A Review of 3-Nitrooxypropanol for Enteric Methane Mitigation from Ruminant Livestock
Abstract
:Simple Summary
Abstract
1. Introduction
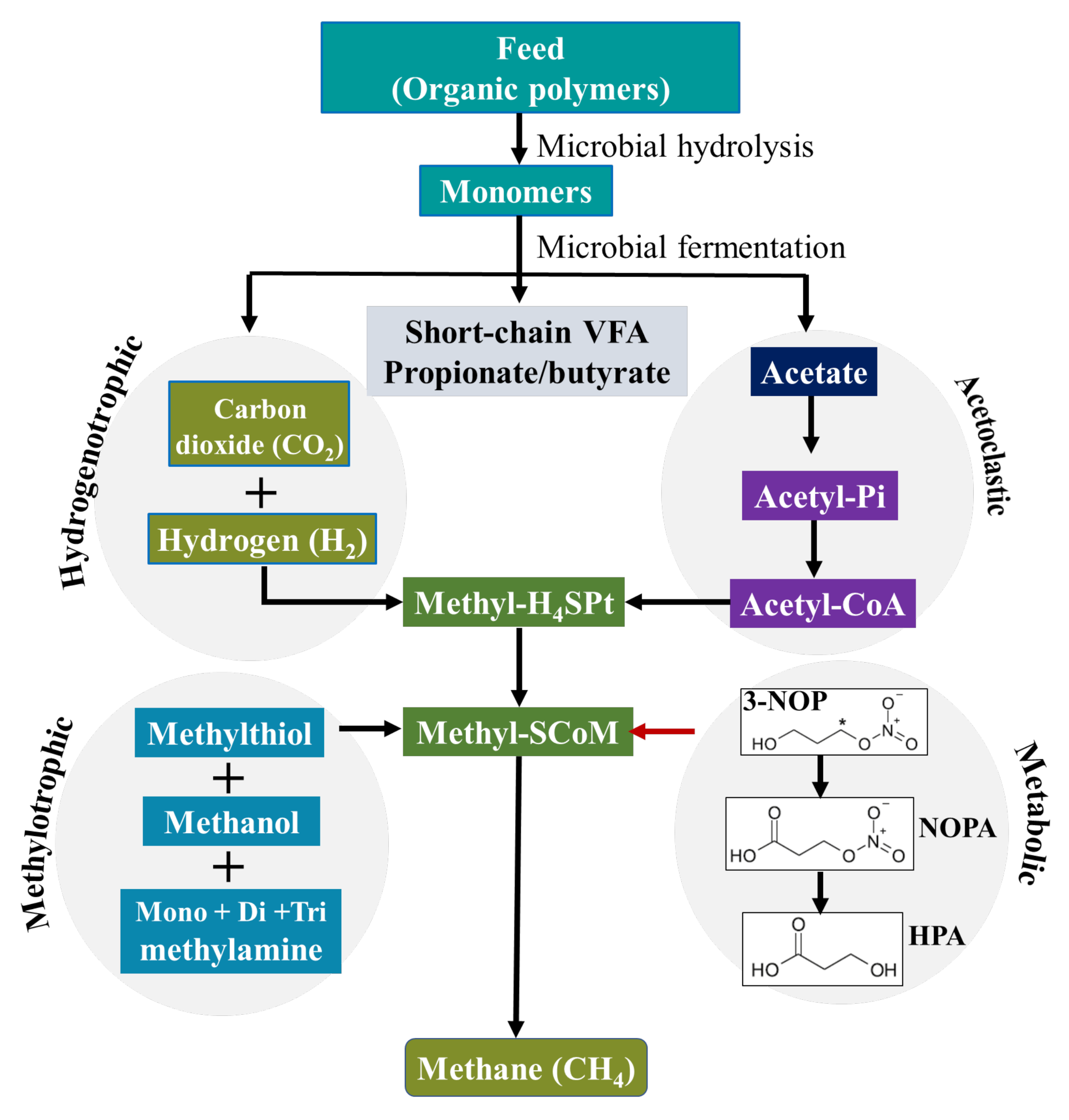
2. 3-Nitrooxypropanol, Mode of Action and Safety
3. Effects on Rumen Fermentation and Methanogenesis
4. Mitigation of Enteric CH4 Using 3-Nitrooxypropanol
4.1. Method of Providing 3-Nitrooxypropanol to Animals
4.2. Efficacy and Uncertanty
4.3. Effectiveness of 3-Nitrooxypropanol in Long-Term Studies
5. Effects of 3-Nitrooxypropanol on Dry Matter Intake, Digestibility and Animal Productivity
5.1. Effects of 3-Nitrooxypropanol on Dry Matter Intake and Digestibility
5.2. Effects of 3-Nitrooxypropanol on Animal Productivity
6. Practical Considerations for Use on Farm
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edenhofer, O. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, MA, USA, 2015; Volume 3. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Amim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.; Vellinga, T.; Opio, C.; Steinfeld, H. Productivity gains and greenhouse gas emissions intensity in dairy systems. Livest. Sci. 2011, 139, 100–108. [Google Scholar] [CrossRef]
- Reisinger, A.; Clark, H.; Cowie, A.L.; Emmet-Booth, J.; Gonzalez Fischer, C.; Herrero, M.; Howden, M.; Leahy, S. How necessary and feasible are reductions of methane emissions from livestock to support stringent temperature goals? Phil. Trans. R. 2021, A379, 20200452. [Google Scholar] [CrossRef] [PubMed]
- Rogelj, J.; Shindell, D.; Jiang, K.; Fifita, S.; Forster, P.; Ginzburg, V.; Handa, C.; Kheshgi, H.; Kobayashi, S.; Kriegler, E. Mitigation pathways compatible with 1.5 °C in the context of sustainable development. In Global Warming of 1.5 °C; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018; pp. 93–174. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019. [Google Scholar]
- Monaco, A.; Ross, K.; Waskow, D.; Ge, M. How Methane Emissions Contribute to Climate Change. 2021. Available online: https://www.wri.org/insights/methane-gas-emissions-climate-change (accessed on 20 November 2021).
