Assessment of Testicular Lhcgr mRNA Expression Correlated with Testis and Seminal Vesicle Activities in the Libyan jird (Meriones libycus, Rodentia: Muridae) during Breeding Season Compared with Nonbreeding Season
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Histology
2.3. mRNA Extraction and Reverse Transcription
2.4. Polymerase Chain Reaction (PCR)
2.5. Sequencing
2.6. Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
3.1. Seasonal Changes of Testes Structure and Seminal Vesicles
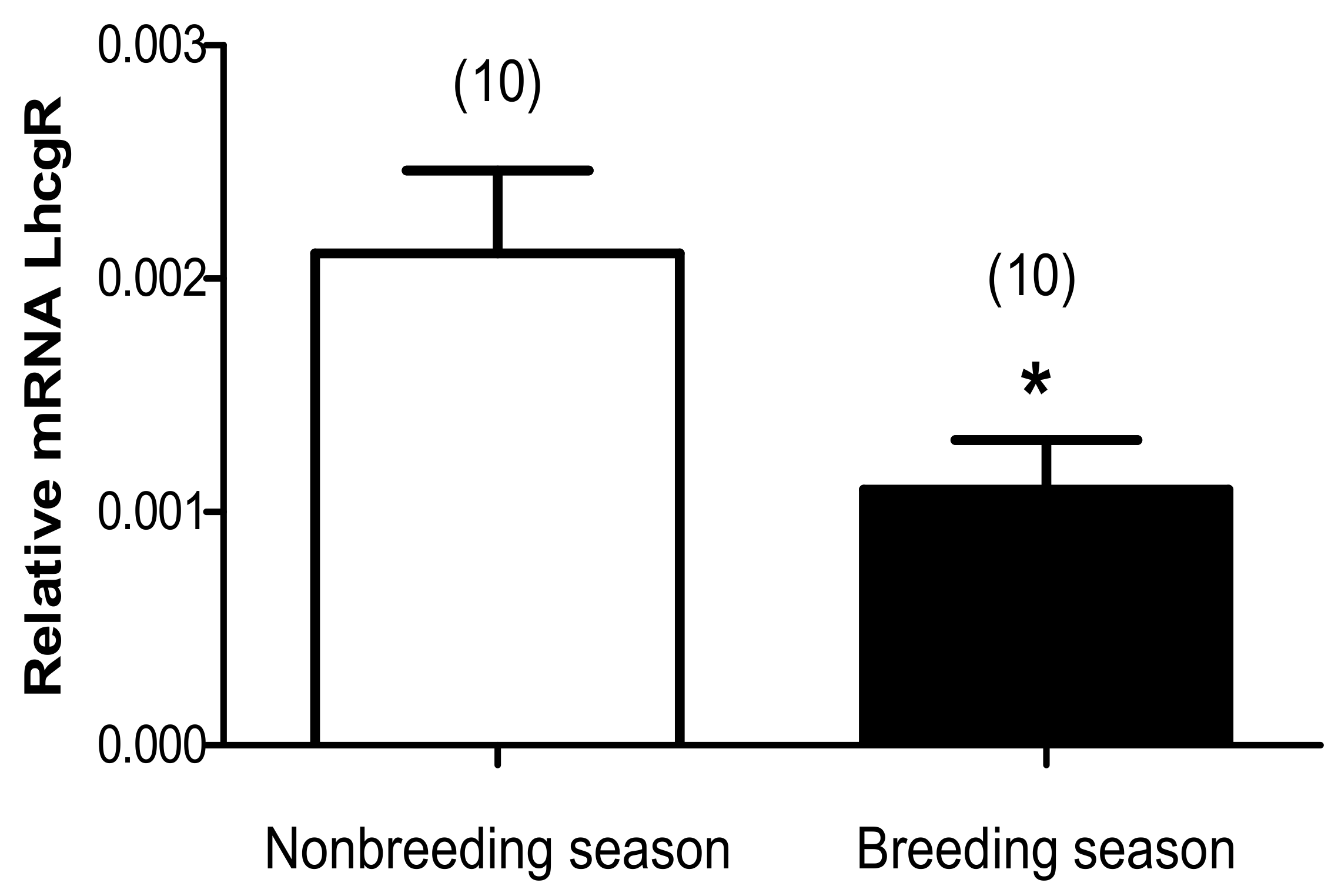
3.2. Seasonal Changes in Relative mRNA Expression of Lhcgr
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Fasano, S. Evolutionary aspects of cellular communication in the vertebrate hypothalamohypophysio-gonadal axis. Int. Rev. Cytol. 2002, 218, 69–141. [Google Scholar] [PubMed]
- Fink, G. Neuroendocrine regulation of pituitary function. In General Principles: Neuroendocrinology in Physiology and Medicine; Humana Press USA: Totowa, NJ, USA, 2000; pp. 107–134. [Google Scholar]
- Thompson, E.L.; Patterson, M.; Murphy, K.G.; Smith, K.L.; Dhillo, W.S.; Todd, J.F.; Ghtei, J.F.; Bloom, S.R. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J. Neuroendocrinol. 2004, 16, 850–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahab, M.; Mastronardi, C.; Seminara, S.B.; Crowley, W.F.; Ojeda, S.R.; Plant, T.M. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc. Natl. Acad. Sci. USA 2005, 102, 2129–2134. [Google Scholar] [CrossRef] [Green Version]
- Libuchi, R.; Kamine, A.; Shimozuru, M.; Nio-Kobayashi, J.; Watanabe, G.; Taya, K.; Tsubota, T. Changes in plasma gonadotropins, inhibin and testosterone concentrations and testicular gonadotropin receptor mRNA expression during testicular active, regressive and recrudescent phase in the captive Japanese black bear (Ursus thibetanus japonicus). Jpn. J. Vet. Res. 2010, 57, 185–196. [Google Scholar]
- Mondain-Monval, M.; Smith, A.J.; Simon, P.; Møller, O.M.; Scholler, R.; McNeilly, A.S. Effect of melatonin implantation on the seasonal variation of FSH secretion in the male blue fox (Alopex lagopus). J. Reprod. Fertil. 1988, 8, 345–354. [Google Scholar] [CrossRef]
- Lincoln, G.A.; Lincoln, C.E.; McNeilly, A.S. Seasonal cycles in the blood plasma concentration of FSH, inhibin and testosterone, and testicular size in rams of wild, feral and domesticated breeds of sheep. J. Reprod. Fertil. 1990, 88, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Barnes, B.M. Annual cycles of gonadotropins and androgens in the hibernating golden-mantled ground squirrel. Gen. Comp. Endocrinol. 1986, 62, 13–22. [Google Scholar] [CrossRef]
- Schams, D.; Barth, D. Annual profiles of reproductive hormones in peripheral plasma of the male roe deer (Capreolus capreolus). J. Reprod. Fertil. 1982, 66, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Monfort, S.L.; Brown, J.L.; Wood, T.C.; Wemmer, C.; Vargas, A.; Williamson, L.R.; Wildt, D.E. Seasonal patterns of basal and GnRH Induced LH, FSH and testosterone secretion in Eld’s deer stags (Cervus eldi thamin). J. Reprod. Fertil. 1993, 98, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Hart, D.W.; Alghamdi, A.A.; Bennett, N.C.; Mohammed, O.B.; Amor, N.M.; Alagaili, A.N. The pattern of reproduction in the Libyan jird (Meriones libycus; Rodentia: Muridae) from central Saudi Arabia in the absence of rainfall. Can. J. Zool. 2019, 97, 210–219. [Google Scholar] [CrossRef]
