Systems Biology–Derived Genetic Signatures of Mastitis in Dairy Cattle: A New Avenue for Drug Repurposing
Abstract
Simple Summary
Abstract
1. Introduction
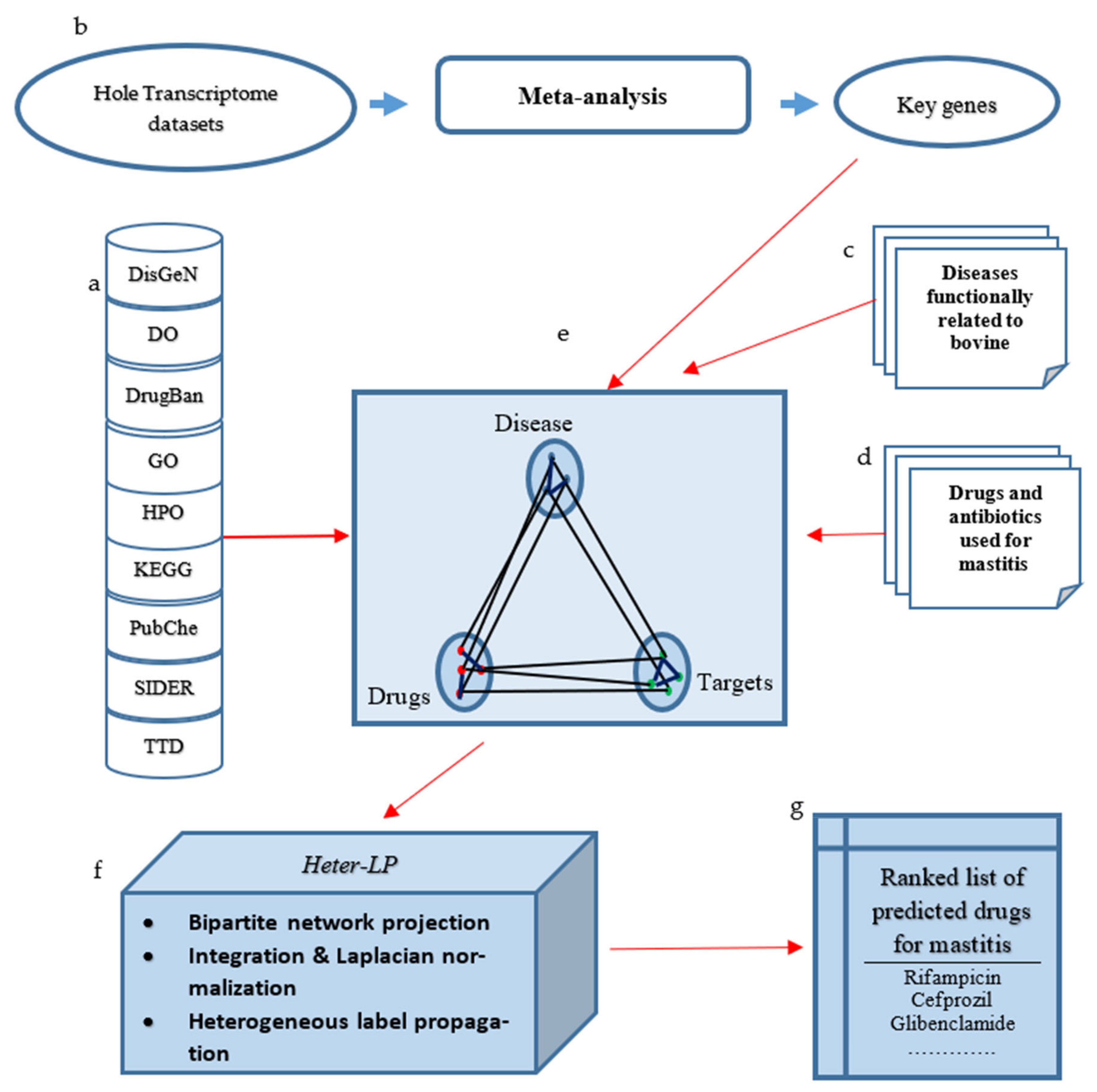
2. Materials and Methods
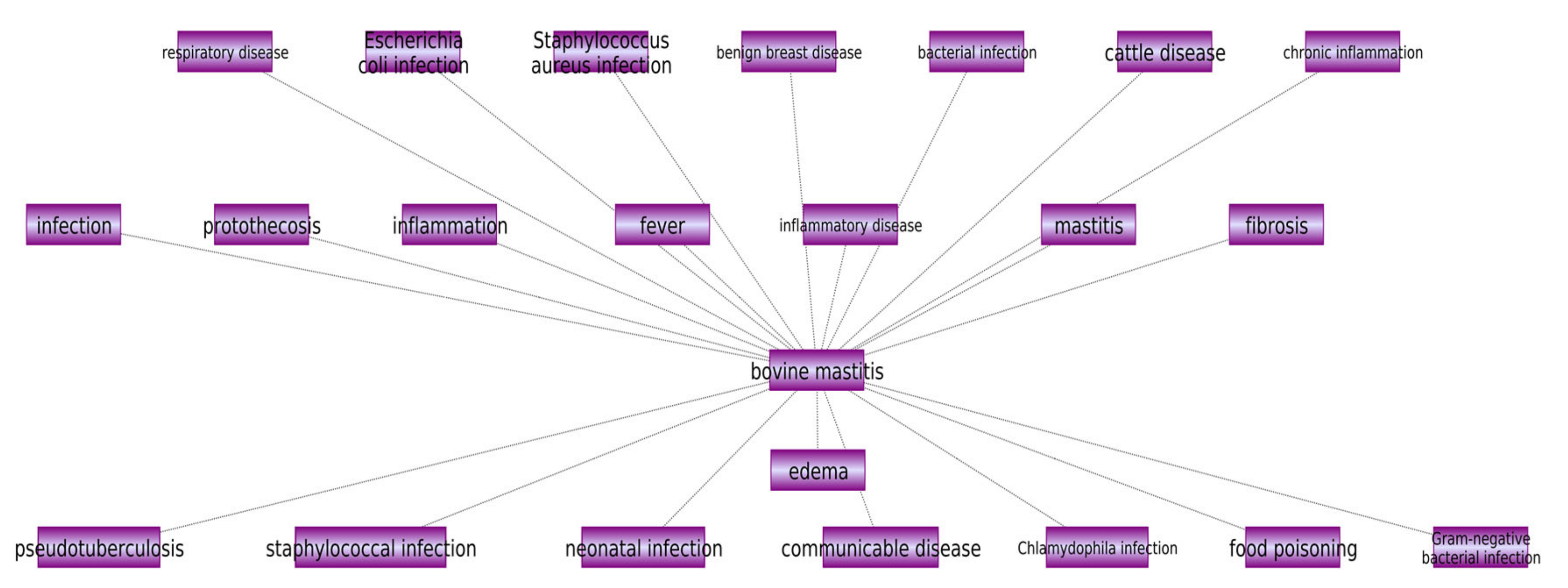
2.1. The Input Network Construction
- 1.
- Key genes with a robust bio-signature in response to bovine mastitis, especially in E. coli infection:
- 2.
- Functionally related diseases or biological processes associated with bovine mastitis:
- 3.
