Effects of Dietary Resveratrol Supplementation on Growth Performance and Anti-Inflammatory Ability in Ducks (Anas platyrhynchos) through the Nrf2/HO-1 and TLR4/NF-κB Signaling Pathways
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethics Approval
2.3. Ducks and Husbandry
2.4. Sample Collection
2.5. Histopathological Analysis of Liver
2.6. Transmission Electron Microscopy (TEM)
2.7. Assay of the Antioxidant Levels of the Plasma and the Liver
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Effect of Dietary RES on the Growth Performance in Ducks
3.2. Effect of Dietary RES on the Plasma Biochemistry in Ducks
3.3. Effect of Dietary RES on the Expression of Inflammatory Cytokines in Systemic and Duck Liver
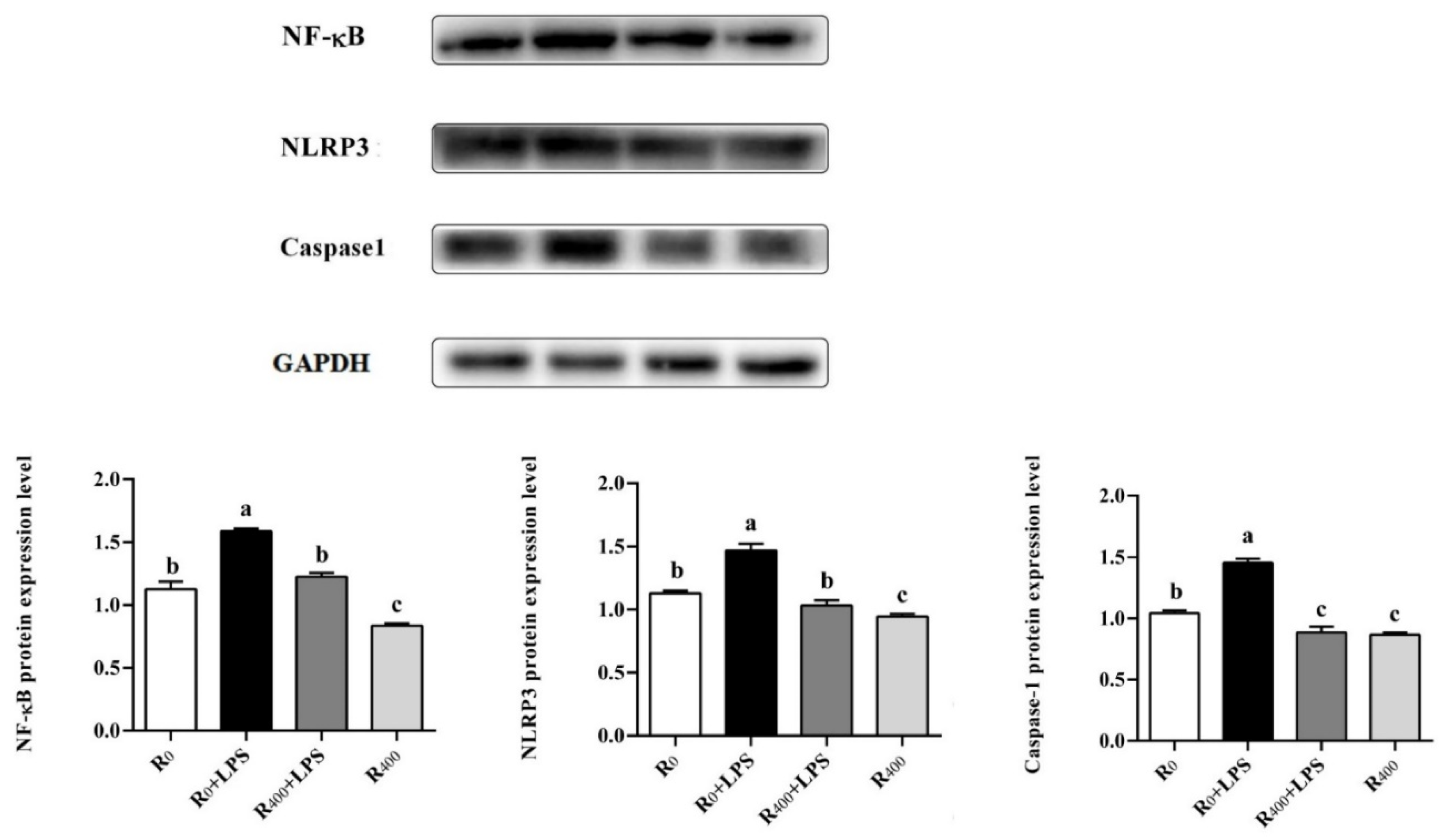
3.4. Effect of Dietary RES on Regulation of TLR4/NF-κB Signaling Pathway
3.5. Effect of Dietary RES on Regulation of Nrf2/HO-1 Signaling Pathway and Anti-Oxidase Activity in Duck
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakutis, B.; Monstviliene, E.; Januskeviciene, G. Analyses of airborne contamination with bacteria, endotoxins and dust in livestock barns and poultry houses. Acta Vet. Brno. 2004, 73, 283–289. [Google Scholar] [CrossRef][Green Version]
- Wu, B.; Qin, L.; Wang, M.; Zhou, T.; Dong, Y.; Chai, T. The composition of microbial aerosols, PM2.5, and PM10 in a duck house in Shandong province, China. Poult. Sci. 2019, 98, 5913–5924. [Google Scholar] [CrossRef]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, G.; Huang, X.; Li, C.; Huang, X.; Zhang, X.; Lin, Q.; Liu, S.; Dai, Q. Evaluation of serum antioxidative status, immune status and intestinal condition of linwu duck challenged by lipopolysaccharide with various dosages and replications. Poult. Sci. 2021, 100, 101199. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Wick, M.; Lilburn, M.S. Effect of embryonic thermal manipulation on heat shock protein 70 (HSP70) expression and subsequent immune response to post-hatch lipopolysaccharide challenge in Pekin ducklings. Poult. Sci. 2019, 98, 722–733. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Jin, S.; Pang, Q.; Shan, A.; Feng, X. Dietary resveratrol alleviated lipopolysaccharide-induced ileitis through Nrf2 and NF-kappaB signalling pathways in ducks (Anas platyrhynchos). Anim. Physiol. Anim. Nutr. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, H.; Jiao, Y.; Pang, Q.; Wang, Y.; Wang, M.; Shan, A.; Feng, X. Dietary curcumin alleviated acute ileum damage of ducks (anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef]
