Do Live Weight, Body Condition Score, Back Muscle or Back-Fat Reserves Create the Suspicion of Goats Infected with Eimeria or Trichostrongylids?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Flock Management
2.2. Sampling Procedure and Parasitological Methods
2.3. Statistical Evaluation
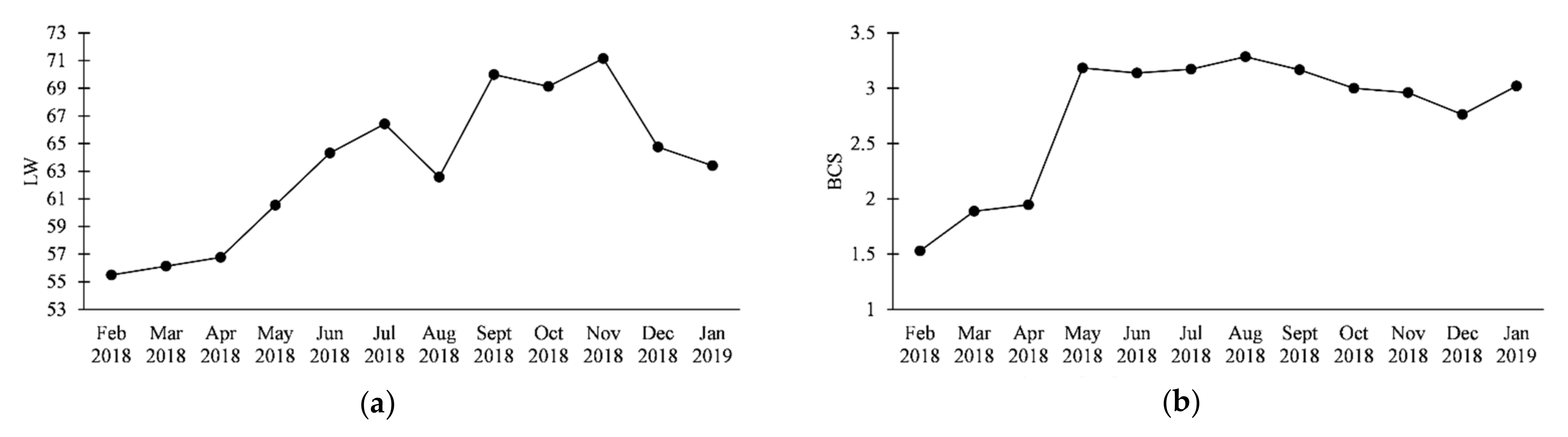
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chartier, C.; Paraud, C. Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin. Res. 2012, 103, 84–92. [Google Scholar] [CrossRef]
- Holm, S.A.; Sörensen, C.R.; Thamsborg, S.M.; Enemark, H.L. Gastrointestinal nematodes and anthelmintic resistance in Danish goat herds. Parasite 2014, 21, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriánová, I.A.; Kopecký, O.; Šlosárková, S.; Vadlejch, J. Comparison of internal parasitic fauna in dairy goats at conventional and organic farms in the Czech Republic. Small Rumin. Res. 2019, 175, 126–132. [Google Scholar] [CrossRef]
- Koudela, B.; Boková, A. Coccidiosis in goats in the Czech Republic. Vet. Parasitol. 1998, 76, 261–267. [Google Scholar] [CrossRef]
- Hoste, H.; Sotiraki, S.; Landau, S.Y.; Jackson, F.; Beveridge, I. Goat–Nematode interactions: Think differently. Trends Parasitol. 2010, 26, 376–381. [Google Scholar] [CrossRef]
- Hoste, H.; Sotiraki, S.; Torres-Acosta, J.F.J. Control of Endoparasitic Nematode Infections in Goats. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 163–173. [Google Scholar] [CrossRef]
- Goolsby, M.K.; Leite-Browning, M.L.; Browning, R., Jr. Evaluation of parasite resistence commonly used commercial anthelmintics in meat goats. J. Anim. Sci. 2016, 94 (Suppl. 2), S189–S190. [Google Scholar] [CrossRef]
- Kenyon, F.; Jackson, F. Targeted flock/herd and individual ruminant treatment approaches. Vet. Parasitol. 2012, 186, 10–17. [Google Scholar] [CrossRef]
- Fthenakis, G.C.; Papadopoulos, E. Impact of parasitism in goat production. Small Rumin. Res. 2018, 163, 21–23. [Google Scholar] [CrossRef]
- Bath, G.F.; van Wyk, J.A. The Five Point Check© for targeted selective treatment of internal parasites in small ruminants. Small Rumin. Res. 2009, 86, 6–13. [Google Scholar] [CrossRef]
- Cabaret, J.; Gonnord, V.; Cortet, J.; Sauvé, C.; Ballet, J.; Tournadre, H.; Benoit, M. Indicators for Internal Parasitic Infections in Organic Flocks: The Diarrhoea Score (Disco) Proposal for Lambs; Organic Farming and European Rural Development: Odense, Denmark, 2006; pp. 552–553. [Google Scholar]
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef]
- Odden, A.; Enemark, H.L.; Ruiz, A.; Robertson, L.J.; Ersdal, C.; Nes, S.K.; Tømmerberg, V.; Stuen, S. Controlled efficacy trial confirming toltrazuril resistance in a field isolate of ovine Eimeria spp. Parasites Vectors 2018, 11, 394. [Google Scholar] [CrossRef]
- Ptáček, M.; Milerski, M.; Schmidová, J.; Ducháček, J.; Tančin, V.; Uhrinčať, M.; Hakl, J.; Stádník, L. Relationship between body mass index, body energy reserves, milk, and meat production of original Wallachian sheep. Small Rumin. Res. 2018, 165, 131–133. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Maloney, S.K.; Blache, D. Review of sheep body condition score in relation to production characteristics. N. Z. J. Agric. Res. 2014, 57, 38–64. [Google Scholar] [CrossRef]