- Khammar, F.; Brudieux, R. Seasonal changes in testicular contents and plasma concentrations of androgens in the desert gerbil (Gerbillus gerbillus). J. Reprod. Fertil. 1987, 80, 89–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaime, A.; Laraki, M.; Gautier, J.Y.; Garnier, D.H. Seasonal variation of androgens and several sexual parameters in male Meriones shawi in southern Morocco. Gen. Comp. Endocrinol. 1992, 86, 289–296. [Google Scholar] [CrossRef]
- El-Bakry, H.A.; Zahran, W.M.; Bartness, T.J. Photoperiodic responses of four wild-trapped desert rodent species. Am. J. Physiol. 1998, 275, R2012–R2022. [Google Scholar] [CrossRef] [PubMed]
- Belhocine, M.; Gernigon-Spychalowicz, T.; Robert, A.M.; Schoevaert, D.; Bennazzoug, Y.; Exbrayat, J.M. Ecophysiological responses of the Seminal vesicle of Libyan Jird (Meriones libycus) to the Saharan conditions: Histological, morphometric and immunohistochemical analysis. Histol. Histopathol. 2007, 22, 603–615. [Google Scholar] [PubMed]
- Boufermes, R.; Richard, N.; Le Moguen, K.; Amirat, Z.; Khammar, F.; Kottler, M.L. Seasonal expression of KiSS-1 and the pituitary gonadotropins LHβ and FSHβ in adult male Libyan Jird (Meriones libycus). Anim. Reprod. Sci. 2014, 147, 56–63. [Google Scholar] [CrossRef]
- Saalu, L.C.; Akunna, G.G.; Oyewopo, A.O. The histomorphometric evidences of Vernonia amygdalina leaf extract-induced testicular toxicity. Int. J. Morphol. 2013, 31, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2 (-delta delta c (t)), method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Saez, F.J.; Madrid, F.; Canteras, M.; Pasteur, L.M. Testicular histomorphometry and the proliferative and apoptotic activities of the seminiferous epithelium in Syrian hamster (Mesocricetus auratus) during regression owing to short photoperiod. Andrology 2015, 3, 598–610. [Google Scholar] [CrossRef]
- Gernigon, T.; Berger, M.; Lécher, P. Seasonal variations in the ultrastructure and production of androgen-dependent proteins in the seminal vesicles of a Saharian rodent (Psammomys obesus). J. Endocrinol. 1994, 142, 37–46. [Google Scholar] [CrossRef]
- Godoy Pieri, N.C.; da Silva Santos, P.R.; Santos Roballo, K.C.; Flamini, M.A.; Barbeito, C.G.; Ambrosio, C.E.; Miglino, M.A.; Dos Santos Martin, D. Seasonal variations cause morphological changes and altered spermatogenesis in the testes of viscacha (Lagostomus maximus). Anim. Reprod. Sci. 2014, 149, 316–324. [Google Scholar] [CrossRef]