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group14 I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; Arias, P., Bellouin, N., Coppola, E., Jones, R., Krinner, G., Marotzke, J., Naik, V., Palmer, M., Plattner, G.-K., Rogelj, J., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Beauchemin, K.A.; Ungerfeld, E.; Eckard, R.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, s2–s16. [Google Scholar] [CrossRef] [Green Version]
- Breider, I.S.; Wall, E.; Garnsworthy, P.C. Short communication: Heritability of methane production and genetic correlations with milk yield and body weight in Holstein-Friesian dairy cows. J. Dairy Sci. 2019, 102, 7277–7281. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, X.; Xue, B.; Peng, Q.; Wang, Z.; Yan, T.; Wang, L. Immunization against rumen methanogenesis by vaccination with a new recombinant protein. PLoS ONE 2015, 10, e0140086. [Google Scholar] [CrossRef] [Green Version]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; de Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 2021, 16, e0247820. [Google Scholar]
- Henderson, G.; Cook, G.; Ronimus, R. Enzyme- and gene-based approaches for developing methanogen-specific compounds to control ruminant methane emissions: A review. Anim. Prod. Sci. 2016, 58, 1017–1026. [Google Scholar] [CrossRef]
- Hristov, A.; Oh, J.; Giallongo, F.; Frederick, T.; Harper, M.; Weeks, H.; Branco, A.; Moate, P.; Deighton, M.; Williams, S.; et al. An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc. Natl. Acad. Sci. USA 2015, 112, 10663–10668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duin, E.; Wagner, T.; Shima, S.; Prakash, D.; Cronin, B.; Yáñez-Ruiz, D.; Duval, S.; Rümbeli, R.; Stemmler, R.; Thauer, R.; et al. Mode of action uncovered for the specific reduction of methane emissions from ruminants by the small molecule 3-nitrooxypropanol. Proc. Natl. Acad. Sci. USA 2016, 113, 6172–6177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayanegara, A.; Sarwono, K.A.; Kondo, M.; Matsui, H.; Ridla, M.; Laconi, E.B.; Nahrowi. Use of 3-nitrooxypropanol as feed additive for mitigating enteric methane emissions from ruminants: A meta-analysis. Ital. J. Anim. Sci. 2018, 17, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, J.; Bannink, A.; France, J.; Kebreab, E.; van Gastelen, S. Short communication: Antimethanogenic effects of 3-nitrooxypropanol depend on supplementation dose, dietary fiber content, and cattle type. J. Dairy Sci. 2018, 101, 9041–9047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungerfeld, E.M. Inhibition of rumen methanogenesis and ruminant productivity: A meta-analysis. Front. Vet. Sci. 2018, 5, 113. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.; Baek, Y.; Lee, S.; Seo, J. The effects of dietary supplementation with 3-nitrooxypropanol on enteric methane emissions, rumen fermentation, and production performance in ruminants: A meta-analysis. J. Anim. Sci. Technol. 2020, 62, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Arndt, C.; Hristov, A.; Price, W.; McClelland, S.; Pelaez, A.; Cueva, S.; Oh, J.; Bannink, A.; Bayat, A.; Crompton, L. Strategies to Mitigate Enteric Methane Emissions by Ruminants-a Way to Approach the 2.0 °C Target; preprint 20210085288; CABI: Wallingford, UK, 2021. [Google Scholar] [CrossRef]
- Almeida, A.K.; Hegarty, R.S.; Cowie, A. Meta-analysis quantifying the potential of dietary additives and rumen modifiers for methane mitigation in ruminant production systems. Anim. Nutr. 2021, 7, 1219–1230. [Google Scholar] [CrossRef]
- Ogawa, T.; Nakazato, A.; Sato, M.; Hatayama, K. Synthesis of 2-and 3-nitrooxypropanol by chemoselective reduction of methyl 2-and 3-nitrooxypropionoate. Synthesis 1990, 1990, 459–460. [Google Scholar] [CrossRef]
- Duval, S.; Kindermann, M. Use of Nitrooxy Organic Molecules in Feed for Reducing Enteric Methane Emissions in Ruminants, and/or to Improve Ruminant Performance. International Patent WO2012084629A1, 28 April 2012. [Google Scholar]
- Thiel, A.; Rümbeli, R.; Mair, P.; Yeman, H.; Beilstein, P. 3-NOP: ADME studies in rats and ruminating animals. Food Chem. Toxicol. 2019, 125, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Gingell, R.; Kirkpatrick, J.B.; Steup, D.R. Subchronic toxicity study of 1, 3-propanediol administered orally to rats. Int. J. Toxicol. 2000, 19, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Thiel, A.; Schoenmakers, A.; Verbaan, I.; Chenal, E.; Etheve, S.; Beilstein, P. 3-NOP: Mutagenicity and genotoxicity assessment. Food Chem. Toxicol. 2019, 123, 566–573. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Waldrip, H.M.; Parker, D.; Todd, R.W.; Brauer, D. Dietary mitigation of enteric methane emissions from ruminants: A review of plant tannin mitigation options. Anim. Nutr. 2020, 6, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Honan, M.; Feng, X.; Tricarico, J.M.; Kebreab, E. Feed additives as a strategic approach to reduce enteric methane production in cattle: Modes of action, effectiveness and safety. Anim. Prod. Sci. 2021, 1, 15. [Google Scholar] [CrossRef]