- Milerski, M. Metodika Prodádění Ultrazvukových Měření Zmasilosti a Protučnělosti Jehňat a Kůzlat; Praha Uhříněves: Prague, Czech Republic, 2007; pp. 1–12. [Google Scholar]
- Ptáček, M.; Ducháček, J.; Stádník, L.; Beran, J. Mutual relationships among body condition score, live weight, and backfat tissue development in meat sheep. Acta Vet. Brno 2014, 83, 341–346. [Google Scholar] [CrossRef] [Green Version]
- Chay-Canul, A.J.; Garcia-Herrera, R.A.; Ojeda-Robertos, N.F.; Marcias-Cruz, U.; Vicente-Pérez, R.; Meza-Villalvazo, V.M. Relation between body condition score and subcutaneous fat and muscle area measurement by ultrasound in Pelibuey ewes. Emir. J. Food Agric. 2019, 31, 53–58. [Google Scholar]
- Mendizabal, J.A.; Delfa, R.; Arana, A.; Purroy, A. Body condition score and fat mobilization as management tools for goats on native pastures. Small Rumin. Res. 2011, 98, 121–127. [Google Scholar] [CrossRef]
- Russel, A.J.F.; Doney, J.M.; Gunn, R.G. Subjective assessment of body fat in live sheep. J. Agric. Sci. 1969, 72, 451–454. [Google Scholar] [CrossRef]
- Hervieu, J.; Morand-Fehr, P.; Schmidely, P.; Fedele, V.; Delfa, R. Mesures anatomiques permettant d’expliquer les variations des notes sternales, lombaires et caudales utilisées pour estimer l’état corporel des chévres laitičres [Body measurements explaining variations in scores for the sternal, lumbar, and caudal regions used to estimate body condition in dairy goats]. Options Méditerranéennes Série A Séminaires Méditerranéens 1991, 13, 43–56. [Google Scholar]
- Cornelius, M.P.; Jacobson, C.; Besier, R.B. Body condition score as a selection tool for targeted selective treatment-based nematode control strategies in Merino ewes. Vet. Parasitol. 2014, 206, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Laurenson, Y.C.; Kahn, L.P.; Bishop, S.C.; Kyriazakis, I. Which is the best phenotypic trait for use in a targeted selective treatment strategy for growing lambs in temperate climates? Vet. Parasitol. 2016, 226, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Roepstorff, S.; Nansen, P. Epidemiology, Diagnosis and Control of Helminth Parasites of Swine 3; FAO: Rome, Italy, 1998; pp. 1–161. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Taylor, M.; Catchpole, J.; Marshall, R.; Norton, C.; Green, J. Eimeria species of sheep. In Biotechnology, Guidelines on Techniques in Coccidiosis Research. European Commission COST 89/820; Eckert, J., Braun, R., Shirley, M., Coudert, P., Eds.; Office for Official Publications of the European Communities: Luxembourg, 1995; pp. 25–39. [Google Scholar]
- Taylor, M.; Coop, R.; Wall, R. Veterinary Parasitology, 3rd ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 1–874. [Google Scholar]
- van Wyk, J.A.; Mayhew, E. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort J. Vet. Res. 2013, 80, 1–14. [Google Scholar] [CrossRef]
- Eknæs, M.; Kolstad, K.; Volden, H.; Hove, K. Changes in body reserves and milk quality throughout lactation in dairy goats. Small Rumin. Res. 2006, 63, 1–11. [Google Scholar] [CrossRef]
- Dønnem, I.; Eknæs, M.; Randby, Å.T. Energy status, measured by computer tomography (CT)-scanning, and milk quality of dairy goats fed rations with various energy concentrations. Lives. Sci. 2011, 142, 235–244. [Google Scholar] [CrossRef]