- Relevant drugs and antibiotics to E. coli mastitis:
2.2. Running Heter-LP
3. Results
3.1. Basic Similarities and Relations
3.2. Disease Genes
3.3. Disease Similarity Data
3.4. Drugs and Disease
3.5. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Bar, D.; Tauer, L.W.; Bennett, G.; González, R.N.; Hertl, J.A.; Schukken, Y.H.; Schulte, H.F.; Welcome, F.L.; Gröhn, Y.T. The cost of generic clinical mastitis in dairy cows as estimated by using dynamic programming. J. Dairy Sci. 2008, 91, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Pakdel, A. Bovine Mastitis: Etiology and Epidemiology, challenges, current trends and future perspectives in monitoring, detection and treatment. In Mastitis Symptoms, Triggers and Treatment; NOVA: New York, NY, USA, 2019; ISBN 978-1-53616-124-3. [Google Scholar]
- Bradley, A. Bovine mastitis: An evolving disease. Vet. J. 2002, 164, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.; Larry Smith, K. Coliform mastitis. Vet. Res. 2003, 34, 507–519. [Google Scholar] [CrossRef]
- Zadoks, R.; Fitzpatrick, J. Changing trends in mastitis. Ir. Vet. J. 2009, 62 (Suppl. 4), S59–S70. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Paape, M.J.; Lee, J.-W.; Zhao, X.; Hope, J.C.; Rainard, P. Escherichia coli and Staphylococcus aureus Elicit Differential Innate Immune Responses following Intramammary Infection. Clin. Diagn. Lab. Immunol. 2004, 11, 463–472. [Google Scholar] [CrossRef]
- Burvenich, C.; Bannerman, D.D.; Lippolis, J.D.; Peelman, L.; Nonnecke, B.J.; Kehrli, M.E., Jr.; Paape, M.J. Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J. Dairy Sci. 2007, 90 (Suppl. 1), E39–E54. [Google Scholar] [CrossRef]
- Hagiwara, S.; Mori, K.; Nagahata, H. Predictors of fatal outcomes resulting from acute Escherichia coli mastitis in dairy cows. J. Vet. Med. Sci. 2016, 78, 905–908. [Google Scholar] [CrossRef][Green Version]
- Bramley, A.J.; Dodd, F.H. Reviews of the progress of dairy science: Mastitis control--progress and prospects. J. Dairy Res. 1984, 51, 481–512. [Google Scholar] [CrossRef]
- Mestorino, N.; Errecalde, J.O. Pharmacokinetic–Pharmacodynamic Considerations for Bovine Mastitis Treatment; IntechOpen: London, UK, 2012. [Google Scholar]
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis detection: Current trends and future perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef]
- Lewis, J.D. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011, 140, 1817–1826. [Google Scholar] [CrossRef]
- Sharifi, S.; Pakdel, A.; Ebrahimi, M.; Reecy, J.M.; Fazeli Farsani, S.; Ebrahimie, E. Integration of machine learning and meta-analysis identifies the transcriptomic bio-signature of mastitis disease in cattle. PLoS ONE 2018, 13, e0191227. [Google Scholar] [CrossRef]
- Tiwari, J.G.; Babra, C.; Tiwari, H.K.; Williams, V.; Wet, S.D.; Gibson, J.; Paxman, A.; Morgan, E.; Costantino, P.; Sunagar, R.; et al. Trends In Therapeutic and Prevention Strategies for Management of Bovine Mastitis: An Overview. J. Vaccines Vaccin. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Camerlink, I.; Ellinger, L.; Bakker, E.J.; Lantinga, E.A. Homeopathy as replacement to antibiotics in the case of Escherichia coli diarrhoea in neonatal piglets. Homeopathy 2010, 99, 57–62. [Google Scholar] [CrossRef]
- Suojala, L.; Kaartinen, L.; Pyorala, S. Treatment for bovine Escherichia coli mastitis—an evidence-based approach. J. Vet. Pharmacol. Therap. 2013, 36, 521–531. [Google Scholar] [CrossRef]