- Chaves, E.M.; Aguilera-Merlo, C.; Cruceño, A.; Fogal, T.; Piezzi, R.; Scardapane, P.L.; Dominguez, S. Seasonal morphological variations and age related changes of the seminal vesicle of viscacha (Lagostomus maximus maximus): An ultrastructural and immunohistochemical study. Anat. Rec. 2012, 295, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.; Aguilera-Merlo, C.; Cruceño, A.; Fogal, T.; Mohamed, F. Morphological study of the prostate gland in viscacha (Lagostomus maximus maximus) during periods of maximal and minimal reproductive activity. Anat. Rec. 2015, 298, 1919–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Y.; Hsu, M.C.; Tseng, T.H.; Wu, L.S.; Yang, K.T.; Chiu, C.H. Kisspeptin expression in mouse Leydig cells correlates with age. J. Chin. Med. Assoc. 2015, 78, 249–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, S.; Adeshina, I.; Chen, H.; Zirkin, B.R.; Hussain, M.A.; Wondisford, F.A.; Wolfe, A.; Radovick, S. Developmental and endocrine regulation of kisspeptin expression in mouse leydig cells. Endocrinology 2015, 156, 514–1522. [Google Scholar] [CrossRef] [Green Version]
- Tariq, A.R.; Shabab, M. Effect of kisspeptin challenge on testosterone and inhibin secretion from in vitro testicular tissue of adult male rhesus monkey (Macaca mulatta). Andrologia 2017, 49, 125–190. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G.A. Long-term stimulatory effects of a continuous infusion of LHRH agonist on testicular function in male red deer (Cervus elaphus). J. Reprod. Fertil. 1987, 80, 57–261. [Google Scholar] [CrossRef] [Green Version]
- El Omari, B.; Lacroix, A.; Saboureau, M. Daily and seasonal variations in plasma LH and testosterone concentrations in the adult male hedgehog (Erinaceus europaeus). J. Reprod. Fertil. 1989, 86, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Fowler, P.A.; Racey, P.A. Effect of melatonin administration and long day-length on endocrine cycles in the hedgehog (Erinaceus europaeus). J. Pineal Res. 1990, 8, 193–204. [Google Scholar] [CrossRef]
- Saboureau, M.; Boissin, J. Peripheral metabolism of testosterone during the annual reproductive cycle in the male hedgehog: A hibernating mammal. Can. J. Zool. 1983, 61, 2849–2855. [Google Scholar] [CrossRef]
- Reiter, R.J.; Richardson, B.A.; Johnson, L.Y.; Ferguson, B.N.; Dinh, D.T. Melatonin rhythm: Reduction in aging Syrian hamsters. Science 1980, 210, 1372–1373. [Google Scholar] [CrossRef]
- Hoffmann, K. The role of the pineal gland in the photoperiodic control of seasonal cycles in hamsters. In Biological Clocks in Seasonal Reproductive Cycles; Follett, B.K., Follett, D.E., Eds.; Wright: Bristol, UK, 1981; pp. 237–250. [Google Scholar]
- Brown, J.I.; Wildt, D.E.; Raath, J.R.; De Vos, V.; Janssen, D.I.; Citino, S.B.; Howard, J.G.; Bush, M. Seasonal variation in pituitary-gonadal function in free ranging impala (Aepyceros melampus). J. Reprod. Fertil. 1991, 93, 497–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khammar, F.; Brudieux, R. Seasonal changes in plasma concentrations of gonadotropins and the responsiveness of the pituitary and testis to GnRH in desert rodent, the sand rat (Psammomys obesus). Reprod. Nutr. Dév. 1991, 31, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, U.B.; Jakubowiak, A.; Steinberger, A.; Chin, W.W. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 1997, 138, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Dalkin, A.C.; Haisenleder, D.J.; Ortolano, G.A.; Ellis, T.R.; Marshall, J.C. The frequency of Gonadotropin-Releasing-Hormone stimulation differentially regulates gonadotropins subunit messenger ribonucleic acid expression. Endocrinology 1989, 125, 917–924. [Google Scholar] [CrossRef]