- Wolin, M.J. Fermentation in the rumen and human large intestine. Science 1981, 213, 1463–1468. [Google Scholar] [CrossRef]
- van Gastelen, S.; Dijkstra, J.; Binnendijk, G.; Duval, S.; Heck, J.; Kindermann, M.; Zandstra, T.; Bannink, A. 3-Nitrooxypropanol decreases methane emissions and increases hydrogen emissions of early lactation dairy cows, with associated changes in nutrient digestibility and energy metabolism. J. Dairy Sci. 2020, 103, 8074–8093. [Google Scholar] [CrossRef] [PubMed]
- Haisan, J.; Sun, Y.; Guan, L.; Beauchemin, K.; Iwaasa, A.; Duval, S.; Barreda, D.; Oba, M. The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation. J. Dairy Sci. 2014, 97, 3110–3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, C.K.; Humphries, D.J.; Kirton, P.; Kindermann, M.; Duval, S.; Steinberg, W. Effects of 3-nitrooxypropanol on methane emission, digestion, and energy and nitrogen balance of lactating dairy cows. J. Dairy Sci. 2014, 97, 3777–3789. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rémond, D.; Ortigues, I.; Jouany, J.-P. Energy substrates for the rumen epithelium. Proc. Nutr. Soc. 2007, 54, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Melgar, A.; Harper, M.T.; Oh, J.; Giallongo, F.; Young, M.E.; Ott, T.L.; Duval, S.; Hristov, A.N. Effects of 3-nitrooxypropanol on rumen fermentation, lactational performance, and resumption of ovarian cyclicity in dairy cows. J. Dairy Sci. 2020, 103, 410–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilde, M.; von Soosten, D.; Hüther, L.; Meyer, U.; Zeyner, A.; Dänicke, S. Effects of 3-nitrooxypropanol and varying concentrate feed proportions in the ration on methane emission, rumen fermentation and performance of periparturient dairy cows. Arch. Anim. Nutr. 2021, 75, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Alemu, A.W.; Pekrul, L.K.; Shreck, A.L.; Booker, C.W.; McGinn, S.M.; Kindermann, M.; Beauchemin, K.A. 3-Nitrooxypropanol decreased enteric methane production from growing beef cattle in a commercial feedlot: Implications for sustainable beef cattle production. Front. Anim. Sci. 2021, 2, 641590. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. A theoretical comparison between two ruminal electron sinks. Front. Microbiol. 2013, 4, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Perez, A.; Okine, E.; McGinn, S.; Guan, L.; Oba, M.; Duval, S.; Kindermann, M.; Beauchemin, K. The potential of 3-nitrooxypropanol to lower enteric methane emissions from beef cattle. J. Amim. Sci. 2014, 92, 4682–4693. [Google Scholar]
- Haisan, J.; Sun, Y.; Guan, L.; Beauchemin, K.A.; Iwaasa, A.; Duval, S.; Kindermann, M.; Barreda, D.R.; Oba, M. The effects of feeding 3-nitrooxypropanol at two doses on milk production, rumen fermentation, plasma metabolites, nutrient digestibility, and methane emissions in lactating Holstein cows. Anim. Prod. Sci. 2017, 57, 282–289. [Google Scholar] [CrossRef]
- Pitta, D.; Melgar, A.; Hristov, A.; Indugu, N.; Narayan, K.; Pappalardo, C.; Hennessy, M.; Vecchiarelli, B.; Kaplan-Shabtai, V.; Kindermann, M.; et al. Temporal changes in total and metabolically active ruminal methanogens in dairy cows supplemented with 3-nitrooxypropanol. J. Dairy Sci. 2021, 104, 8721–8735. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, G.; Duval, S.; Kindermann, M.; Schirra, H.J.; Denman, S.E.; McSweeney, C.S. 3-NOP vs. halogenated compound: Methane production, ruminal fermentation and microbial community response in forage fed cattle. Front. Microbiol. 2018, 9, 1582. [Google Scholar] [CrossRef] [PubMed]
- Gruninger, R.J.; Zhang, X.M.; Smith, M.L.; Kung, L.; Vyas, D.; McGinn, S.M.; Kindermann, M.; Wang, M.; Tan, Z.L.; Beauchemin, K.A. Application of 3-nitrooxypropanol and canola oil to mitigate enteric methane emissions of beef cattle results in distinctly different effects on the rumen microbial community. Res. Sq. 2021, 1, 1–33. [Google Scholar] [CrossRef]
- Zhang, X.M.; Gruninger, R.J.; Alemu, A.W.; Wang, M.; Tan, Z.L.; Kindermann, M.; Beauchemin, K.A. 3-Nitrooxypropanol supplementation had little effect on fiber degradation and microbial colonization of forage particles when evaluated using the in situ ruminal incubation technique. J. Dairy Sci. 2020, 103, 8986–8997. [Google Scholar] [CrossRef]