- Lehtolainen, T.; Shwimmer, A.; Shpigel, N.Y.; Honkanen-Buzalski, T.; Pyorala, S. In vitro antimicrobial susceptibility of Escherichia coli isolates from clinical bovine mastitis in Finland and Israel. J. Dairy Sci. 2003, 86, 3927–3932. [Google Scholar] [CrossRef]
- Lotfi Shahreza, M.; Ghadiri, N.; Mousavi, S.R.; Varshosaz, J.; Green, J.R. A review of network-based approaches to drug repositioning. Brief Bioinform. 2018, 19, 878–892. [Google Scholar] [CrossRef]
- Lotfi, S.M.; Ghadiri, N.; Green, J.R. A computational drug repositioning method applied to rare diseases: Adrenocortical carcinoma. Sci. Rep. 2020, 10, 8846. [Google Scholar] [CrossRef]
- Lotfi, S.M.; Ghadiri, N.; Mousavi, S.R.; Varshosaz, J.; Green, J.R. Heter-LP: A heterogeneous label propagation algorithm and its application in drug repositioning. J. Biomed. Inform. 2017, 68, 167–183. [Google Scholar] [CrossRef]
- Bader, G.D.; Betel, D.; Hogue, C.W.V. BIND: The Biomolecular Interaction Network Database. Nucleic Acids Res. 2003, 31, 248–250. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology. Tool for the unification of biology. Gene. Ontol. Consort. 2000, 25, 25–29. [Google Scholar]
- Nikitin, A.; Egorov, S.; Daraselia, N.; Mazo, I. Pathway studio--the analysis and navigation of molecular networks. Bioinformatics 2003, 19, 2155–2157. [Google Scholar] [CrossRef]
- Sharifi, S.; Pakdel, A.; Ebrahimie, E.; Aryan, Y.; Reecy, J.M. Prediction of key regulators and downstream targets of E. coli induced mastitis. J. Appl. Genet. 2019, 60, 367–373. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Chen, Z. Identification of Key Candidate Genes in Dairy Cow in Response to Escherichia coli Mastitis by Bioinformatical Analysis. Front. Genet. 2019, 10, 1251. [Google Scholar] [CrossRef]
- Younis, S.; Javed, Q.; Blumenberg, M. Meta-Analysis of Transcriptional Responses to Mastitis-Causing Escherichia coli. PLoS ONE 2016, 11, e0148562. [Google Scholar] [CrossRef]
- Wagner, S.A. The Effects of anti-Inflammatory Drugs on Clinical Signs, Milk Production, and Mammary Epithelial Cells in Cows with Endotoxin-Induced Mastitis. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2003. [Google Scholar]
- Oliver, S.P.; Murinda, S.E.; Jayarao, B.M. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: A comprehensive review. Foodborne Pathog. Dis. 2011, 8, 337–355. [Google Scholar] [CrossRef]
- Srinivasan, V.; Gillespie, B.E.; Lewis, M.J.; Nguyen, L.T.; Headrick, S.I.; Schukken, Y.H.; Oliver, S.P. Phenotypic and genotypic antimicrobial resistance patterns of Escherichia coli isolated from dairy cows with mastitis. Vet. Microbiol. 2007, 124, 319–328. [Google Scholar] [CrossRef]
- Lohuis, J.A.; Van Leeuwen, W.; Verheijden, J.H.; Van Miert, A.S.; Brand, A. Effect of dexamethasone on experimental Escherichia coli mastitis in the cow. J. Dairy Sci. 1988, 71, 2782–2789. [Google Scholar] [CrossRef]
- Wagner, S.A.; Apley, M.D. Effects of two anti-inflammatory drugs on physiologic variables and milk production in cows with endotoxin-induced mastitis. Am. J. Vet. Res. 2004, 65, 64–68. [Google Scholar] [CrossRef]
- McDougall, S.; Bryan, M.A.; Tiddy, R.M. Effect of treatment with the nonsteroidal anti-inflammatory meloxicam on milk production, somatic cell count, probability of re-treatment, and culling of dairy cows with mild clinical mastitis. J. Dairy Sci. 2009, 92, 4421–4431. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.E.; Ceri, H.; Morck, D.W.; Buret, A.G.; Read, R.R. Biofilm bacteria: Formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 2002, 66, 86–92. [Google Scholar] [PubMed]