- Soares, M.J.; Hoffmann, J.C. Seasonal reproduction in the mongoose, Herpestes auropunctatus II. Testicular responsiveness to luteinizing hormone. Gen. Comp. Endocrinol. 1982, 47, 226–234. [Google Scholar] [CrossRef]
- Sanford, L.M.; Howland, B.E.; Palmer, W.M. Seasonal changes in the secretion of gonadotropic hormones and in the endocrine response of the pituitary of male sheep in the absence of gonadal influence. Can. J. Physiol. Pharmacol. 1984, 62, 834–839. [Google Scholar] [CrossRef]
- Barnes, B.M.; Kretzmann, M.; Zucker, I.; Licht, P. Plasma androgen and gonadotropin levels during hibernation and testicular maturation in golden-mantled ground squirrels. Biol. Reprod. 1988, 38, 616–622. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, T. Thyroid hormone and seasonal regulation of reproduction. Front. Neuroendocrinol. 2013, 34, 157–166. [Google Scholar] [CrossRef]
- Hazlerigg, D.; Simonneaux, V. Seasonal regulation of reproduction in mammals. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 1575–1604. [Google Scholar]
 ) is high (700 µm ± 806 µm) with regular outline Leyding cells (
) is high (700 µm ± 806 µm) with regular outline Leyding cells (  ) are observed in the inter-tubular space. (b) The spermatogenesis is very active and numerous spermatozoa (SPZ) are accumulated in the lumen of the seminiferous tubule. (c) Leydig cells (L) are large and arranged around blood vessels (Vx), and the basement membrane (⊇) is regular, intact, and thin. (d) Leydig cells (L) are located in the vicinity of the seminiferous tubules (
) are observed in the inter-tubular space. (b) The spermatogenesis is very active and numerous spermatozoa (SPZ) are accumulated in the lumen of the seminiferous tubule. (c) Leydig cells (L) are large and arranged around blood vessels (Vx), and the basement membrane (⊇) is regular, intact, and thin. (d) Leydig cells (L) are located in the vicinity of the seminiferous tubules (  ). (a): ×100; (b): ×400; (c,d): ×1250.
). (a): ×100; (b): ×400; (c,d): ×1250.
 ) is high (700 µm ± 806 µm) with regular outline Leyding cells (
) is high (700 µm ± 806 µm) with regular outline Leyding cells (  ) are observed in the inter-tubular space. (b) The spermatogenesis is very active and numerous spermatozoa (SPZ) are accumulated in the lumen of the seminiferous tubule. (c) Leydig cells (L) are large and arranged around blood vessels (Vx), and the basement membrane (⊇) is regular, intact, and thin. (d) Leydig cells (L) are located in the vicinity of the seminiferous tubules (
) are observed in the inter-tubular space. (b) The spermatogenesis is very active and numerous spermatozoa (SPZ) are accumulated in the lumen of the seminiferous tubule. (c) Leydig cells (L) are large and arranged around blood vessels (Vx), and the basement membrane (⊇) is regular, intact, and thin. (d) Leydig cells (L) are located in the vicinity of the seminiferous tubules (  ). (a): ×100; (b): ×400; (c,d): ×1250.