- Jin, S.; Pang, Q.; Yang, H.; Diao, X.; Feng, X. Effects of dietary resveratrol supplementation on the chemical composition, oxidative stability and meat quality of ducks (anas platyrhynchos). Food Chem. 2021, 363, 130263. [Google Scholar] [CrossRef]
- He, S.; Li, S.; Arowolo, M.A.; Yu, Q.; Chen, F.; Hu, R.; He, J. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim. Sci. J. 2019, 90, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Luo, P.; Chen, S.; Deng, Z.; Fu, X.; Xu, D.; Tian, Y.; Huang, Y.; Liu, W. Resveratrol sustains intestinal barrier integrity, improves antioxidant capacity, and alleviates inflammation in the jejunum of ducks exposed to acute heat stress. Poult. Sci. 2021, 100, 101459. [Google Scholar] [CrossRef] [PubMed]
- Tafti, Z.A.; Mahmoodi, M.; Hajizadeh, M.R.; Ezzatizadeh, V.; Piryaei, A. Conditioned media derived from human adipose tissue mesenchymal stromal cells improves primary hepatocyte maintenance. Cell J. 2021, 23, 143–144. [Google Scholar] [CrossRef]
- Kim, D.; Mun, S.; Lee, J.; Park, A.; Seok, A.; Chun, Y.T.; Kang, H.G. Proteomics analysis reveals differential pattern of widespread protein expression and novel role of histidine-rich glycoprotein and lipopolysaccharide-binding protein in rheumatoid arthritis. Int. J. Biol. Macromol. 2018, 109, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Wei, H.; Hagen, T.; Frei, B. α-Lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, Z.; Chen, Y.; Zhang, Y.; Rong, G.; Mu, C.; Xu, Q.; Chen, G. Molecular cloning and functional analysis of the duck TLR4 gene. Int. J. Mol. Sci. 2013, 14, 18615–18628. [Google Scholar] [CrossRef]
- Brown, K.; Park, S.; Kanno, T.; Franzoso, G.; Siebenlist, U. Mutual regulation of the transcriptional activator NF-κB and its inhibitor, IκB-α. Proc. Natl. Acad. Sci. USA 1993, 90, 2532–2536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, B.; Cao, S.; Wang, Y.; Wu, D. Silybin attenuates LPS-induced lung injury in mice by inhibiting NF-κB signaling and NLRP3 activation. Int. J. Mol. Med. 2017, 39, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hou, L.; Sun, J.; Zeng, B.; Xi, Q.; Luo, J.; Chen, T.; Zhang, Y. Porcine, milk exosome miRNAs attenuate LPS-Induced apoptosis through inhibiting TLR4/NF-κB and p53 pathways in intestinal epithelial cells. Agric. Food Chem. 2019, 67, 9477–9491. [Google Scholar] [CrossRef]
