.L.; Kung, L.; Vyas, D.; McGinn, S.M.; Kindermann, M.; Wang, M.; Tan, Z.L.; Beauchemin, K.A. Application of 3-nitrooxypropanol and canola oil to mitigate enteric methane emissions of beef cattle results in distinctly different effects on the rumen microbial community. Res. Sq. 2021, 1, 1–33. [Google Scholar] [CrossRef]
- Zhang, X.M.; Gruninger, R.J.; Alemu, A.W.; Wang, M.; Tan, Z.L.; Kindermann, M.; Beauchemin, K.A. 3-Nitrooxypropanol supplementation had little effect on fiber degradation and microbial colonization of forage particles when evaluated using the in situ ruminal incubation technique. J. Dairy Sci. 2020, 103, 8986–8997. [Google Scholar] [CrossRef]
- Lopes, J.; de Matos, L.; Harper, M.; Giallongo, F.; Oh, J.; Gruen, D.; Ono, S.; Kindermann, M.; Duval, S.; Hristov, A. Effect of 3-nitrooxypropanol on methane and hydrogen emissions, methane isotopic signature, and ruminal fermentation in dairy cows. J. Dairy Sci. 2016, 99, 5335–5344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, N.; Kennelly, J. Effects of feeding frequency on ruminal parameters, plasma insulin, milk yield, and milk composition in Holstein cows. J. Dairy Sci. 1990, 73, 1857–1863. [Google Scholar] [CrossRef]
- Penner, G.B.; Aschenbach, J.R.; Gäbel, G.; Rackwitz, R.; Oba, M. Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal pH and the susceptibility to subacute ruminal acidosis in sheep. J. Nutr. 2009, 139, 1714–1720. [Google Scholar] [CrossRef] [Green Version]
- Romero-Perez, A.; Okine, E.; McGinn, S.; Guan, L.; Oba, M.; Duval, S.; Kindermann, M.; Beauchemin, K. Sustained reduction in methane production from long-term addition of 3-nitrooxypropanol to a beef cattle diet. J. Amim. Sci. 2015, 93, 1780–1791. [Google Scholar]
- Van Wesemael, D.; Vandaele, L.; Ampe, B.; Cattrysse, H.; Duval, S.; Kindermann, M.; Fievez, V.; De Campeneere, S.; Peiren, N. Reducing enteric methane emissions from dairy cattle: Two ways to supplement 3-nitrooxypropanol. J. Dairy Sci. 2019, 102, 1780–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, D.; McGinn, S.; Duval, S.; Kindermann, M.; Beauchemin, K. Effects of sustained reduction of enteric methane emissions with dietary supplementation of 3-nitrooxypropanol on growth performance of growing and finishing beef cattle. J. Amim. Sci. 2016, 94, 2024–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Fernández, G.; Abecia, L.; Arco, A.; Cantalapiedra-Hijar, G.; Martín-García, A.; Molina-Alcaide, E.; Kindermann, M.; Duval, S.; Yáñez-Ruiz, D. Effects of ethyl-3-nitrooxy propionate and 3-nitrooxypropanol on ruminal fermentation, microbial abundance, and methane emissions in sheep. J. Dairy Sci. 2014, 97, 3790–3799. [Google Scholar] [CrossRef] [Green Version]
- Vyas, D.; McGinn, S.; Duval, S.; Kindermann, M.; Beauchemin, K. Optimal dose of 3-nitrooxypropanol for decreasing enteric methane emissions from beef cattle fed high-forage and high-grain diets. Anim. Prod. Sci. 2016, 58, 1049–1055. [Google Scholar] [CrossRef]
- Alemu, A.; Shreck, A.; Booker, C.; McGinn, S.; Pekrul, L.; Kindermann, M.; Beauchemin, K. Use of 3-nitrooxypropanol in a commercial feedlot to decrease enteric methane emissions from cattle fed a corn-based finishing diet. J. Amim. Sci. 2021, 99, skaa394. [Google Scholar] [CrossRef]
- Vyas, D.; Alemu, A.; McGinn, S.; Duval, S.; Kindermann, M.; Beauchemin, K. The combined effects of supplementing monensin and 3-nitrooxypropanol on methane emissions, growth rate, and feed conversion efficiency in beef cattle fed high-forage and high-grain diets. J. Amim. Sci. 2018, 96, 2923–2938. [Google Scholar] [CrossRef] [PubMed]
- McGinn, S.; Flesch, T.; Beauchemin, K.; Shreck, A.; Kindermann, M. Micrometeorological methods for measuring methane emission reduction at beef cattle feedlots: Evaluation of 3-Nitrooxypropanol feed additive. J. Environ. Qual. 2019, 48, 1454–1461. [Google Scholar] [CrossRef]
- Zhang, X.; Smith, M.; Gruninger, R.; Kung, L.; Vyas, D.; McGinn, S.; Kindermann, M.; Wang, M.; Tan, Z.; Beauchemin, K. Combined effects of 3-nitrooxypropanol and canola oil supplementation on methane emissions, rumen fermentation and biohydrogenation, and total tract digestibility in beef cattle. J. Amim. Sci. 2021, 99, skab081. [Google Scholar] [CrossRef] [PubMed]
- Melgar, A.; Welter, K.; Nedelkov, K.; Martins, C.; Harper, M.; Oh, J.; Räisänen, S.; Chen, X.; Cueva, S.; Duval, S.; et al. Dose-response effect of 3-nitrooxypropanol on enteric methane emissions in dairy cows. J. Dairy Sci. 2020, 103, 6145–6156. [Google Scholar] [CrossRef]
- Meale, S.; Popova, M.; Saro, C.; Martin, C.; Bernard, A.; Lagree, M.; Yáñez-Ruiz, D.; Boudra, H.; Duval, S.; Morgavi, D. Early life dietary intervention in dairy calves results in a long-term reduction in methane emissions. Sci. Rep. 2021, 11, 3003. [Google Scholar] [CrossRef] [PubMed]