). (a): ×100; (b): ×400; (c,d): ×1250.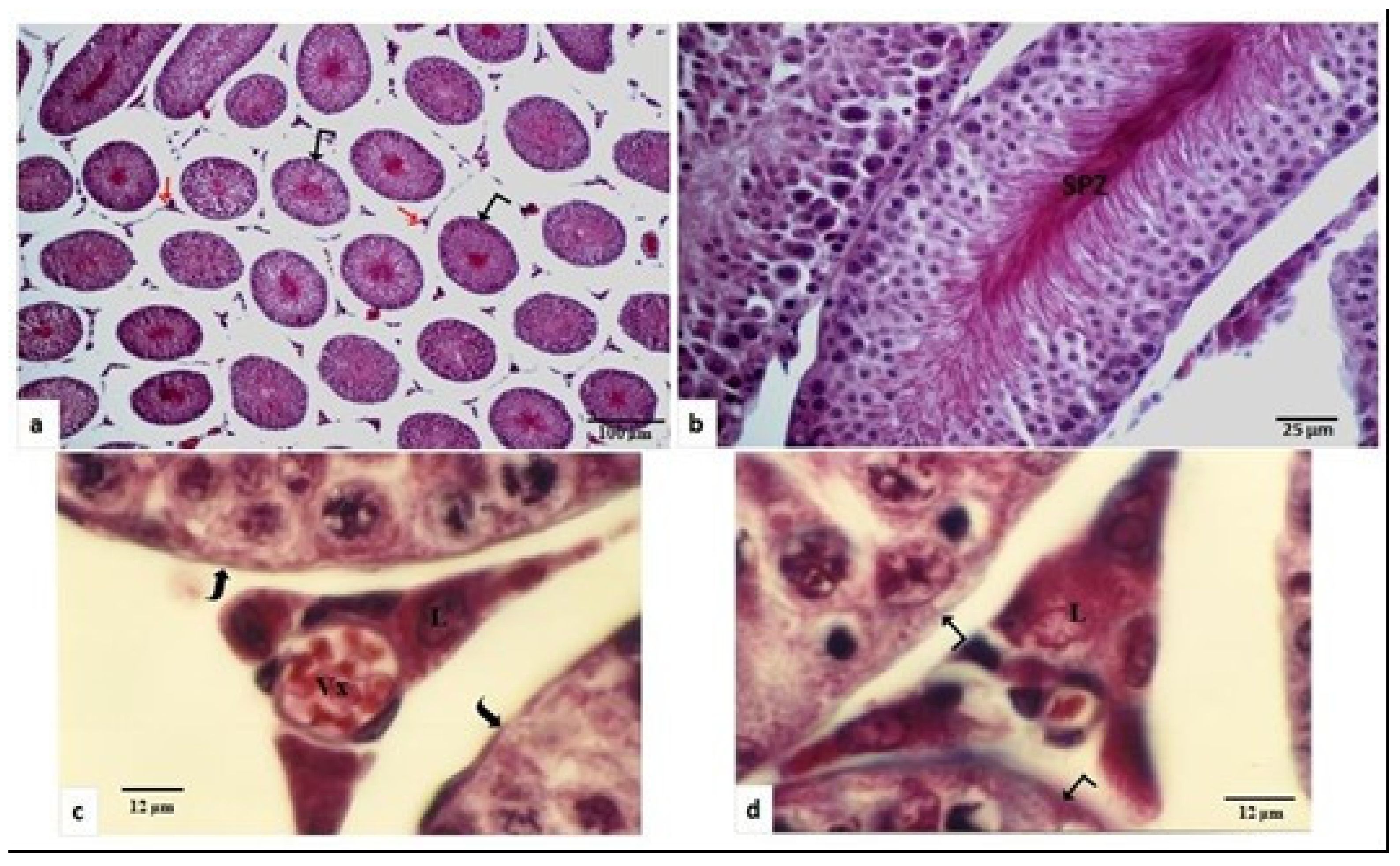
 ) is thick and pleated and the tunica albuginea (➨) is greatly dense. (c) Leydig cell (L) are of greatly reduced size and seminiferous tubules are bordered by a deep basement membrane (
) is thick and pleated and the tunica albuginea (➨) is greatly dense. (c) Leydig cell (L) are of greatly reduced size and seminiferous tubules are bordered by a deep basement membrane (  ). (d) Spermatogenesis stopped at the stage of spermatogonia and tubular lumen completely disappeared. (a,b,d): ×400; (c):×1250.