- Shanmugam, N.; Reddy, M.A.; Guha, M.; Natarajan, R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 2003, 52, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.M.; Xing, H.J.; Cai, J.Z.; Zhang, H.F.; Xu, S.W. H2S exposure-induced oxidative stress promotes LPS-mediated hepatocyte autophagy through the PI3K/AKT/TOR pathway. Ecotoxicol. Environ. Saf. 2020, 209, 111801. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, X.; Tian, X.; Yang, M.; Li, X. Procyanidin B2 promotes intestinal injury repair and attenuates colitis-associated tumorigenesis suppression of oxidative stress in mice. Antioxid. Redox Signal. 2020, 35, 75–92. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Q.; Li, Y.; Zhang, Y.; Wu, Y. MitoQ modulates lipopolysaccharide-induced intestinal barrier dysfunction via regulating Nrf2 signaling. Mediat. Inflamm. 2020, 1-9, 3276148. [Google Scholar] [CrossRef]
- Tong, W.; Chen, X.; Song, X.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; He, C.; Liang, X.; et al. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp. Ther. Med. 2020, 19, 1824–1834. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, T.; Li, J.H.; Miao, S.Y.; Xiao, X.Z. The effects of resveratrol on inflammation and oxidative stress in a rat model of chronic obstructive pulmonary disease. Molecules 2017, 22, 1529. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, D.; Liu, K.; Hou, L.; Zhang, W. Systematic review and meta-analysis of the protective effect of resveratrol on multiple organ injury induced by sepsis in animal models. Biomed. Rep. 2019, 10, 55–62. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yun, S.M.; Song, M.Y.; Jung, K.; Kim, E.H. Cyanidin chloride induces apoptosis by inhibiting NF-kappaB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants 2020, 9, 285. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Shan, A.; Feng, X. Avian host defense cathelicidins: Structure, expression, biological functions, and potential therapeutic applications. Poult. Sci. 2020, 99, 6434–6445. [Google Scholar] [CrossRef]
- Bai, H.; Bao, Q.; Zhang, Y.; Song, Q.; Liu, B.; Zhong, L.; Zhang, X.; Wang, Z.; Jiang, Y.; Xu, Q.; et al. Research note: Effects of the rearing method and stocking density on carcass traits and proximate composition of meat in small-sized meat ducks. Poult. Sci. 2020, 99, 2011–2016. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; McKinnon, J.; Wyrsch, E.; Hammond, J.M.; Charles, I.G.; Djordjevic, S.P. Genomic interplay in bacterial communities: Implications for growth promoting practices in animal husbandry. Front Microbiol. 2014, 5, 394. [Google Scholar] [CrossRef]
