Ovine Toll-like Receptor 9 (TLR9) Gene Variation and Its Association with Flystrike Susceptibility
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Identification of Flystrike
2.3. Blood Collection
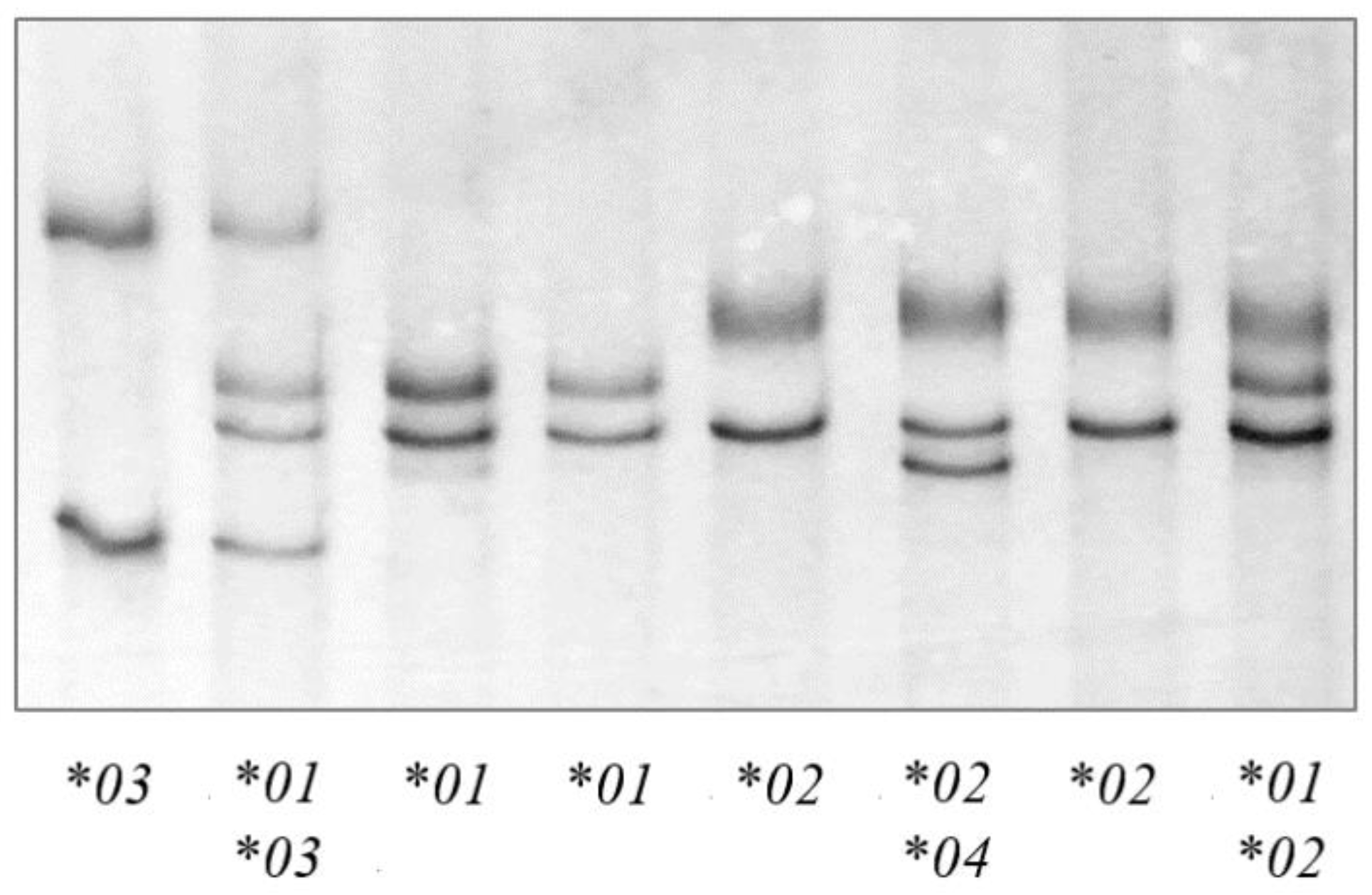
2.4. PCR-SSCP Analysis and Genotyping of Ovine TLR9
2.5. Sequencing of New Alleles and Sequence Analysis
2.6. Statistical Analyses
3. Results
3.1. Alleles of TLR9 and Their Frequencies
3.2. Associations between the Variables and Flystrike Occurrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anstead, C.A.; Perry, T.; Richards, S.; Korhonen, P.K.; Young, N.D.; Bowles, V.M.; Batterham, P.; Robin, B.; Gasser, R.B. The Battle against Flystrike–Past Research and New Prospects through Genomics. Adv. Parasitol. 2017, 98, 227–281. [Google Scholar] [PubMed]
- Smith, W.J.; Li, Y.; Ingham, A.; Collis, E.; McWilliam, S.M.; Dixon, T.J.; Norris, B.J.; Mortimer, S.I.; Moore, R.J.; Reverter, A. A genomics-informed, SNP associated study reveals FBLN1 and FABP4 as contributing to resistance to fleecerot in Australian Merino sheep. BMC Vet. Res. 2010, 6, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDiarmid, J.; Clarke, R.; McClure, S.; Bowen, F.; Burrell, D. Use of a monoclonal antibody to ovine IgE for fly strike studies in sheep. Int. J. Parasitol. 1995, 25, 1505–1507. [Google Scholar] [CrossRef]
- O’Meara, T.J.; Nesa, M.; Sandeman, R.M. Antibody responses to Lucilia cuprina in sheep selected for resistance or susceptibility to L. cuprina. Parasite Immunol. 1997, 19, 535–543. [Google Scholar] [CrossRef]
- Kumagai, Y.; Akira, S. Identification and functions of pattern-recognition receptors. J. Allergy Clin. Immunol. 2010, 125, 985–992. [Google Scholar] [CrossRef]
- Kang, J.Y.; Lee, J.O. Structural biology of the Toll-like receptor family. Annu. Rev. Biochem. 2011, 80, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [Green Version]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Bewick, A.J.; Vogel, K.J.; Moore, A.J.; Schmitz, R.J. Evolution of DNA Methylation across Insects. Mol. Biol. Evol. 2017, 34, 654–665. [Google Scholar] [CrossRef] [Green Version]
- Török, H.P.; Glas, J.; Tonenchi, L.; Bruennler, G.; Folwaczny, M.; Folwaczny, C. Crohn’s disease is associated with a toll-likereceptor-9 polymorphism. Gastroenterology 2004, 127, 365–366. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, C.; Zhou, Z.; Pei, F. TLR9 polymorphisms and systemic lupus erythematosus risk: An update meta-analysis study. Rheumatol. Int. 2016, 36, 585–595. [Google Scholar] [CrossRef]
- Omar, A.H.; Yasunami, M.; Yamazaki, A.; Shibata, H.; Ofori, M.F.; Akanmori, B.D.; Shuaibu, M.N.; Kikuchi, M.; Hirayama, K. Toll-like receptor 9 (TLR9) polymorphism associated with symptomatic malaria: A cohort study. Malar. J. 2012, 11, 168. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, L.; Xiao, S.; Chen, W.; Hu, F.; Li, F.; Guo, P.; Chen, X.; Cai, W.; Tang, X. The association of TLR2, TLR3, and TLR9 gene polymorphisms with susceptibility to Talaromycosis among Han Chinese AIDS patients in Guangdong. Front. Cell Infect. Microbiol. 2021, 11, 625461. [Google Scholar] [CrossRef] [PubMed]