- Ziv, G.; Shem-Tov, M.; Ascher, F. Combined effect of ampicillin, colistin and dexamethasone administered intramuscularly to dairy cows on the clinico-pathological course of E. coli-endotoxin mastitis. Vet. Res. 1998, 29, 89–98. [Google Scholar] [PubMed]
- Barlow, J. Mastitis therapy and antimicrobial susceptibility: A multispecies review with a focus on antibiotic treatment of mastitis in dairy cattle. J. Mamar. Gland Biol. Neoplasia 2011, 16, 383–407. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Mohammadi-Dehcheshmeh, M.; Ebrahimie, E.; Petrovski, K.R. Comprehensive analysis of machine learning models for prediction of sub-clinical mastitis: Deep Learning and Gradient-Boosted Trees outperform other models. Comput. Biol. Med. 2019, 114, 103456. [Google Scholar] [CrossRef]
- Ebrahimie, E.; Ebrahimi, F.; Ebrahimi, M.; Tomlinson, S.; Petrovski, K.R. A large-scale study of indicators of sub-clinical mastitis in dairy cattle by attribute weighting analysis of milk composition features: Highlighting the predictive power of lactose and electrical conductivity. J. Dairy Res. 2018, 85, 193–200. [Google Scholar] [CrossRef]
- Ebrahimie, E.; Ebrahimi, F.; Ebrahimi, M.; Tomlinson, S.; Petrovski, K.R. Hierarchical pattern recognition in milking parameters predicts mastitis prevalence. Comput. Electron. Agric. 2018, 147, 6–11. [Google Scholar] [CrossRef]
- Jamali, A.A.; Ferdousi, R.; Razzaghi, S.; Li, J.; Safdari, R.; Ebrahimie, E. DrugMiner: Comparative analysis of machine learning algorithms for prediction of potential druggable proteins. Drug Discov. Today 2016, 21, 718–724. [Google Scholar] [CrossRef]
- KayvanJoo, A.H.; Ebrahimi, M.; Haqshenas, G. Prediction of hepatitis C virus interferon/ribavirin therapy outcome based on viral nucleotide attributes using machine learning algorithms. BMC Res. Notes 2014, 7, 1–11. [Google Scholar] [CrossRef][Green Version]
- Farouk, O.; Abdelkhalek, M.; Abdallah, A.; Shata, A.; Senbel, A.; Attia, E.; Elghaffar, M.A.; Mesbah, M.; Soliman, N.; Amin, M.; et al. Rifampicin for Idiopathic Granulomatous Lobular Mastitis: A Promising Alternative for Treatment. World J. Surg. 2017, 41, 1313–1321. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Miller, S.I. Antibiotic Resistance and Regulation of the Gram-Negative Bacterial Outer Membrane Barrier by Host Innate Immune Molecules. MBio 2016, 7, e01541-16. [Google Scholar] [CrossRef]
- Erskine, R.J.; Walker, R.D.; Bolin, C.A.; Bartlett, P.C.; White, D.G. Trends in antibacterial susceptibility of mastitis pathogens during a seven-year period. J. Dairy Sci. 2002, 85, 1111–1118. [Google Scholar] [CrossRef]
- Simard, J.M.; Geng, Z.; Woo, S.K.; Ivanova, S.; Tosun, C.; Melnichenko, L.; Gerzanich, V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2009, 29, 317–330. [Google Scholar] [CrossRef]
- Zhang, W.; Fievez, L.; Cheu, E.; Bureau, F.; Rong, W.; Zhang, F.; Zhang, Y.; Advenier, C.; Gustin, P. Anti-inflammatory effects of formoterol and ipratropium bromide against acute cadmium-induced pulmonary inflammation in rats. Eur. J Pharmacol. 2010, 628, 171–178. [Google Scholar] [CrossRef]
- Kvetny, J.; Heldgaard, P.E.; Bladbjerg, E.M.; Gram, J. Subclinical hypothyroidism is associated with a low-grade inflammation, increased triglyceride levels and predicts cardiovascular disease in males below 50 years. Clin. Endocrinol. 2004, 61, 232–238. [Google Scholar] [CrossRef]