- Anwar, M.M.; Ramadan, T.A.; Taha, T.A. Serum metabolites, milk yield, and physiological responses during the first week after kidding in Anglo-Nubian, Angora, Baladi, and Damascus goats under subtropical conditions. J. Anim. Sci. 2012, 90, 4795–4806. [Google Scholar] [CrossRef]
- Ghosh, C.P.; Datta, S.; Mandal, D.; Das, A.K.; Roy, D.C.; Roy, A.; Tudu, N.K. Body condition scoring in goat: Impact and significance. J. Entomol. Zool. Stud. 2019, 7, 554–560. [Google Scholar]
- Houdijk, J.G.M.; Kyriazakis, I.; Kidane, A.; Athanasiadou, S. Manipulating small ruminant parasite epidemiology through the combination of nutritional strategies. Vet. Parasitol. 2012, 186, 38–50. [Google Scholar] [CrossRef]
- Leathwick, D.M.; Besier, R.B. The management of anthelmintic resistance in grazing ruminants in Australasia—Strategies and experiences. Vet. Parasitol. 2014, 204, 44–54. [Google Scholar] [CrossRef]
- Besier, R.B.; Love, R.J.; Lyon, J.; van Burgel, J. A targeted selective treatment approach for effective and sustainable sheep worm management: Investigations in Western Australia. Anim. Prod. Sci. 2010, 50, 1034–1042. [Google Scholar] [CrossRef]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangoura, B.; Bardsley, K.D. Ruminant Coccidiosis. Vet. Clin. N. Am. Food Anim. Prac. 2020, 36, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Besier, R.B. Targeted treatment strategies for sustainable worm control in small ruminants. Trop. Biomed. 2008, 25, 9–17. [Google Scholar] [PubMed]
- Bessell, P.R.; Sargison, N.D.; Mirende, K.; Dash, R.; Prasad, S.; Al-Riyami, L.; Gammon, N.; Stuke, K.; Wooley, R.; Barbaruah, M.; et al. The impact of anthelmintic drugs on weight gain of smallholder goats in subtropical regions. Prev. Vet. Med. 2018, 159, 72–81. [Google Scholar] [CrossRef]
- Torres-Acosta, J.F.J.; Hoste, H. Alternative or improved methods to limit gastro-intestinal parasitism in grazing sheep and goats. Small Rumin. Res. 2008, 77, 159–173. [Google Scholar] [CrossRef]

| February April | May July | August October | November December | |
|---|---|---|---|---|
| Grazing pasture | - | Ad libitum | Ad libitum | - |
| Concentrated supplement | 2 kg | 1.6 kg | 1.4 kg | - |
| Haylage | 6.1 kg | 1.5 kg | 1.5 kg | 6.1 kg |
| Hay | ad libitum | ad libitum | ad libitum | ad libitum |
| Mineral licks | ad libitum | ad libitum | ad libitum | ad libitum |
| Month | Eimeria sp. | Strongylid Nematodes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prev 1 (%) | GM 2 (OPG 7) | AM 3 (OPG 7) | Sd 4 (OPG 7) | Min 5 (OPG 7) | Max 6 (OPG 7) | Prev 1 (%) | GM 2 (EPG 8) | AM 3 (EPG 8) | Sd 4 (EPG 8) | Min 5 (EPG 8) | Max 6 (EPG 8) | |
| Feb | 80 | 72 | 71 | 63.8 | 0 | 200 | 93 | 243 | 329 | 276.7 | 0 | 1140 |
| Mar | 65 | 36 | 28 | 28.9 | 0 | 100 | 100 | 455 | 836 | 998.2 | 20 | 4380 |
| Apr | 65 | 60 | 49 | 52.8 | 0 | 180 | 100 | 826 | 1298 | 1297.7 | 80 | 3040 |
| May | 43 | 34 | 19 | 29.0 | 0 | 120 | 100 | 3063 | 4541 | 4541.4 | 120 | 13,740 |
| Jun | 78 | 95 | 105 | 138.1 | 0 | 640 | 100 | 4308 | 6264 | 6264.4 | 340 | 20,980 |
| Jul | 32 | 115 | 64 | 63.9 | 0 | 180 | 89 | 1902 | 2536 | 2535.7 | 0 | 10,220 |
| Aug | 14 | 79 | 14 | 41.5 | 0 | 200 | 100 | 1146 | 1837 | 1837.1 | 40 | 4660 |
