Dynamics of Known Long Non-Coding RNAs during the Maternal-to-Zygotic Transition in Rabbit
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Manipulation and Sample Collection
2.3. RNA Sequencing and Data Processing
2.4. Mapping, Filtering, and Quantification
2.5. Screening of DElncRNAs
2.6. Co-Expresssion Analysis of mRNA and lncRNA
2.7. Validation of DE lncRNAs Using RT-qPCR
2.8. Statistical Analysis
3. Results
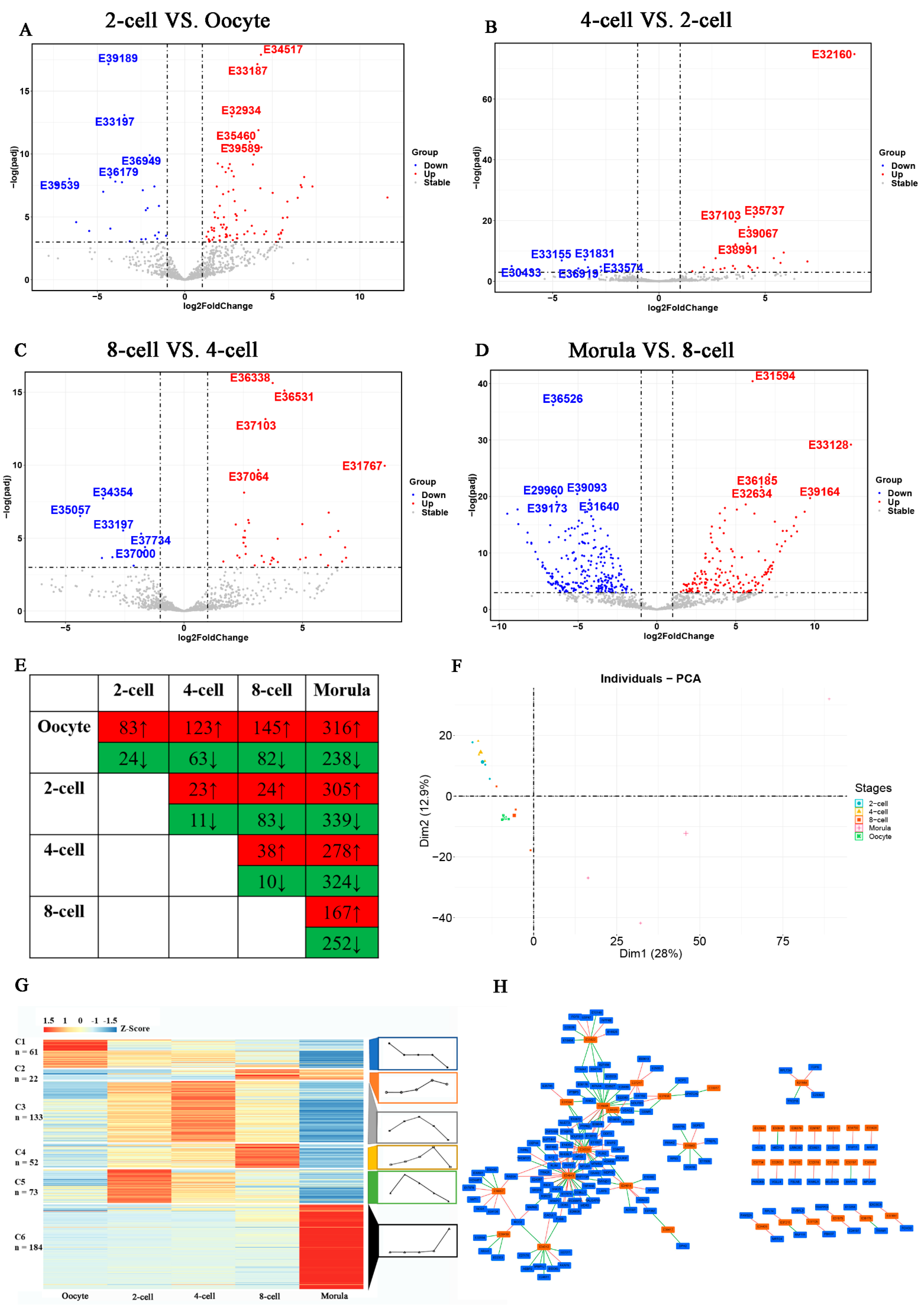
3.1. Temporal Expression Profile of lncRNA during Rabbit Pre-Implantation Development
3.2. Maternal lncRNAs Degradation
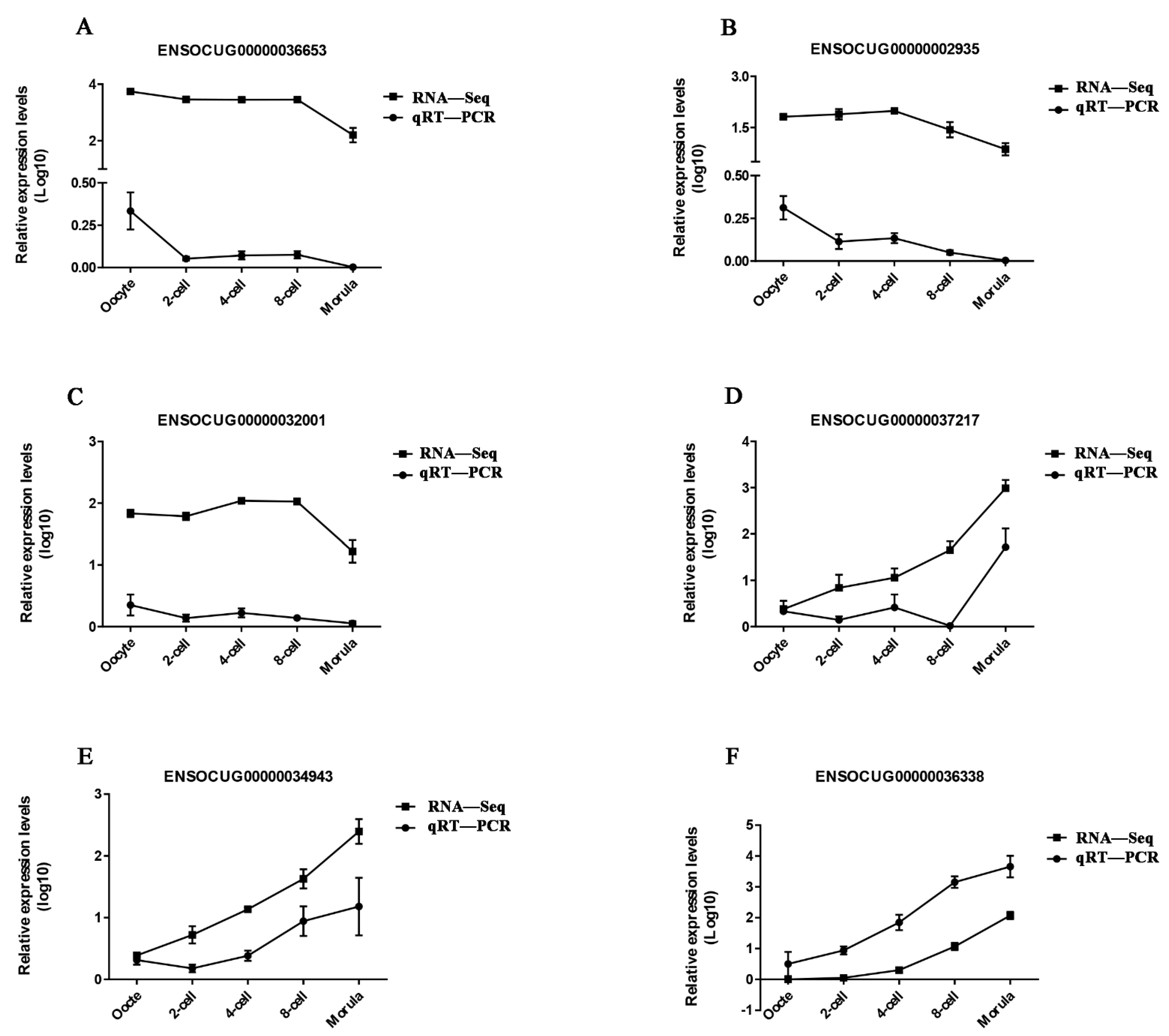
3.3. Validation of DE lncRNAs by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, S.; Liang, X.; Ren, X.; Shi, Y.; Su, H.; Li, Y.; Du, K.; Wang, J.; Jia, X.; Chen, S.; et al. Integrated Analysis of mRNA and miRNA Expression Profiles in the Ovary of Oryctolagus cuniculus in Response to Gonadotrophic Stimulation. Front. Endocrinol. 2019, 10, 744. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Fasolo, F.; Di Gregoli, K.; Maegdefessel, L.; Johnson, J.L. Non-coding RNAs in cardiovascular cell biology and atherosclerosis. Cardiovasc. Res. 2019, 115, 1732–1756. [Google Scholar] [CrossRef]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 2018, 39, 2704–2716. [Google Scholar] [CrossRef] [Green Version]
- Laubichler, M.D.; Davidson, E.H. Boveri’s long experiment: Sea urchin merogones and the establishment of the role of nuclear chromosomes in development. Dev. Biol. 2008, 314, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hamatani, T.; Carter, M.G.; Sharov, A.A.; Ko, M.S. Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell. 2004, 6, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Léandri, R.D.; Archilla, C.; Bui, L.C.; Peynot, N.; Liu, Z.; Cabau, C.; Chastellier, A.; Renard, J.P.; Duranthon, V. Revealing the dynamics of gene expression during embryonic genome activation and first differentiation in the rabbit embryo with a dedicated array screening. Physiol. Genom. 2009, 36, 98–113. [Google Scholar] [CrossRef]
- Tang, F.; Kaneda, M.; O’Carroll, D.; Hajkova, P.; Barton, S.C.; Sun, Y.A.; Lee, C.; Tarakhovsky, A.; Lao, K.; Surani, M.A. Maternal microRNAs are essential for mouse zygotic development. Genes. Dev. 2007, 21, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Lin, J.; Liu, M.; Li, R.; Tian, B.; Zhang, X.; Xu, B.; Liu, M.; Zhang, X.; Li, Y.; et al. Highly sensitive sequencing reveals dynamic modifications and activities of small RNAs in mouse oocytes and early embryos. Sci. Adv. 2016, 2, e1501482. [Google Scholar] [CrossRef] [Green Version]