- Aksoy, L.; Kolay, E.; Ağılönü, Y.; Aslan, Z.; Kargıoğlu, M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci. 2013, 20, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Lançon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a chemoprotective agent: A promising molecule for fighting cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Z.; Yin, X.; Zheng, X.; Li, Q.; Zhang, H.; Zheng, L.; Feng, X. Resveratrol-induced brown fat-like phenotype in 3T3-L1 adipocytes partly via mTOR pathway. Food Nutr. Res. 2020, 64, 3656. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, F.; Li, Z.; Jin, X.; Zhang, C. Effects of resveratrol on growth performance, intestinal development, and antioxidant status of broilers under heat stress. Animals 2021, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Toschi, A.; Piva, A.; Grilli, E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr. Res. Rev. 2020, 33, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Reddavide, R.; Cisternino, A.M.; Inguaggiato, R.; Rotolo, O.; Zinzi, I.; Veronese, N.; Guerra, V.; Fucilli, F.; Di Giovanni, G.; Leandro, G. Non-alcoholic fatty liver disease is associated with higher metabolic expenditure in overweight and obese subjects: A case-control study. Nutrients 2019, 11, 1830. [Google Scholar] [CrossRef]
- Hadfield, J.M.; Bowdridge, E.C.; Holásková, I.; Elsasser, T.H.; Dailey, R.A. Breed-specific differences in the immune response to lipopolysaccharide in ewes. J. Anim. Sci. 2018, 96, 4220–4228. [Google Scholar] [CrossRef] [PubMed]
- Pietro, C.; Gianni, T.; Serge, M.; Roberto, F.; Antonio, P.; Marilena, R.; Caterina, F.; Luisa, C.; Stefano, F.; Giacomo, G.; et al. Albumin replacement in patients with severe sepsis or septic shock. J. Emerg. Med. 2014, 47, 257–258. [Google Scholar] [CrossRef]
- Islam, M.S.; Miao, L.; Yu, H.; Han, Z.; Sun, H. Ethanol extract of illicium henryi attenuates LPS-Induced acute kidney injury in mice via regulating inflammation and oxidative stress. Nutrients 2019, 11, 1412. [Google Scholar] [CrossRef]
- Chao, H.; Yue, S.; Qian, Z. Current understanding of inflammatory responses in acute kidney injury. Curr. Gene Ther. 2017, 17, 405–410. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Qi, X.; Zhang, X.; Ren, K. Resveratrol protects against post-contrast acute kidney injury in aabbits with diabetic nephropathy. Front Pharmacol. 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.N.; Li, Y.; Jia, P.L.; Ji, S.L.; Chen, Y.P.; Wang, T. Protective effects of pterostilbene against hepatic damage, redox imbalance, mitochondrial dysfunction, and endoplasmic reticulum stress in weanling piglets. J. Anim. Sci. 2020, 98, skaa328. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Wang, L.Y.; Liu, G.H.; Tang, D.Z.; Fan, X.X.; Zhao, J.P.; Jiao, H.C.; Wang, X.J.; Sun, S.H.; Lin, H. Leucine alters immunoglobulin a secretion and inflammatory cytokine expression induced by lipopolysaccharide via the nuclear factor-κb pathway in intestine of chicken embryos. Animal 2018, 12, 1903–1911. [Google Scholar] [CrossRef]