- Lopes, J.; de Matos, L.; Harper, M.; Giallongo, F.; Oh, J.; Gruen, D.; Ono, S.; Kindermann, M.; Duval, S.; Hristov, A. Effect of 3-nitrooxypropanol on methane and hydrogen emissions, methane isotopic signature, and ruminal fermentation in dairy cows. J. Dairy Sci. 2016, 99, 5335–5344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, N.; Kennelly, J. Effects of feeding frequency on ruminal parameters, plasma insulin, milk yield, and milk composition in Holstein cows. J. Dairy Sci. 1990, 73, 1857–1863. [Google Scholar] [CrossRef]
- Penner, G.B.; Aschenbach, J.R.; Gäbel, G.; Rackwitz, R.; Oba, M. Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal pH and the susceptibility to subacute ruminal acidosis in sheep. J. Nutr. 2009, 139, 1714–1720. [Google Scholar] [CrossRef] [Green Version]
- Romero-Perez, A.; Okine, E.; McGinn, S.; Guan, L.; Oba, M.; Duval, S.; Kindermann, M.; Beauchemin, K. Sustained reduction in methane production from long-term addition of 3-nitrooxypropanol to a beef cattle diet. J. Amim. Sci. 2015, 93, 1780–1791. [Google Scholar]
- Van Wesemael, D.; Vandaele, L.; Ampe, B.; Cattrysse, H.; Duval, S.; Kindermann, M.; Fievez, V.; De Campeneere, S.; Peiren, N. Reducing enteric methane emissions from dairy cattle: Two ways to supplement 3-nitrooxypropanol. J. Dairy Sci. 2019, 102, 1780–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, D.; McGinn, S.; Duval, S.; Kindermann, M.; Beauchemin, K. Effects of sustained reduction of enteric methane emissions with dietary supplementation of 3-nitrooxypropanol on growth performance of growing and finishing beef cattle. J. Amim. Sci. 2016, 94, 2024–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Fernández, G.; Abecia, L.; Arco, A.; Cantalapiedra-Hijar, G.; Martín-García, A.; Molina-Alcaide, E.; Kindermann, M.; Duval, S.; Yáñez-Ruiz, D. Effects of ethyl-3-nitrooxy propionate and 3-nitrooxypropanol on ruminal fermentation, microbial abundance, and methane emissions in sheep. J. Dairy Sci. 2014, 97, 3790–3799. [Google Scholar] [CrossRef] [Green Version]
- Vyas, D.; McGinn, S.; Duval, S.; Kindermann, M.; Beauchemin, K. Optimal dose of 3-nitrooxypropanol for decreasing enteric methane emissions from beef cattle fed high-forage and high-grain diets. Anim. Prod. Sci. 2016, 58, 1049–1055. [Google Scholar] [CrossRef]
- Alemu, A.; Shreck, A.; Booker, C.; McGinn, S.; Pekrul, L.; Kindermann, M.; Beauchemin, K. Use of 3-nitrooxypropanol in a commercial feedlot to decrease enteric methane emissions from cattle fed a corn-based finishing diet. J. Amim. Sci. 2021, 99, skaa394. [Google Scholar] [CrossRef]
- Vyas, D.; Alemu, A.; McGinn, S.; Duval, S.; Kindermann, M.; Beauchemin, K. The combined effects of supplementing monensin and 3-nitrooxypropanol on methane emissions, growth rate, and feed conversion efficiency in beef cattle fed high-forage and high-grain diets. J. Amim. Sci. 2018, 96, 2923–2938. [Google Scholar] [CrossRef] [PubMed]
- McGinn, S.; Flesch, T.; Beauchemin, K.; Shreck, A.; Kindermann, M. Micrometeorological methods for measuring methane emission reduction at beef cattle feedlots: Evaluation of 3-Nitrooxypropanol feed additive. J. Environ. Qual. 2019, 48, 1454–1461. [Google Scholar] [CrossRef]
- Zhang, X.; Smith, M.; Gruninger, R.; Kung, L.; Vyas, D.; McGinn, S.; Kindermann, M.; Wang, M.; Tan, Z.; Beauchemin, K. Combined effects of 3-nitrooxypropanol and canola oil supplementation on methane emissions, rumen fermentation and biohydrogenation, and total tract digestibility in beef cattle. J. Amim. Sci. 2021, 99, skab081. [Google Scholar] [CrossRef] [PubMed]
- Melgar, A.; Welter, K.; Nedelkov, K.; Martins, C.; Harper, M.; Oh, J.; Räisänen, S.; Chen, X.; Cueva, S.; Duval, S.; et al. Dose-response effect of 3-nitrooxypropanol on enteric methane emissions in dairy cows. J. Dairy Sci. 2020, 103, 6145–6156. [Google Scholar] [CrossRef]
- Meale, S.; Popova, M.; Saro, C.; Martin, C.; Bernard, A.; Lagree, M.; Yáñez-Ruiz, D.; Boudra, H.; Duval, S.; Morgavi, D. Early life dietary intervention in dairy calves results in a long-term reduction in methane emissions. Sci. Rep. 2021, 11, 3003. [Google Scholar] [CrossRef] [PubMed]