- Sahakyan, A.; Yang, Y.; Plath, K. The Role of Xist in X-Chromosome Dosage Compensation. Trends Cell Biol. 2018, 28, 999–1013. [Google Scholar] [CrossRef]
- Monnier, P.; Martinet, C.; Pontis, J.; Stancheva, I.; Ait-Si-Ali, S.; Dandolo, L. H19 lncRNA controls gene expression of the Imprinted Gene Network by recruiting MBD1. Proc. Natl. Acad. Sci. USA 2013, 110, 20693–20698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hangauer, M.J.; Vaughn, I.W.; McManus, M.T. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013, 9, e1003569. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef]
- Managadze, D.; Lobkovsky, A.E.; Wolf, Y.I.; Shabalina, S.A.; Rogozin, I.B.; Koonin, E.V. The vast, conserved mammalian lincRNome. PLoS Comput. Biol. 2013, 9, e1002917. [Google Scholar] [CrossRef] [PubMed]
- Postepska-Igielska, A.; Giwojna, A.; Gasri-Plotnitsky, L.; Schmitt, N.; Dold, A.; Ginsberg, D.; Grummt, I. LncRNA Khps1 Regulates Expression of the Proto-oncogene SPHK1 via Triplex-Mediated Changes in Chromatin Structure. Mol. Cell. 2015, 60, 626–636. [Google Scholar] [CrossRef]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesana, M.; Cacchiarelli, D.; Legnini, I.; Santini, T.; Sthandier, O.; Chinappi, M.; Tramontano, A.; Bozzoni, I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011, 147, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrieri, C.; Cimatti, L.; Biagioli, M.; Beugnet, A.; Zucchelli, S.; Fedele, S.; Pesce, E.; Ferrer, I.; Collavin, L.; Santoro, C.; et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature 2012, 491, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Karlic, R.; Ganesh, S.; Franke, V.; Svobodova, E.; Urbanova, J.; Suzuki, Y.; Aoki, F.; Vlahovicek, K.; Svoboda, P. Long non-coding RNA exchange during the oocyte-to-embryo transition in mice. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2017, 24, 129–141. [Google Scholar]
- Yan, L.; Yang, M.; Guo, H.; Yang, L.; Wu, J.; Li, R.; Liu, P.; Lian, Y.; Zheng, X.; Yan, J.; et al. Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat. Struct. Mol. Biol. 2013, 20, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Dinnyés, A.; Tian, X.C.; Yang, X. Cloning of rabbits. In Principles of Cloning; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Picelli, S.; Björklund, Å.K.; Faridani, O.R.; Sagasser, S.; Winberg, G.; Sandberg, R. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 2013, 10, 1096–1098. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Dinger, M.E.; Amaral, P.P.; Mercer, T.R.; Pang, K.C.; Bruce, S.J.; Gardiner, B.B.; Askarian-Amiri, M.E.; Ru, K.; Soldà, G.; Simons, C.; et al. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 2008, 18, 1433–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, A.; Green, K.; Dawson, C.; Redrup, L.; Huynh, K.D.; Lee, J.T.; Hemberger, M.; Reik, W. Epigenetic dynamics of the Kcnq1 imprinted domain in the early embryo. Development 2006, 133, 4203–4210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Huang, K.; Luo, Y.; Li, S. Identification and functional analysis of long non-coding RNAs in mouse cleavage stage embryonic development based on single cell transcriptome data. BMC Genom. 2014, 15, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.Z.; Du, K.; Hu, S.Q.; Chen, S.Y.; Jia, X.B.; Cai, M.C.; Shi, Y.; Wang, J.; Lai, S.J. Genome-wide identification and characterization of long non-coding RNAs during postnatal development of rabbit adipose tissue. Lipids Health Dis. 2018, 17, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, L.; Lei, M.; Li, C.; Zhang, X.; Ren, Y.; Zheng, J.; Guo, Z.; Zhang, C.; Yang, C.; Mei, X.; et al. Identification of Long Non-Coding RNAs Related to Skeletal Muscle Development in Two Rabbit Breeds with Different Growth Rate. Int. J. Mol. Sci. 2018, 19, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Chen, Y.; Hu, S.; Yang, N.; Wang, M.; Liu, M.; Li, J.; Xiao, Y.; Wu, X. Systematic Analysis of Non-coding RNAs Involved in the Angora Rabbit (Oryctolagus cuniculus) Hair Follicle Cycle by RNA Sequencing. Front. Genet. 2019, 10, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manes, C. The participation of the embryonic genome during early cleavage in the rabbit. Dev. Biol. 1973, 32, 453–459. [Google Scholar] [CrossRef]
- Christians, E.; Rao, V.H.; Renard, J.P. Sequential acquisition of transcriptional control during early embryonic development in the rabbit. Dev. Biol. 1994, 164, 160–172. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Liu, Y.; Wu, Q.; Li, D.; Zhang, L.; Wu, X.; Wang, R.; Zhang, D.; Gao, S.; Li, W. Long non-coding RNAs potentially function synergistically in the cellular reprogramming of SCNT embryos. BMC Genome 2018, 19, 631. [Google Scholar] [CrossRef]
- Bultman, S.J.; Gebuhr, T.C.; Pan, H.; Svoboda, P.; Schultz, R.M.; Magnuson, T. Maternal BRG1 regulates zygotic genome activation in the mouse. Genes Dev. 2006, 20, 1744–1754. [Google Scholar] [CrossRef]