- Kayisoglu, O.; Weiss, F.; Niklas, C.; Pierotti, I.; Bartfeld, S. Location-specific cell identity rather than exposure to GI microbiota defines many innate immune signalling cascades in the gut epithelium. Gut 2020, 70, 687–697. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef]
- Thi, T.V.; Hyoung-Kyu, K.; Long, L.T.; Sung-Ryul, L.; My, H.T.; Hee, K.T.; Hye-Jin, H.; Nari, K.; Ha, K.S.; Soo, K.K. NecroX-5 prevents hypoxia/reoxygenation injury by inhibiting the mitochondrial calcium uniporter. Cardiovasc. Res. 2012, 94, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Limon-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. 2019, 674, 137–147. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Wen, Y.; Li, B.; Zhao, Y.; Xing, J.; Chen, Y. Nrf2 inhibits periodontal ligament stem cell apoptosis under excessive oxidative stress. Int. J. Mol. Sci. 2017, 18, 1076. [Google Scholar] [CrossRef]
- Teixeira, T.M.; Costa, D.C.D.; Resende, A.C.; Soulage, C.O.; Bezerra, F.F.; Daleprane, J.B. Activation of nrf2-antioxidant signaling by 1,25-dihydroxycholecalciferol prevents leptin-induced oxidative stress and inflammation in human endothelial cells. J. Nutr. 2017, 147, jn239475. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Y.J.; Liu, W.W.; Shi, A.W.; Ning, G. Salidroside suppresses huvecs cell injury induced by oxidative stress through activating the nrf2 signaling pathway. Molecules 2016, 21, 1033. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Xing, T.; Pan, X.; Zhang, L.; Gao, F. Hepatic Oxidative Stress, Apoptosis, and Inflammation in Broiler Chickens With Wooden Breast Myopathy. Front Physiol. 2021, 12, 659777. [Google Scholar] [CrossRef]
- Kang, E.S.; Kim, G.H.; Kim, H.J.; Woo, I.S.; Ham, S.A.; Jin, H.; Kim, M.Y.; Kim, H.J.; Lee, J.H.; Chang, K.C.; et al. Nrf2 regulates curcumin-induced aldose reductase expression indirectly via nuclear factor-kappa B. Int. J. Mol. Med. 2008, 22, 349. [Google Scholar] [CrossRef]
- Banning, A.; Brigelius-Flohé, R. NF-kappaB, Nrf2, and HO-1 interplay in redox-regulated VCAM-1 expression. Antioxid. Redox Signal. 2005, 7, 889–899. [Google Scholar] [CrossRef] [PubMed]

| Experimental Groups | Basal Diet | RES (mg/kg Basal Diet) | Number Ducks per Treatment Diet |
|---|---|---|---|
| Growth performance experiment R0 | Corn-soybean | 0 | 140 |
| R400 | Corn-soybean | 400 | 140 |