- Abbas, A.M.; Sakr, H.F. Effect of magnesium sulfate and thyroxine on inflammatory markers in a rat model of hypothyroidism. Can. J. Physiol. Pharmacol. 2016, 94, 426–432. [Google Scholar] [CrossRef]
- Uzkeser, H.; Cadirci, E.; Halici, Z.; Odabasoglu, F.; Polat, B.; Yuksel, T.N.; Ozaltin, S.; Atalay, F. Anti-inflammatory and antinociceptive effects of salbutamol on acute and chronic models of inflammation in rats: Involvement of an antioxidant mechanism. Mediat. Inflamm. 2012, 2012, 438912. [Google Scholar] [CrossRef]
- Zhu, H.; Lemos, H.; Bhatt, B.; Islam, B.N.; Singh, A.; Gurav, A.; Huang, L.; Browning, D.D.; Mellor, A.; Fulzele, S.; et al. Carbidopa, a drug in use for management of Parkinson disease inhibits T cell activation and autoimmunity. PLoS ONE 2017, 12, e0183484. [Google Scholar] [CrossRef]
| Sub-Network | Using Criterion | Resource | Number of Nodes | |
|---|---|---|---|---|
| In Each Resource | In Total | |||
| Drugs | Chemical substructure similarities | PubChem 1 | 1103 | 5089 |
| Side effect similarities | SIDER 2 | 888 | ||
| Anatomical Therapeutic Chemical (ATC) code similarities | KEGG 3 | 4867 | ||
| Diseases | Disease-gene similarities | DisGeNET 4 | 3295 | 9886 |
| Similarities based on ICD-10 classification 5 | KEGG | 1366 | ||
| Semantic similarities based on Disease Ontology (DO) 7 | DOSE package in R 6 | 6560 | ||
| Semantic similarities based on GO 9 | GOSemSim package in R 8 | 1550 | ||
| Targets | Semantic similarities based on HPO 10 | HPOSim package in R 11 | 979 | 2940 |
| Semantic similarities based on DO | DOSE package in R | 1092 | ||
| Similarities based on KEGG | KEGG | 1132 | ||
| Drug-disease | __ | Therapeutic Target Database (TTD) 12 | Drugs: 6931 | Drugs: 7382 |
| Diseases: 1418 | ||||
| KEGG | Drugs: 1052 | Diseases: 1970 | ||
| Diseases: 592 | ||||
| Drug-target | __ | DrugBank 13 | Drugs: 1521 | Drugs: 3350 |
| Targets: 1346 | ||||
| KEGG | Drugs: 2440 | Targets: 1415 | ||
| Targets: 335 | ||||
| Disease-target | __ | DisGeNET | Diseases: 577 | Diseases: 1838 |
| Targets: 2403 | ||||
| KEGG | Diseases: 1271 | Targets: 4066 | ||
| Targets: 2563 | ||||
| Mastitis-Associated Genes | Reference | Technique |
|---|---|---|
| CXCL2, CXCL8, GRO1, CFB, ZC3H12A, CCL20, NFKBIZ, S100A9, S100A8, PDE4B, CASP4, HP | [14] | meta-analysis of microarray data |
| MAPK1, TP53 (p53), SP1, MAPK14, INS, EGF, AKT1, IFNG, MAPK3, MAPK8, VEGFA, MMP2, BCL2, IL10 | [26] | meta-analysis of microarray data |
| MMP9, IL18, GAPDH, CXCL8, IL6, IL1B, TLR2, GRO1, ICAM1, VCAM1, CXCL2, CCL20, CXCL6, IL8RB, IL1A, CCL3, CCL2, NFKBIA, IL1RN, TIMP1 | [27] | integration of three microarray datasets |
| BCL2,BNBD-9-LIKE, BOLA-RDA, C1S, C2,C3, C4BPA, C6, CCDC80, CCL20, CCL3, CCL4, CCL5, CCR5, CD14, CFB, CMTM8, COL17A1, COL1A2, COTL1, CRISPLD2, CXCL11, CXCL16, CYBA, DEFB10, DEFB4A, EGFLAM, FCER1G, FGL1, FGR, FMOD, FN1, HAPLN1, HMOX1, IL1A, IL1B, ITGB6, KERA, KIT, LAP, LBP, LOC504773, LOXL1, LOXL4, LPL, LPO, LTF, LUM, LYZ2, MFAP4, MFGE8, MSR1, MSTN, MYOC, NCF1, NFKBIZ, NOS2, NTN4, OGN, OLR1, ORM1, POSTN, PRELP, PRSS2, PTAFR, PTX3, PYCARD, RAB27A, RSAD2, S100A12, SAA3, SELP, SERPINA3-1, SERPINF1, SERPINF2, SRGN, TAP1, TFF3, TGFB2, THBS1, TLR2, VEGFC, VLDLR, VNN1 | [28] | meta-analysis of microarray data |
| Row | Drug or Antibiotic | Reference |
|---|---|---|
| 1 | Ampicillin | [19] |
| 2 | Aspirin | [29] |
| 3 | Ceftazidime | [19] |