- Melgar, A.; Lage, C.; Nedelkov, K.; Räisänen, S.; Stefenoni, H.; Fetter, M.; Chen, X.; Oh, J.; Duval, S.; Kindermann, M.; et al. Enteric methane emission, milk production, and composition of dairy cows fed 3-nitrooxypropanol. J. Dairy Sci. 2021, 104, 357–366. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, C.; Pechtl, H.A.; Hettick, J.M.; Campler, M.R.; Pairis-Garcia, M.D.; Beauchemin, K.A.; Celi, P.; Duval, S.M. Effects of 3-nitrooxypropanol on enteric methane production, rumen fermentation, and feeding behavior in beef cattle fed a high-forage or high-grain diet. J. Amim. Sci. 2019, 97, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Samsonstuen, S.; Åby, B.A.; Crosson, P.; Beauchemin, K.A.; Aass, L. Mitigation of greenhouse gas emissions from beef cattle production systems. Acta Agric. Scand. Sect. A Anim. Sci. 2020, 69, 220–232. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; Okine, E.K.; Guan, L.L.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. Effects of 3-nitrooxypropanol on methane production using the rumen simulation technique (Rusitec). Anim. Feed Sci. Technol. 2015, 209, 98–109. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; Okine, E.K.; Guan, L.L.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. Effects of 3-nitrooxypropanol and monensin on methane production using a forage-based diet in Rusitec fermenters. Anim. Feed Sci. Technol. 2016, 220, 67–72. [Google Scholar] [CrossRef]
- Guyader, J.; Ungerfeld, E.M.; Beauchemin, K.A. Redirection of metabolic hydrogen by inhibiting methanogenesis in the rumen simulation technique (RUSITEC). Front. Microbiol. 2017, 8, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Pérez, A.; Okine, E.K.; Guan, L.L.; Duval, S.M.; Kindermann, M.; Beauchemin, K.A. Rapid Communication: Evaluation of methane inhibitor 3-nitrooxypropanol and monensin in a high-grain diet using the rumen simulation technique (Rusitec). J. Amim. Sci. 2017, 95, 4072–4077. [Google Scholar]
- Alvarez-Hess, P.S.; Moate, P.J.; Williams, S.R.O.; Jacobs, J.L.; Beauchemin, K.A.; Hannah, M.C.; Durmic, Z.; Eckard, R.J. Effect of combining wheat grain with nitrate, fat or 3-nitrooxypropanol on in vitro methane production. Anim. Feed Sci. Technol. 2019, 256, 114237. [Google Scholar] [CrossRef]
- Schilde, M.; von Soosten, D.; Hüther, L.; Kersten, S.; Meyer, U.; Zeyner, A.; Dänicke, S. Dose–response effects of 3-nitrooxypropanol combined with low-and high-concentrate feed proportions in the dairy cow ration on fermentation parameters in a rumen simulation technique. Animals 2021, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Wittenberg, K.M.; Ominski, K.H.; Krause, D.O. Efficacy of ionophores in cattle diets for mitigation of enteric methane1. J. Amim. Sci. 2006, 84, 1896–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klop, G.; Dijkstra, J.; Dieho, K.; Hendriks, W.H.; Bannink, A. Enteric methane production in lactating dairy cows with continuous feeding of essential oils or rotational feeding of essential oils and lauric acid. J. Dairy Sci. 2017, 100, 3563–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, M.S. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J. Dairy Sci. 2000, 83, 1598–1624. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Metabolic hydrogen flows in rumen fermentation: Principles and possibilities of interventions. Front. Microbiol. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaxter, K.L.; Czerkawski, J. Modification of the methane production of the sheep by supplementation of ITS diet. J. Sci. Food Agric. 1966, 17, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Nkemka, V.N.; Beauchemin, K.A.; Hao, X. Treatment of feces from beef cattle fed the enteric methane inhibitor 3-nitrooxypropanol. Water Sci. Technol. 2019, 80, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.; Thomas, B.; Stoeckli, J.; Beauchemin, K.; McAllister, T.; Larney, F.; Hao, X. Greenhouse gas and ammonia emissions from stored manure from beef cattle supplemented 3-nitrooxypropanol and monensin to reduce enteric methane emissions. Sci. Rep. 2020, 10, 19310. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.L.; Hao, X.; Gross, C.D.; Beauchemin, K.A.; Chang, S.X. Effect of manure from cattle fed 3-Nitrooxypropanol on anthropogenic greenhouse gas emissions depends on soil type. Agronomy 2021, 11, 371. [Google Scholar] [CrossRef]
- Alvarez-Hess, P.S.; Little, S.M.; Moate, P.J.; Jacobs, J.L.; Beauchemin, K.A.; Eckard, R.J. A partial life cycle assessment of the greenhouse gas mitigation potential of feeding 3-nitrooxypropanol and nitrate to cattle. Agric. Syst. 2019, 169, 14–23. [Google Scholar] [CrossRef]
- Kebreab, E.; Feng, X. Strategies to Reduce Methane Emissions from Enteric and Lagoon Sources; Contract 17RD018; Prepared for State of California Air Resources Board Research Division: Sacramento, CA, USA, 2021; p. 57. [Google Scholar]
- FAO. Global Livestock Environmental Assessment Model (GLEAM) [online]. 2017. Available online: www.fao.org/gleam/en/ (accessed on 21 November 2021).