| Pathological experiment | |||
| R0 | Corn-soybean | 0 | 8 |
| R0 + LPS | Corn-soybean | 0 | 8 |
| R400 + LPS | Corn-soybean | 400 | 8 |
| R400 | Corn-soybean | 400 | 8 |
| Items | Groups | SEM | p-Value | |
|---|---|---|---|---|
| R0 | R400 | |||
| Initial BW (g) | 35.13 ± 0.41 | 35.12 ± 0.36 | 0.37 | 0.754 |
| Final BW (g) | 647.83 ± 20.20 | 661.43 ± 15.09 | 9.41 | 0.005 |
| ADFI (g/day) | 61.08 ± 1.89 | 59.61 ± 1.63 | 1.69 | 0.727 |
| ADG (g/day) | 21.88 ± 0.27 | 22.37 ± 0.19 | 0.33 | 0.005 |
| F/G (g/g) | 2.79 ± 0.10 | 2.66 ± 0.08 | 0.97 | 0.182 |
| Items | Groups | |||
|---|---|---|---|---|
| R0 | R0 + LPS | R400 + LPS | R400 | |
| TP (g/L) | 30.60 ± 4.45 | 29.40 ± 4.61 | 33.60 ± 4.60 | 31.40 ± 2.19 |
| ALB (g/L) | 21.04 ± 0.66 a | 17.52 ± 0.85 b | 19.56 ± 2.18 a | 21.12 ± 1.87 a |
| GLOB (g/L) | 13.02 ± 1.32 | 12.38 ± 1.20 | 14.74 ± 1.89 | 13.66 ± 1.43 |
| A/G | 1.46 ± 0.08 a | 1.19 ± 0.09 c | 1.34 ± 0.10 b | 1.49 ± 0.09 a |
| TB (μmol/L) | 1.14 ± 0.24 b | 1.66 ± 0.21 a | 1.32 ± 0.21 b | 1.17 ± 0.13 b |
| ALP (KDa) | 478.8 ± 31.2 | 470.2 ± 32.37 | 525.4 ± 48.39 | 503.4 ± 41.03 |
| ALT (U/L) | 32.80 ± 0.98 b | 40.00 ± 6.06 a | 34.40 ± 2.42 b | 30.60 ± 2.49 c |
| AST (U/L) | 31.50 ± 0.98 b | 40.80 ± 3.44 a | 34.00 ± 2.44 b | 31.80 ± 4.78 b |
| Cr (μmol/L) | 22.00 ± 4.05 b | 28.60 ± 4.03 a | 23.60 ± 2.06 b | 20.06 ± 3.92 b |
| BUN (mmol/L) | 0.18 ± 0.07 b | 0.40 ± 0.06 a | 0.24 ± 0.05 b | 0.22 ± 0.04 b |
| Items | Groups | |||
|---|---|---|---|---|
| R0 | R0 + LPS | R400 + LPS | R400 | |
| The serum contents of inflammatory factors | ||||
| IL-1β (pg/mL) | 23.51 ± 3.12 b | 31.14 ± 0.73 a | 21.08 ± 1.47 b | 17.12 ± 1.03 c |
| IL-6 (pg/mL) | 123.24 ± 7.21 b | 159.65 ± 4.22 a | 128.94 ± 6.85 b | 115.10 ± 8.39 c |
| TNF-α (pg/mL) | 63.08 ± 9.73 b | 76.35 ± 5.01 a | 59.27 ± 3.19 b | 50.36 ± 3.69 c |
| The liver contents of inflammatory factors | ||||
| IL-1β (pg/mg protein) | 2.26 ± 0.17 b | 2.96 ± 0.37 a | 2.58 ± 0.58 b | 1.83 ± 0.32 c |
| IL-6 (pg/mg protein) | 7.38 ± 0.31 c | 8.96 ± 0.29 a | 8.18 ± 0.48 b | 5.69 ± 0.45 d |
| TNF-α (pg/mg protein) | 61.84 ± 4.05 b | 69.18 ± 4.65 a | 65.38 ± 2.56 b | 50.36 ± 3.69 c |
| Items | Groups | |||
|---|---|---|---|---|
| R0 | R0 + LPS | R400 + LPS | R400 | |
| TLR4 | 1.01 ± 0.16 b | 1.63 ± 0.51 a | 0.85 ± 0.12 b | 0.79 ± 0.13 b |
| NF-κB | 1.00 ± 0.04 b | 1.78 ± 0.50 a | 1.09 ± 0.11 b | 0.75 ± 0.18 b |
| p53 | 1.01 ± 0.18 b | 3.06 ± 1.07 a | 1.20 ± 0.20 b | 0.85 ± 0.25 b |
| NLRP3 | 1.06 ± 0.13 b | 1.70 ± 0.23 a | 1.10 ± 0.40 b | 0.85 ± 0.08 b |
| TXNIP | 1.01 ± 0.14 b | 1.43 ± 0.38 a | 0.81 ± 0.13 b | 0.68 ± 0.16 c |