- Melgar, A.; Lage, C.; Nedelkov, K.; Räisänen, S.; Stefenoni, H.; Fetter, M.; Chen, X.; Oh, J.; Duval, S.; Kindermann, M.; et al. Enteric methane emission, milk production, and composition of dairy cows fed 3-nitrooxypropanol. J. Dairy Sci. 2021, 104, 357–366. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, C.; Pechtl, H.A.; Hettick, J.M.; Campler, M.R.; Pairis-Garcia, M.D.; Beauchemin, K.A.; Celi, P.; Duval, S.M. Effects of 3-nitrooxypropanol on enteric methane production, rumen fermentation, and feeding behavior in beef cattle fed a high-forage or high-grain diet. J. Amim. Sci. 2019, 97, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Samsonstuen, S.; Åby, B.A.; Crosson, P.; Beauchemin, K.A.; Aass, L. Mitigation of greenhouse gas emissions from beef cattle production systems. Acta Agric. Scand. Sect. A Anim. Sci. 2020, 69, 220–232. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; Okine, E.K.; Guan, L.L.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. Effects of 3-nitrooxypropanol on methane production using the rumen simulation technique (Rusitec). Anim. Feed Sci. Technol. 2015, 209, 98–109. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; Okine, E.K.; Guan, L.L.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. Effects of 3-nitrooxypropanol and monensin on methane production using a forage-based diet in Rusitec fermenters. Anim. Feed Sci. Technol. 2016, 220, 67–72. [Google Scholar] [CrossRef]
- Guyader, J.; Ungerfeld, E.M.; Beauchemin, K.A. Redirection of metabolic hydrogen by inhibiting methanogenesis in the rumen simulation technique (RUSITEC). Front. Microbiol. 2017, 8, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Pérez, A.; Okine, E.K.; Guan, L.L.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. Rapid Communication: Evaluation of methane inhibitor 3-nitrooxypropanol and monensin in a high-grain diet using the rumen simulation technique (Rusitec). J. Amim. Sci. 2017, 95, 4072–4077. [Google Scholar]
- Alvarez-Hess, P.S.; Moate, P.J.; Williams, S.R.O.; Jacobs, J.L.; Beauchemin, K.A.; Hannah, M.C.; Durmic, Z.; Eckard, R.J. Effect of combining wheat grain with nitrate, fat or 3-nitrooxypropanol on in vitro methane production. Anim. Feed Sci. Technol. 2019, 256, 114237. [Google Scholar] [CrossRef]
- Schilde, M.; von Soosten, D.; Hüther, L.; Kersten, S.; Meyer, U.; Zeyner, A.; Dänicke, S. Dose–response effects of 3-nitrooxypropanol combined with low-and high-concentrate feed proportions in the dairy cow ration on fermentation parameters in a rumen simulation technique. Animals 2021, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Wittenberg, K.M.; Ominski, K.H.; Krause, D.O. Efficacy of ionophores in cattle diets for mitigation of enteric methane1. J. Amim. Sci. 2006, 84, 1896–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klop, G.; Dijkstra, J.; Dieho, K.; Hendriks, W.H.; Bannink, A. Enteric methane production in lactating dairy cows with continuous feeding of essential oils or rotational feeding of essential oils and lauric acid. J. Dairy Sci. 2017, 100, 3563–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, M.S. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J. Dairy Sci. 2000, 83, 1598–1624. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Metabolic hydrogen flows in rumen fermentation: Principles and possibilities of interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaxter, K.L.; Czerkawski, J. Modification of the methane production of the sheep by supplementation of ITS diet. J. Sci. Food Agric. 1966, 17, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Nkemka, V.N.; Beauchemin, K.A.; Hao, X. Treatment of feces from beef cattle fed the enteric methane inhibitor 3-nitrooxypropanol. Water Sci. Technol. 2019, 80, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.; Thomas, B.; Stoeckli, J.; Beauchemin, K.; McAllister, T.; Larney, F.; Hao, X. Greenhouse gas and ammonia emissions from stored manure from beef cattle supplemented 3-nitrooxypropanol and monensin to reduce enteric methane emissions. Sci. Rep. 2020, 10, 19310. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.L.; Hao, X.; Gross, C.D.; Beauchemin, K.A.; Chang, S.X. Effect of manure from cattle fed 3-Nitrooxypropanol on anthropogenic greenhouse gas emissions depends on soil type. Agronomy 2021, 11, 371. [Google Scholar] [CrossRef]
- Alvarez-Hess, P.S.; Little, S.M.; Moate, P.J.; Jacobs, J.L.; Beauchemin, K.A.; Eckard, R.J. A partial life cycle assessment of the greenhouse gas mitigation potential of feeding 3-nitrooxypropanol and nitrate to cattle. Agric. Syst. 2019, 169, 14–23. [Google Scholar] [CrossRef]
- Kebreab, E.; Feng, X. Strategies to Reduce Methane Emissions from Enteric and Lagoon Sources; Contract 17RD018; Prepared for State of California Air Resources Board Research Division: Sacramento, CA, USA, 2021; p. 57. [Google Scholar]
- FAO. Global Livestock Environmental Assessment Model (GLEAM) [online]. 2017. Available online: www.fao.org/gleam/en/ (accessed on 21 November 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).