| Type 1 | Equation 2 | Source |
|---|---|---|
| all | CH4/DMI (g/kg DMI) = −38.7 (±6.3) × 3-NOP + 20.2 (±1.25) (R2 = 0.59, n = 39, p < 0.01) | [16] |
| all | CH4/DMI (g/kg DMI) = −0.00158 (±0.000544) × 3-NOP + 12.3 (p < 0.05) | [17] |
| all | CH4/DMI (g/kg DMI) = −0.041 (±0.0047) × 3-NOP + 20.636 (±1.02) (R2 = 0.74, n = 54, p < 0.01) | [19] |
| beef | CH4/DMI (g/kg DMI) = −0.037 (±0.0043) × 3-NOP + 21.365 (±1.48) (R2 = 0.80, n = 35, p < 0.01) | [19] |
| dairy | CH4/DMI (g/kg DMI) = −0.073 (±0.0084) × 3-NOP + 20.068 (±1.16) (R2 = 0.92, n = 16, p < 0.01) | [19] |
| long-term | CH4/DMI (g/kg DMI) = −0.053 (±0.0055) × 3-NOP + 21.379 (±2.11) (R2 = 0.91, n = 19, p < 0.01) | [19] |
| all | CH4/DOM (g/kg DOM) = −54.6 (±13.3) × 3-NOP + 30.6 (±1.32) (R2 = 0.68, n = 10, p < 0.01) | [16] |
| all | CH4/milk (g/kg milk) = −29.5 (±11.9) × 3-NOP + 14.0 (±1.90) (R2 = 0.46, n = 12, p < 0.05) | [16] |
| all | CH4/BW (g/kg BW) = −0.94 (±0.19) × 3-NOP + 0.486 (±0.04) (R2 = 0.42, n = 39, p < 0.01) | [16] |
| all | CH4 (g/d) = −0.00176 (±0.000411) × 3-NOP + 12.3 (p < 0.05) | [17] |
| all | CH4 (% of GEI) = −10.3 × 3-NOP + 6.16 (R2 = 0.49, n = 29, p < 0.01) | [16] |
| Reference | Animal | Diet and Level 1 | 3-Nitrooxypropanol (3-NOP) | Effects 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg DM 2 | Length of Experimental Period 3 | VFA | Ammonia Nitrogen | CH4 Yield 5 | CH4 Measurement | H2 Production | Digestibility 6 | Microbes 6 | |||
| Haisan et al. [32] | Dairy | Silage: concentrate (60:40) | 130 | 28-d periods | ↓ acetate and acetate-to-propionate ratio | NR | ↓ (60% relative to a control diet) | Sulfur hexafluoride tracer technique | NR | NR | ↓ Methanogens |
| Reynolds et al. [33] | Dairy | Silage: concentrate (51:49) | 25 and 124 | 5-wk | ↓ acetate and acetate-to-propionate ratio | – | ↓ (7%, 9.8% relative to a control diet, g/d) | Respiration chambers | NR | ↓ DM, OM, ADF, nitrogen, and energy by the higher dose | NR |
| Hristov et al. [14] | Dairy | TMR | 40, 60, and 80 | 12-wk | NR | NR | ↓ (25%, 31%, 32% relative to a control diet, g/d) | GreenFeed system | ↑ 0.48, 0.96, and 1.27 g/d, respectively | NR | NR |
| Lopes et al. [46] | Dairy | Forage: concentrate (55:45) | 60 | Two 14-d periods | ↓ acetate and acetate-to-propionate ratio | ↓ | ↓ (34%, relative to a control diet) | GreenFeed system | ↑ 1.3 g/d | NR | ↓ Ruminococcus and Clostridium spp. |
| Haisan et al. [41] | Dairy | Silage: concentrate (60:40) | 68 and 132 | Three 28-d periods | ↓ acetate | – | ↓ (23–37% relative to a control diet) | Sulfur hexafluoride tracer technique | NR | ↑ DM, NDF at high dose | # Methanogens, protozoa, and bacteria |
| Van Wesemael et al. [50] | Dairy | Silage: concentrate (66:34) | 75 7 | 10-wk | NR | NR | ↓ (21–23% relative to a control diet) | GreenFeed units | NR | NR | NR |
| Melgar et al. [36] | Dairy | Forage: concentrate (58:42) | 60 | 15-wk | ↓ acetate, total VFA | – | ↓ (21%, relative to a control diet) | GreenFeed system | ↑ 48-fold relative to control diets | ↑ crude protein | NR |
| Melgar et al. [58] | Dairy | Forage: concentrate (60:40) | 40, 60, 80, 100, 150, and 200 | 31 d | NR | NR | ↓ (16–36%, relative to a control diet) | GreenFeed system | ↑ 6- to 10-fold relative to control diets | NR | NR |
| d 3 ante partum until 115 DIM | Dairy | Forage: concentrate (60:40) | 51 | NR | NR | ↓ (17%, relative to a control diet) | Climate respiration chambers | ↑ 11-fold | ↑ DM, OM, NDF and gross energy | NR | |
| Meale et al. [59] | Dairy | Milk and concentrate | 3 mg/kg BW | 14-wk | No effect | NR | ↓ (11.6–17.5% relative to control calves, g/d) | GreenFeed system | NR | NR | ↓ rumen bacteria and archaeal at 60 weeks of age |