| Caspase-1 | 1.00 ± 0.09 b | 1.31 ± 0.37 a | 0.92 ± 0.14 b | 0.65 ± 0.12 c |
| IL-6 | 1.02 ± 0.23 b | 1.57 ± 0.40 a | 0.83 ± 0.17 b | 0.63 ± 0.13 c |
| TNF-α | 1.01 ± 0.11 b | 1.88 ± 0.67 a | 1.12 ± 0.14 b | 0.59 ± 0.19 c |
| Items | Groups | |||
|---|---|---|---|---|
| R0 | R0 + LPS | R400 + LPS | R400 | |
| Nrf2 | 1.00 ± 0.10 b | 0.70 ± 0.16 c | 1.02 ± 0.10 b | 1.48 ± 0.26 a |
| Keap1 | 1.02 ± 0.23 b | 1.65 ± 0.32 a | 1.28 ± 0.41 b | 0.95 ± 0.16 b |
| HO-1 | 1.00 ± 0.08 b | 0.86 ± 0.17 c | 1.24 ± 0.33 b | 1.86 ± 0.52 a |
| SOD1 | 1.05 ± 0.20 b | 0.63 ± 0.16 b | 0.86 ± 0.28 b | 1.92 ± 0.52 a |
| GCLC | 1.01 ± 0.19 b | 0.89 ± 0.26 b | 1.28 ± 0.23 a | 1.26 ± 0.26 a |
| GCLM | 1.01 ± 0.11 b | 0.78 ± 0.16 c | 1.16 ± 0.18 a | 1.33 ± 0.31 a |
| Items | Groups | |||
|---|---|---|---|---|
| R0 | R0 + LPS | R400 + LPS | R400 | |
| GST (U/mg protein) | 18.14 ± 1.46 c | 23.61 ± 1.40 a | 20.11 ± 2.02 b | 16.91 ± 0.82 c |
| GSH-PX (U/mg protein) | 383.38 ± 27.40 b | 311.63 ± 51.87 c | 342.97 ± 33.51 b | 422.38 ± 54.62 a |
| T-SOD (U/mg protein) | 375.53 ± 39.38 a | 330.26 ± 11.94 c | 302.03 ± 22.77 b | 381.01 ± 43.64 a |
| MDA (nmol/mg) | 1.06 ± 0.14 c | 1.40 ± 0.05 a | 1.44 ± 0.10 b | 1.12 ± 0.34 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wang, Y.; Liu, M.; Liu, X.; Jiao, Y.; Jin, S.; Shan, A.; Feng, X. Effects of Dietary Resveratrol Supplementation on Growth Performance and Anti-Inflammatory Ability in Ducks (Anas platyrhynchos) through the Nrf2/HO-1 and TLR4/NF-κB Signaling Pathways. Animals 2021, 11, 3588. https://doi.org/10.3390/ani11123588
Yang H, Wang Y, Liu M, Liu X, Jiao Y, Jin S, Shan A, Feng X. Effects of Dietary Resveratrol Supplementation on Growth Performance and Anti-Inflammatory Ability in Ducks (Anas platyrhynchos) through the Nrf2/HO-1 and TLR4/NF-κB Signaling Pathways. Animals. 2021; 11(12):3588. https://doi.org/10.3390/ani11123588
Chicago/Turabian StyleYang, Hao, Yingjie Wang, Mengru Liu, Xiao Liu, Yihan Jiao, Sanjun Jin, Anshan Shan, and Xingjun Feng. 2021. "Effects of Dietary Resveratrol Supplementation on Growth Performance and Anti-Inflammatory Ability in Ducks (Anas platyrhynchos) through the Nrf2/HO-1 and TLR4/NF-κB Signaling Pathways" Animals 11, no. 12: 3588. https://doi.org/10.3390/ani11123588
APA StyleYang, H., Wang, Y., Liu, M., Liu, X., Jiao, Y., Jin, S., Shan, A., & Feng, X. (2021). Effects of Dietary Resveratrol Supplementation on Growth Performance and Anti-Inflammatory Ability in Ducks (Anas platyrhynchos) through the Nrf2/HO-1 and TLR4/NF-κB Signaling Pathways. Animals, 11(12), 3588. https://doi.org/10.3390/ani11123588