). (d) Spermatogenesis stopped at the stage of spermatogonia and tubular lumen completely disappeared. (a,b,d): ×400; (c):×1250.
 ) is thick and pleated and the tunica albuginea (➨) is greatly dense. (c) Leydig cell (L) are of greatly reduced size and seminiferous tubules are bordered by a deep basement membrane (
) is thick and pleated and the tunica albuginea (➨) is greatly dense. (c) Leydig cell (L) are of greatly reduced size and seminiferous tubules are bordered by a deep basement membrane (  ). (d) Spermatogenesis stopped at the stage of spermatogonia and tubular lumen completely disappeared. (a,b,d): ×400; (c):×1250.
). (d) Spermatogenesis stopped at the stage of spermatogonia and tubular lumen completely disappeared. (a,b,d): ×400; (c):×1250.
 ), epithelial fold axis (
), epithelial fold axis (  ), supranuclear zona of epithelial cells (*), lumen (L), connective fibers (↯), fibromuscular wall (fw). Staining: (a) Van-Gieson; (d,e) Azan of Heidenhain; (b,c,f) Sirius red. (a,f) ×100; (b,e) ×1250; (c,d) ×400.
), supranuclear zona of epithelial cells (*), lumen (L), connective fibers (↯), fibromuscular wall (fw). Staining: (a) Van-Gieson; (d,e) Azan of Heidenhain; (b,c,f) Sirius red. (a,f) ×100; (b,e) ×1250; (c,d) ×400.
 ), epithelial fold axis (
), epithelial fold axis (  ), supranuclear zona of epithelial cells (*), lumen (L), connective fibers (↯), fibromuscular wall (fw). Staining: (a) Van-Gieson; (d,e) Azan of Heidenhain; (b,c,f) Sirius red. (a,f) ×100; (b,e) ×1250; (c,d) ×400.
), supranuclear zona of epithelial cells (*), lumen (L), connective fibers (↯), fibromuscular wall (fw). Staining: (a) Van-Gieson; (d,e) Azan of Heidenhain; (b,c,f) Sirius red. (a,f) ×100; (b,e) ×1250; (c,d) ×400.
| Primers | Sequence 5′→3′ | Product Size 1(bp) | Exons (E) |
|---|---|---|---|
| LhcgR | F: TGC ACA GTG GAG CCT TCC R: ATT CCG CCA TCT TTG AGG | 329 | F: E1 R: E2 |
| Primers | Sequence 5′→3′ 1(bp) | Product Size 1(bp) | Exons (E) |
|---|---|---|---|
| LhcgR | F: GCT GC GCT TTT AGG AAT TTG R: CCA AAC AAT GTG AAA GCA CA | 86 | F: E1 R: E3 |
| β-actin | F: ATG TTG CCC TGG ACT TTG AGR: CCT CTC ATT GCC AAT GGT GA | 151 | F: E3 R: E4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boufermes, R.; Belhocine, M.; Amirat, Z.; Khammar, F. Assessment of Testicular Lhcgr mRNA Expression Correlated with Testis and Seminal Vesicle Activities in the Libyan jird (Meriones libycus, Rodentia: Muridae) during Breeding Season Compared with Nonbreeding Season. Animals 2021, 11, 320. https://doi.org/10.3390/ani11020320
Boufermes R, Belhocine M, Amirat Z, Khammar F. Assessment of Testicular Lhcgr mRNA Expression Correlated with Testis and Seminal Vesicle Activities in the Libyan jird (Meriones libycus, Rodentia: Muridae) during Breeding Season Compared with Nonbreeding Season. Animals. 2021; 11(2):320. https://doi.org/10.3390/ani11020320
Chicago/Turabian StyleBoufermes, Radia, Mansouria Belhocine, Zaina Amirat, and Farida Khammar. 2021. "Assessment of Testicular Lhcgr mRNA Expression Correlated with Testis and Seminal Vesicle Activities in the Libyan jird (Meriones libycus, Rodentia: Muridae) during Breeding Season Compared with Nonbreeding Season" Animals 11, no. 2: 320. https://doi.org/10.3390/ani11020320