| Sep | 63 | 60 | 49 | 60.1 | 0 | 220 | 100 | 707 | 1200 | 1239.5 | 20 | 5500 |
| Oct | 64 | 115 | 148 | 302.4 | 0 | 1420 | 100 | 389 | 654 | 665.6 | 20 | 2840 |
| Nov | 92 | 184 | 260 | 275.6 | 0 | 1100 | 96 | 364 | 542 | 541.7 | 0 | 1880 |
| Dec | 83 | 90 | 103 | 122.8 | 0 | 540 | 83 | 122 | 144 | 144.4 | 0 | 540 |
| Jan | 70 | 62 | 52 | 52.5 | 0 | 180 | 83 | 190 | 226 | 226.1 | 0 | 740 |
| Statistical Model of Partial Linear Regression | p-Value for Fixed Factors and for EIM as a Covariate in the Model | Pearson Partial Correlation (r) | |||
|---|---|---|---|---|---|
| Season 1 | Age 2 | Breed 3 | b*EIM 4 | ||
| LW 5 = 59.944 (±3.104) *** + 0.328 (± 3.703) × 10−3 × EIM n.s. | <0.0001 | 0.0016 | 0.0007 | 0.9296 | 0.116 n.s. |
| BCS 6 = 2.752 (±0.243) *** − 0.666 (± 0.308) × 10−3 × EIM * | <0.0001 | <0.0001 | 0.1529 | 0.0314 | −0.198 ** |
| MLLT 7 = 23.277 (±1.299) *** − 3.403 (± 1.665) × 10−3 × EIM * | <0.0001 | 0.0019 | 0.0027 | 0.0422 | −0.157 * |
| BT 8 = 3.151 (±0.209) *** − 0.772 (± 0.268) × 10−3 × EIM ** | <0.0001 | <0.0001 | 0.0015 | 0.0043 | −0.195 ** |
| Statistical Model of Partial Linear Regression | p-Value for Fixed Factors and for STR as a Covariate in the Model | Pearson Partial Correlation (r) | |||
|---|---|---|---|---|---|
| Season 1 | Age 2 | Breed 3 | b*STR 4 | ||
| LW 5 = 60.209 (±3.061) *** − 0.359 (±0.265) × 10−3 × STR n.s. | <0.0001 | 0.0013 | 0.0008 | 0.1763 | 0.032 n.s. |
| BCS 6 = 2.681 (±0.241) *** − 0.035 (±0.019) × 10−3 × STR n.s. | <0.0001 | <0.0001 | 0.1931 | 0.0652 | 0.016 n.s. |
| MLLT 7 = 23.092 (±1.295) *** − 0.338 (±0.200) × 10−3 × STR n.s. | <0.0001 | 0.0007 | 0.0034 | 0.0922 | −0.000 n.s. |
| BT 8 = 3.090 (±0.211) *** − 0.037 (±0.033) × 10−3 × STR n.s. | <0.0001 | <0.0001 | 0.0032 | 0.2575 | 0.000 n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptáček, M.; Kyriánová, I.A.; Nápravníková, J.; Ducháček, J.; Husák, T.; Chay-Canul, A.J.; Zaragoza-Vera, C.; Cruz-Bacab, L.; Vadlejch, J. Do Live Weight, Body Condition Score, Back Muscle or Back-Fat Reserves Create the Suspicion of Goats Infected with Eimeria or Trichostrongylids? Animals 2021, 11, 3591. https://doi.org/10.3390/ani11123591
Ptáček M, Kyriánová IA, Nápravníková J, Ducháček J, Husák T, Chay-Canul AJ, Zaragoza-Vera C, Cruz-Bacab L, Vadlejch J. Do Live Weight, Body Condition Score, Back Muscle or Back-Fat Reserves Create the Suspicion of Goats Infected with Eimeria or Trichostrongylids? Animals. 2021; 11(12):3591. https://doi.org/10.3390/ani11123591
Chicago/Turabian StylePtáček, Martin, Iveta Angela Kyriánová, Jana Nápravníková, Jaromír Ducháček, Tomáš Husák, Alfonso J. Chay-Canul, Claudia Zaragoza-Vera, Luis Cruz-Bacab, and Jaroslav Vadlejch. 2021. "Do Live Weight, Body Condition Score, Back Muscle or Back-Fat Reserves Create the Suspicion of Goats Infected with Eimeria or Trichostrongylids?" Animals 11, no. 12: 3591. https://doi.org/10.3390/ani11123591
APA StylePtáček, M., Kyriánová, I. A., Nápravníková, J., Ducháček, J., Husák, T., Chay-Canul, A. J., Zaragoza-Vera, C., Cruz-Bacab, L., & Vadlejch, J. (2021). Do Live Weight, Body Condition Score, Back Muscle or Back-Fat Reserves Create the Suspicion of Goats Infected with Eimeria or Trichostrongylids? Animals, 11(12), 3591. https://doi.org/10.3390/ani11123591