| Melgar et al. [60] | Dairy | Forage: concentrate (58:42) | 60 | 15-wk | NR | NR | ↓ (27%, relative to a control diet) | GreenFeed units | ↑ 6-fold relative to control diets | NR | NR |
| Pitta et al. [42] | Dairy | TMR | 60 | 12-wk | NR | NR | NR | NR | NR | NR | ↓ Methanobrevibacter, Methanosphaera |
| Schilde et al. [37] | Dairy | Silage: concentrate (90:10) | 48 and 51 | d 28 ante partum until d 120 post-partum | ↓ acetate and acetate-to-propionate ratio | ↓ | ↓ (23–35% relative to a control diet) | GreenFeed system | NR | NR | # protozoa |
| Romero-Perez et al. [40] | Beef | Forage: concentrate (60:40) | 47, 144 and 305 | Four 28-d periods | ↓ acetate, acetate-to-propionate ratio | – | ↓ (4–33%, relative to a control diet) | Whole animal metabolic chambers | NR | # | # Methanogens, protozoa, and bacteria |
| Romero-Perez et al. [49] | Beef | Forage: concentrate (60:40) | 280 | 112 d | ↓ acetate, acetate-to-propionate ratio | – | ↓ (59.2%, relative to a control diet) | Whole animal metabolic chambers | NR | NR | ↓ methanogens |
| Vyas et al. [51] | Beef | Silage: concentrate (70:30,8:92) | 100 and 200 | 238 d | NR | NR | ↓ (16–22.9% relative to a backgrounding control diet; 25.8–45.2% relative to a finishing control diet) | Open-circuit calorimetry Chambers | ↑ 2.6- to 5.5-fold (backgrounding phase); 140- to 621.5-fold (finishing phase) relative to control diets | NR | NR |
| Vyas et al. [53] | Beef | Silage: concentrate (65:35,8:92) | 50, 75, 100, 150, and 200 | Two 28-d periods | NR | NR | ↓ (max. 23% and 45% relative to high-forage and high-grain control diets) | Open-circuit calorimetry chambers | ↑ max. 1.03 and 2.77 g/d.animal | NR | NR |
| Martínez-Fernández et al. [43] | Beef | grass hay | 325 | 21 d | ↓ | ↑ | ↓ (38%, relative to a control diet) | Open-circuit respiration chambers | – | ↑DM | ↓ Methanobrevibacter |
| Vyas et al. [55] | Beef | Silage: concentrate (65:35,8:92) | 125 and 200 | 105 d | ↓ acetate and acetate-to-propionate ratio | – | ↓ (37–42% relative to a control diet) | Open-circuit calorimetry chambers | ↑ 2.26 and 7.92 g/animal per day | NR | NR |
| Kim et al. [61] | Beef | Forage: concentrate (65:35) | 100 | Three 21-d periods | ↓ acetate | – | ↓ (18%, relative to high forage control diet) | GreenFeed system | NR | NR | NR |
| McGinn et al. [56] | Beef | Barley silage: barley grain (92:8) | 125 | 120 d | NR | NR | ↓ (70%, relative to a control diet) | Centration ratio and inverse dispersion methods | NR | NR | NR |
| Samsonstuen et al. [62] | Beef | Forage: concentrate (78:22, 47:53, 62:38, 50:50) | 100 and 237 | 34-wk | NR | NR | ↓ (15% and 31% for British breed, 19% and 35 % for Continental breed, kg CO2 eq kg−1 carcass) | HolosNorBeef modle | NR | NR | NR |
| Zhang et al. [45] | Beef | Forage: concentrate (90:10) | 150 | 12 d | NR | NR | ↓ (53%, relative to a control diet) 8 | Gas chromatography | ↑ 780% | # ruminal fiber degradation | ↓ Methanobrevibacter for barley silage |
| Alemu et al. [38] | Beef | Silage: concentrate (70:30) | 150, 175, and 200 | 108 d | NR | NR | ↓ (20%, 25%, and 21% relative to a control diet) | GreenFeed system | ↑ 3.5-, 4-, 4-fold relative to control diets | NR | NR |
| Alemu et al. [54] | Beef | Forage: concentrate (8:92) | 100, 125 and 150 | Three 28-d periods | ↓ acetate: propionate ratio | – | ↓ (52%, 76%, and 63% relative to a control diet) | GreenFeed system | ↑ 4.9-fold | NR | NR |