- Alfano, F.; Peletto, S.; Lucibelli, M.G.; Borriello, G.; Urciuolo, G.; Maniaci, M.G.; Desiato, R.; Tarantino, M.; Barone, A.; Pasquali, P.; et al. Identification of single nucleotide polymorphisms in Toll-like receptor candidate genes associated with tuberculosis infection in water buffalo (Bubalus bubalis). BMC Genet. 2014, 15, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarafidou, T.; Stamatis, C.; Kalozoumi, G.; Spyrou, V.; Fthenakis, G.C.; Billinis, C.; Mamuris, Z. Toll like receptor 9 (TLR9) polymorphism G520R in sheep is associated with seropositivity for small ruminant lentivirus. PLoS ONE 2013, 8, e63901. [Google Scholar]
- Arcangeli, C.; Lucarelli, D.; Torricelli, M.; Sebastiani, C.; Ciullo, M.; Pellegrini, C.; Felici, A.; Costarelli, S.; Giammarioli, M.; Feliziani, F.; et al. First survey of SNPs in TMEM154, TLR9, MYD88 and CCR5 genes in sheep reared in Italy and their association with resistance to SRLVs infection. Viruses 2021, 13, 1290. [Google Scholar] [CrossRef]
- Kakavas, K.V. Sensitivity and applications of the PCR Single-Strand Conformation Polymorphism method. Mol. Biol. Rep. 2021, 48, 3629–3635. [Google Scholar] [CrossRef]
- Zhou, H.; Hickford, J.G.H.; Fang, Q. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal. Biochem. 2006, 354, 159–161. [Google Scholar] [CrossRef]
- Ohto, U.; Shibata, T.; Tanji, H.; Ishida, H.; Krayukhina, E.; Uchiyama, S.; Miyake, K.; Shimizu, T. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 2015, 520, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Rutz, M.; Metzger, J.; Gellert, T.; Luppa, P.; Lipford, G.B.; Wagner, H.; Bauer, S. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur. J. Immunol. 2004, 34, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hickford, J.G.H. Polymorphism of Toll-like receptor 9 (TLR9) gene in sheep. Vet. Immunol. Immunopath. 2008, 121, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.O.; Fang, Q.; Zhou, H.; Hickford, J.G.H. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Anal. Biochem. 2009, 385, 174–175. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Yu, Z.; Dyer, J.; Plowman, J.E.; Hickford, J. Identification of the ovine keratin-associated protein KAP1-2 gene (KRTAP1-2). Exp. Dermatol. 2011, 20, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, J.; Zhou, H.; Gong, H.; Tao, J.; Hickford, J.G.H. Identification of ovine KRTAP28-1 and its association with wool fibre diameter. Animals 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Zhou, H.; Wang, J.; Li, S.; Luo, Y.; Hickford, J.G.H. Characterisation of an ovine keratin associated protein (KAP) gene, which would produce a protein rich in glycine and tyrosine, but lacking in cysteine. Genes 2019, 10, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kijas, J.W.; Townley, D.; Dalrymple, B.P.; Heaton, M.P.; Maddox, J.F.; McGrath, A.; Wilson, P.; Ingersoll, R.G.; McCulloch, R.; McWilliam, S. A genome wide survey of SNP variation reveals the genetic structure of sheep breeds. PLoS ONE 2009, 4, e4668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.; Brinkmann, M.M.; Spooner, E.; Lee, C.C.; Kim, Y.M.; Ploegh, H.L. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 2008, 9, 1407–1414. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Wainwright, M.S.; Comeron, J.M.; Saitou, N.; Sanders, A.R.; Gelernter, J.; Gejman, P.V. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003, 12, 205–216. [Google Scholar] [CrossRef]
- Kimchi-Sarfaty, C.; Oh, J.M.; Kim, I.W.; Sauna, Z.E.; Calcagno, A.M.; Ambudkar, S.V.; Gottesman, M.M. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007, 315, 525–528. [Google Scholar] [CrossRef] [Green Version]
- Komar, A.A.; Lesnik, T.; Reiss, C. Synonymous codon substitutions affect ribosome traffic and protein folding during in vitro translation. FEBS Lett. 1999, 462, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Burrows, L.E.R.; Zhou, H.; Frampton, C.M.A.; Forrest, R.H.J.; Hickford, J.G.H. Ovine FABP4 variation and its association with flystrike susceptibility. Front. Genet. 2021, 12, 675305. [Google Scholar] [CrossRef] [PubMed]

| Allele | Sheep with Flystrike (n = 178) | Sheep without Flystrike (n = 134) | ||
|---|---|---|---|---|
| Number of Sheep Carrying the Allele | Percentage of Sheep Carrying the Allele (%) | Number of Sheep Carrying the Allele | Percentage of Sheep Carrying the Allele (%) | |
| *01 | 28 | 15.7 | 14 | 10.4 |
| *02 | 164 | 92.1 | 128 | 95.5 |
| *03 | 31 | 17.4 | 35 | 26.1 |
| *04 | 6 | 3.4 | 7 | 5.2 |
| Allele | Odds Ratio 1 | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| *01 | 1.195 | 0.575 | 2.566 | 0.638 |
| *02 | 0.414 | 0.126 | 1.241 | 0.126 |
| *03 | 0.495 | 0.268 | 0.900 | 0.022 |
| *04 | 0.585 | 0.182 | 1.824 | 0.351 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhou, H.; Gong, H.; Liu, W.; Fang, Q.; Luo, Y.; Wang, J.; Li, S.; Hu, J.; Hickford, J.G.H. Ovine Toll-like Receptor 9 (TLR9) Gene Variation and Its Association with Flystrike Susceptibility. Animals 2021, 11, 3549. https://doi.org/10.3390/ani11123549
Liu X, Zhou H, Gong H, Liu W, Fang Q, Luo Y, Wang J, Li S, Hu J, Hickford JGH. Ovine Toll-like Receptor 9 (TLR9) Gene Variation and Its Association with Flystrike Susceptibility. Animals. 2021; 11(12):3549. https://doi.org/10.3390/ani11123549
Chicago/Turabian StyleLiu, Xiu, Huitong Zhou, Hua Gong, Wenting Liu, Qian Fang, Yuzhu Luo, Jiqing Wang, Shaobin Li, Jiang Hu, and Jonathan G. H. Hickford. 2021. "Ovine Toll-like Receptor 9 (TLR9) Gene Variation and Its Association with Flystrike Susceptibility" Animals 11, no. 12: 3549. https://doi.org/10.3390/ani11123549
APA StyleLiu, X., Zhou, H., Gong, H., Liu, W., Fang, Q., Luo, Y., Wang, J., Li, S., Hu, J., & Hickford, J. G. H. (2021). Ovine Toll-like Receptor 9 (TLR9) Gene Variation and Its Association with Flystrike Susceptibility. Animals, 11(12), 3549. https://doi.org/10.3390/ani11123549