| 4 | Cephalexin | [19] |
| 5 | Cephapirin (Cefoperazone, Ceftiofur, Cefquinome) | [18] |
| 6 | Chloramphenicol | [30] |
| 7 | Cinoxacin | [31] |
| 8 | Ciprofloxacin | [19,31] |
| 9 | Dexamethasone | [31] |
| 10 | DHS (dihydrostreptomycin sesquisulfate sa) | [19] |
| 11 | Flunixin meglumine | [32] |
| 12 | Fluoroquinolones (enrofloxacin, danofloxacin, marbofloxacin) | [18] |
| 13 | Gentamicin | [19,30] |
| 14 | Isoflupredone acetate | [29] |
| 15 | Ketoprofen | [19] |
| 16 | Meloxicam | [33] |
| 17 | Oxytetracycline | [34] |
| 18 | Penethamate hydriodide | [33] |
| 19 | Polymixin | [35] |
| 20 | Prednisolone | [36] |
| 21 | Tetracycline | [19] |
| 22 | Trimethoprim | [19] |
| 23 | Sulfadoxine | [34] |
| 24 | Sulfamethoxazole | [30] |
| 25 | Sulfadiazine | [19] |
| Row | Drug | Ranking Score | Verification |
|---|---|---|---|
| 1 | Cefoperazone | 0.005000691 | Known drug |
| 2 | Meloxicam | 0.004998696 | Known drug |
| 3 | Cephapirin | 0.003363298 | Known drug |
| 4 | Cephalexin | 0.003362269 | Known drug |
| 5 | Oxytetracycline | 0.003352667 | Known drug |
| 6 | Cinoxacin | 0.003351841 | Known drug |
| 7 | Ketoprofen | 0.003350183 | Known drug |
| 8 | Aspirin | 0.002526886 | Known drug |
| 9 | Ampicillin | 0.001301824 | Known drug |
| 10 | Ceftazidime | 0.001164398 | Known drug |
| 11 | Tetracycline | 0.001162658 | Known drug |
| 12 | Chloramphenicol | 0.000958009 | Known drug |
| 13 | Gentamicin | 0.000937666 | Known drug |
| 14 | Ciprofloxacin | 0.000680685 | Known drug |
| 15 | Dexamethasone | 0.000618516 | Known drug |
| 16 | Prednisolone | 0.000513524 | Known drug |
| 17 | Penicillin G | 8.63 × 10−5 | New drug |
| 18 | Leucovorin | 8.19 × 10−5 | New drug |
| 19 | Rifampicin | 7.91 × 10−5 | New drug |
| 20 | Cefprozil | 7.87 × 10−5 | New drug |
| 21 | Ipratropium | 7.81 × 10−5 | New drug |
| 22 | Cefadroxil | 7.77 × 10−5 | New drug |
| 23 | Clidinium | 7.66 × 10−5 | New drug |
| 24 | Lopinavir | 7.64 × 10−5 | New drug |
| 25 | Glibenclamide | 7.61 × 10−5 | New drug |
| 26 | Thyroxine | 7.57 × 10−5 | New drug |
| 27 | Salbutamol | 7.55 × 10−5 | New drug |
| 28 | Carbidopa | 7.51 × 10−5 | New drug |
| 29 | Benzquinamide | 7.50 × 10−5 | New drug |
| 30 | Diethylpropion | 7.49 × 10−5 | New drug |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharifi, S.; Lotfi Shahreza, M.; Pakdel, A.; Reecy, J.M.; Ghadiri, N.; Atashi, H.; Motamedi, M.; Ebrahimie, E. Systems Biology–Derived Genetic Signatures of Mastitis in Dairy Cattle: A New Avenue for Drug Repurposing. Animals 2022, 12, 29. https://doi.org/10.3390/ani12010029
Sharifi S, Lotfi Shahreza M, Pakdel A, Reecy JM, Ghadiri N, Atashi H, Motamedi M, Ebrahimie E. Systems Biology–Derived Genetic Signatures of Mastitis in Dairy Cattle: A New Avenue for Drug Repurposing. Animals. 2022; 12(1):29. https://doi.org/10.3390/ani12010029
Chicago/Turabian StyleSharifi, Somayeh, Maryam Lotfi Shahreza, Abbas Pakdel, James M. Reecy, Nasser Ghadiri, Hadi Atashi, Mahmood Motamedi, and Esmaeil Ebrahimie. 2022. "Systems Biology–Derived Genetic Signatures of Mastitis in Dairy Cattle: A New Avenue for Drug Repurposing" Animals 12, no. 1: 29. https://doi.org/10.3390/ani12010029
APA StyleSharifi, S., Lotfi Shahreza, M., Pakdel, A., Reecy, J. M., Ghadiri, N., Atashi, H., Motamedi, M., & Ebrahimie, E. (2022). Systems Biology–Derived Genetic Signatures of Mastitis in Dairy Cattle: A New Avenue for Drug Repurposing. Animals, 12(1), 29. https://doi.org/10.3390/ani12010029