| Gruninger et al. [44] | Beef | Forage: concentrate (90:10) | 200 | Four 28-d periods | ↑ propionate percentages | NR | ↓ (28.2%, relative to a control diet) | Open-circuit calorimetry chambers | ↑ 37-fold relative to control diets | NR | ↓ Methanobrevibacter, Methanomicrobium, Methanomethylophilus |
| Zhang et al. [57] | Beef | Forage: concentrate (90:10) | 200 | Four 28-d periods | ↓ acetate, total VFA concentration | – | ↓ (31.6%, relative to a control diet) | Open-circuit calorimetry chambers | ↑ 45-fold relative to control diets | ↑ crude protein and starch digestibility | NR |
| Martínez-Fernández et al. [52] | Sheep | Alfalfa hay and oats (60:40) | 111 | 30 d | ↓ acetate and acetate-to-propionate ratio | – | ↓ (26%, relative to a control diet) | Respiration chambers | NR | # DM | # Methanogenic archaea |
| Reference | Animal (Rumen Fluid) | Diet Substrate and Level 1 | 3-Nitrooxypropanol (3-NOP) | Effects 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/g DM 2 | Persistency Time | VFA | Ammonia Concentration | CH4 Yield | CH4 Measurement | H2 Production | Digestibility | Microbes | |||
| Romero-Perez et al. [63] | cattle | Silage: concentrate (10 g; 60:40) | 0.5, 1 and 2 | 7 d | ↑ except for acetate | – | ↓ (74.6%, 84.2% and 86%, relative to a control diet) 4 | gas chromatograph | ↑ 2.6, 3.05, and 3.18-fold respectively | – DM and OM | ↓ Methanogens in the solid phase |
| Romero-Perez et al. [64] | cattle | Silage: concentrate (10 g; 60:40) | 0.2 | 7 d | NR | NR | ↓ (71.5%, relative to a control diet) 5 | gas chromatograph | ↑1.7-fold relative to control diets | NR | ↓ Methanogens in the solid phase |
| Guyader et al. [65] | cattle | Silage: concentrate (10 g; 60:40) | 0.5 | 19 d | ↓ acetate and isovalerate | ↑ | ↓ (75%, relative to a control diet) | gas-liquid chromatography | ↑ (81%, relative to a control diet) | ↑ DM and OM | NR |
| Romero-Perez et al. [66] | cattle | Silage: concentrate (10 g; 10:90) | 0.2 | 6 d | ↓ acetate | – | ↓ (77.7%, relative to a control diet) 5 | gas chromatograph | ↑ 2.3-fold relative to control diets | – DM | ↓ Methanogens |
| Alvarez-Hess et al. [67] | cattle | Corn grain (0.5 g; 50%) and alfalfa hay (0.5 g; 50%) | 0.08 | 24 h | ↓ acetate-to-propionate ratio | – | ↓ (44%, relative to a control diet) 6 | gas chromatography | NR | – DM | NR |
| Schilde et al. [68] | cattle | Forage: concentrate (12 g; 70:30, 40:60) | 0.07, 0.16, and 1.2 | 48 h | ↓ acetate, iso-butyrate | ↓ | ↓ (17–97%, relative to a control diet) 7 | gas chromatography | 27- and 6.2-fold relative to low- and high-concentrate diets | ↑DM | NR |
| Martínez-Fernández et al. [52] | sheep | alfalfa hay and oats (0.5 g; 60:40) | 8 and 16 | 24 h | ↓ acetate-to-propionate ratio | NR | ↓ (86.1% and 95.4% relative to a control diet) 8 | gas chromatograph | NR | NR | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Beauchemin, K.A.; Dong, R. A Review of 3-Nitrooxypropanol for Enteric Methane Mitigation from Ruminant Livestock. Animals 2021, 11, 3540. https://doi.org/10.3390/ani11123540
Yu G, Beauchemin KA, Dong R. A Review of 3-Nitrooxypropanol for Enteric Methane Mitigation from Ruminant Livestock. Animals. 2021; 11(12):3540. https://doi.org/10.3390/ani11123540
Chicago/Turabian StyleYu, Guanghui, Karen A. Beauchemin, and Ruilan Dong. 2021. "A Review of 3-Nitrooxypropanol for Enteric Methane Mitigation from Ruminant Livestock" Animals 11, no. 12: 3540. https://doi.org/10.3390/ani11123540
APA StyleYu, G., Beauchemin, K. A., & Dong, R. (2021). A Review of 3-Nitrooxypropanol for Enteric Methane Mitigation from Ruminant Livestock. Animals, 11(12), 3540. https://doi.org/10.3390/ani11123540