- Li, L.; Lu, X.; Dean, J. The maternal to zygotic transition in mammals. Mol. Asp. Med. 2013, 34, 919–938. [Google Scholar] [CrossRef] [Green Version]
- Marchesini, M.; Ogoti, Y.; Fiorini, E.; Aktas Samur, A.; Nezi, L.; D’Anca, M.; Storti, P.; Samur, M.K.; Ganan-Gomez, I.; Fulciniti, M.T.; et al. ILF2 Is a Regulator of RNA Splicing and DNA Damage Response in 1q21-Amplified Multiple Myeloma. Cancer Cell 2017, 32, 88–100.e6. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, X.; Dang, Y.; Li, D.; Lu, G.; Chan, W.Y.; Leung, P.C.K.; Zhao, S.; Qin, Y.; Chen, Z.J. Long noncoding RNA HCP5 participates in premature ovarian insufficiency by transcriptionally regulating MSH5 and DNA damage repair via YB1. Nucleic Acids Res. 2020, 48, 4480–4491. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, X.; Wang, L.; Li, J.; Zhao, Y.; Bou, G.; Li, Y.; Jiao, G.; Shen, X.; Wei, R.; et al. A novel long intergenic noncoding RNA indispensable for the cleavage of mouse two-cell embryos. EMBO Rep. 2016, 17, 1452–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, Z.; Kageyama, S.; Aoki, F. Degradation of maternal mRNA in mouse embryos: Selective degradation of specific mRNAs after fertilization. Mol. Reprod Dev. 2005, 72, 281–290. [Google Scholar] [CrossRef]
- Bashirullah, A.; Halsell, S.R.; Cooperstock, R.L.; Kloc, M.; Karaiskakis, A.; Fisher, W.W.; Fu, W.; Hamilton, J.K.; Etkin, L.D.; Lipshitz, H.D. Joint action of two RNA degradation pathways controls the timing of maternal transcript elimination at the midblastula transition in Drosophila melanogaster. EMBO J. 1999, 18, 2610–2620. [Google Scholar] [CrossRef]
- Barckmann, B.; Simonelig, M. Control of maternal mRNA stability in germ cells and early embryos. Biochim. Biophys. Acta 2013, 1829, 714–724. [Google Scholar] [CrossRef]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef] [Green Version]
- Hillier, S.G. Gonadotropic control of ovarian follicular growth and development. Mol. Cell. Endocrinol. 2001, 179, 39–46. [Google Scholar] [CrossRef]
- Arias-Alvarez, M.; García-García, R.M.; Rebollar, P.G.; Gutiérrez-Adán, A.; López-Béjar, M.; Lorenzo, P.L. Ovarian response and embryo gene expression patterns after nonsuperovulatory gonadotropin stimulation in primiparous rabbits does. Theriogenology 2013, 79, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kon, H.; Hokao, R.; Shinoda, M. Fertilizability of Superovulated Eggs by Estrous Stage-independent PMSG/hCG Treatment in Adult Wistar-Imamichi Rats. Exp. Anim. 2014, 63, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.B.; Yang, L.L.; Zhang, T.T.; Wang, Q.; Yin, S.; Luo, S.M.; Shen, W.; Ge, Z.J.; Sun, Q.Y. Multiple superovulations alter histone modifications in mouse early embryos. Reprod. Camb. Engl. 2019, 157, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Smith, T.H.; Battle, S.L.; Ferrell, S.; Hawkins, R.D. Superovulation alters global DNA methylation in early mouse embryo development. Epigenetics 2019, 14, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Market-Velker, B.A.; Zhang, L.; Magri, L.S.; Bonvissuto, A.C.; Mann, M.R. Dual effects of superovulation: Loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum. Mol. Genet. 2010, 19, 36–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denomme, M.M.; Zhang, L.; Mann, M.R. Embryonic imprinting perturbations do not originate from superovulation-induced defects in DNA methylation acquisition. Fertil. Steril. 2011, 96, 734–738.e2. [Google Scholar] [CrossRef] [PubMed]
- Fortier, A.L.; McGraw, S.; Lopes, F.L.; Niles, K.M.; Landry, M.; Trasler, J.M. Modulation of imprinted gene expression following superovulation. Mol. Cell. Endocrinol. 2014, 388, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Huang, K.; Cai, C.; Cai, L.; Jiang, C.Y.; Feng, Y.; Liu, Z.; Zeng, Q.; Cheng, L.; Sun, Y.E.; et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 2013, 500, 593–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Cai, M.; Du, K.; Bai, X.; Tang, L.; Jia, X.; Chen, S.; Wang, J.; Lai, S. Dynamics of Known Long Non-Coding RNAs during the Maternal-to-Zygotic Transition in Rabbit. Animals 2021, 11, 3592. https://doi.org/10.3390/ani11123592
Shi Y, Cai M, Du K, Bai X, Tang L, Jia X, Chen S, Wang J, Lai S. Dynamics of Known Long Non-Coding RNAs during the Maternal-to-Zygotic Transition in Rabbit. Animals. 2021; 11(12):3592. https://doi.org/10.3390/ani11123592
Chicago/Turabian StyleShi, Yu, Mingcheng Cai, Kun Du, Xue Bai, Lipeng Tang, Xianbo Jia, Shiyi Chen, Jie Wang, and Songjia Lai. 2021. "Dynamics of Known Long Non-Coding RNAs during the Maternal-to-Zygotic Transition in Rabbit" Animals 11, no. 12: 3592. https://doi.org/10.3390/ani11123592
APA StyleShi, Y., Cai, M., Du, K., Bai, X., Tang, L., Jia, X., Chen, S., Wang, J., & Lai, S. (2021). Dynamics of Known Long Non-Coding RNAs during the Maternal-to-Zygotic Transition in Rabbit. Animals, 11(12), 3592. https://doi.org/10.3390/ani11123592





