The Autumn Low Milk Yield Syndrome in High Genetic Merit Dairy Cattle: The Possible Role of a Dysregulated Innate Immune Response
Abstract
:Simple Summary
Abstract
1. Introduction
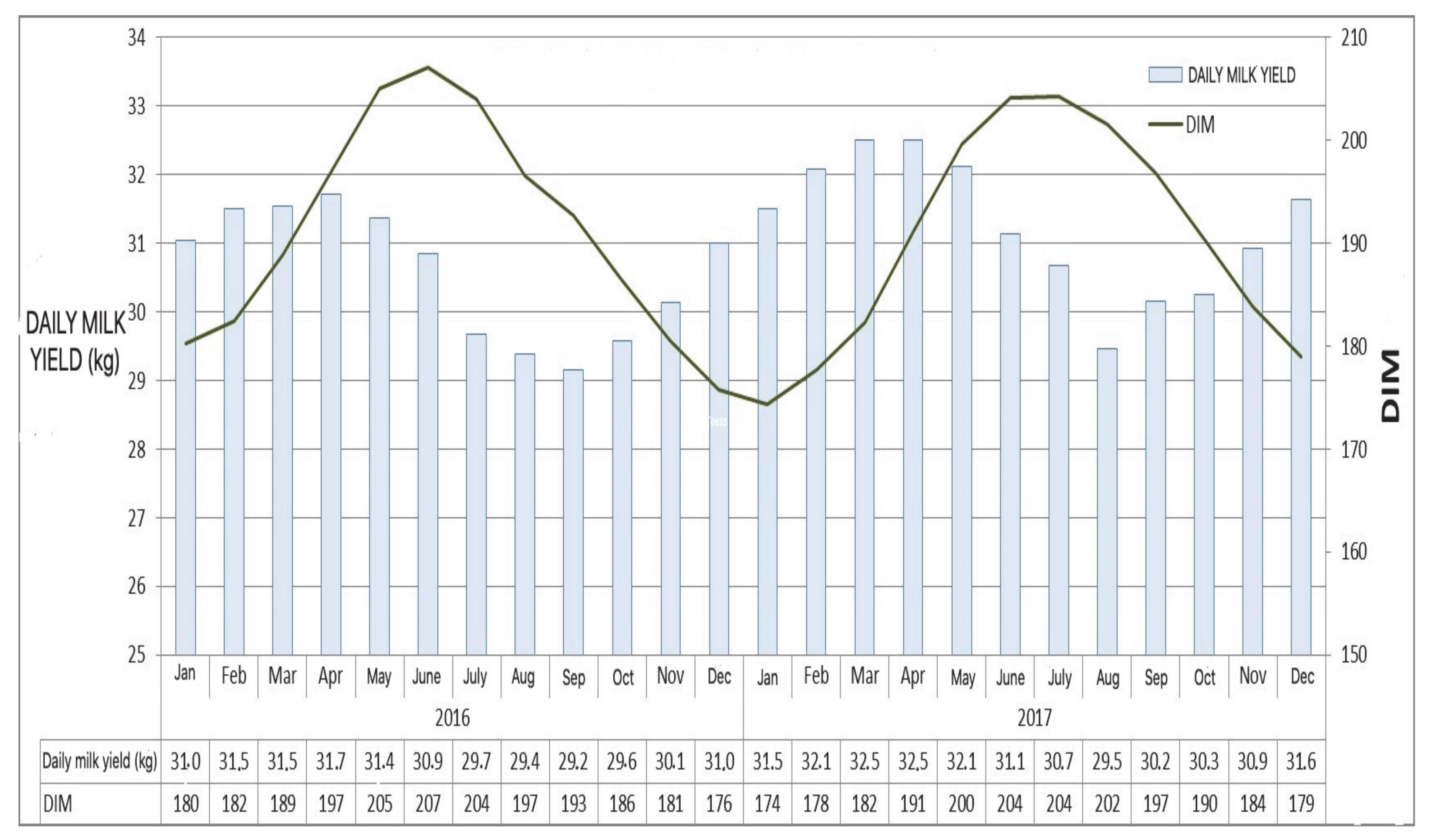
2. Characterization of the Autumn Syndrome
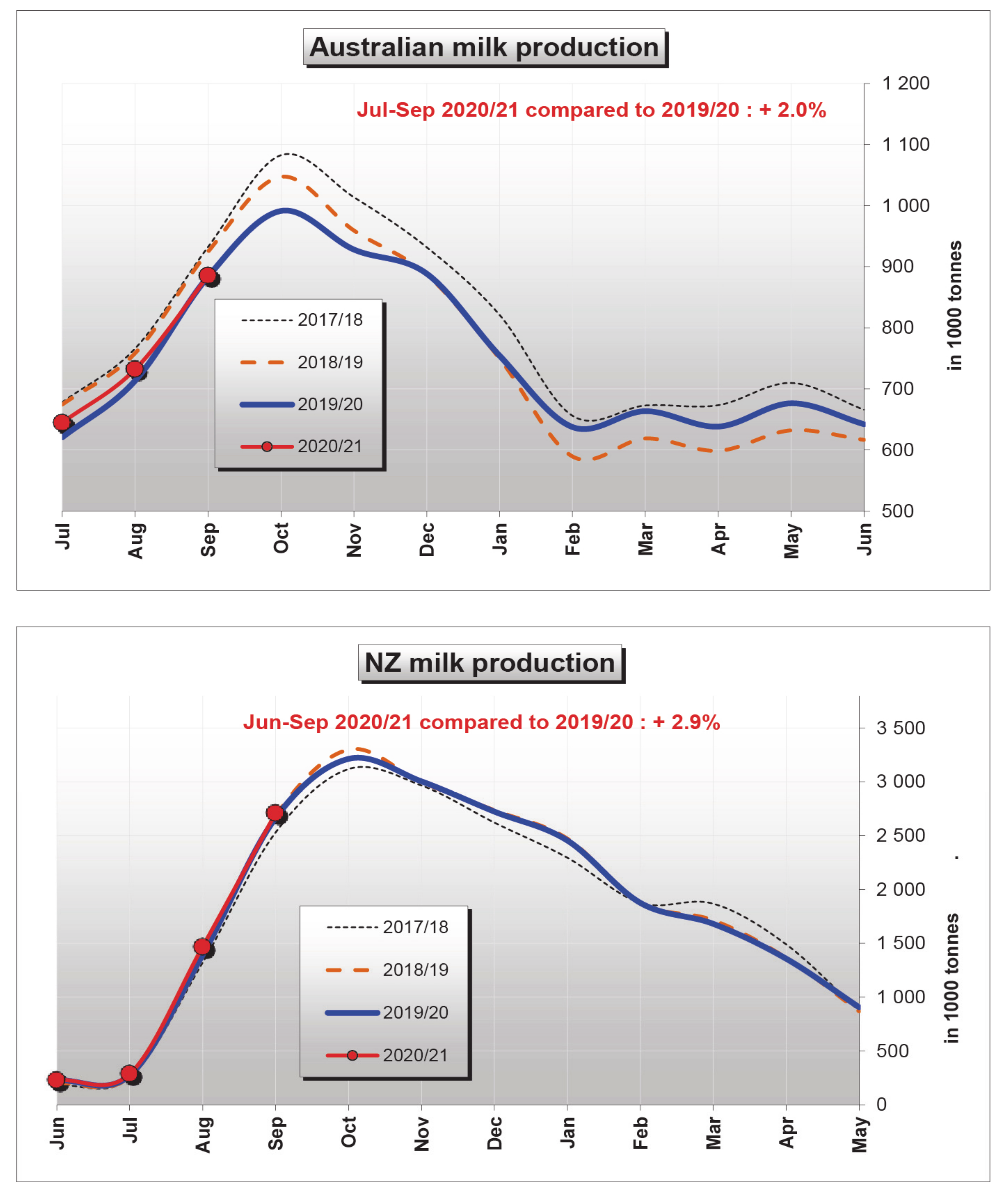
3. Stressors Acting on Dairy Cattle
3.1. Metabolic Stress
3.2. Oxidative Stress
4. Inflammatory State and Thermal Stress
4.1. High Temperature Effects on the Immune System
4.2. Heat Stress and Changes in Liver Function
5. Photoperiod and Heat Stress Actions on Mammary Gland Development
6. Overcrowding in the Dry Cow Box and Animal Welfare
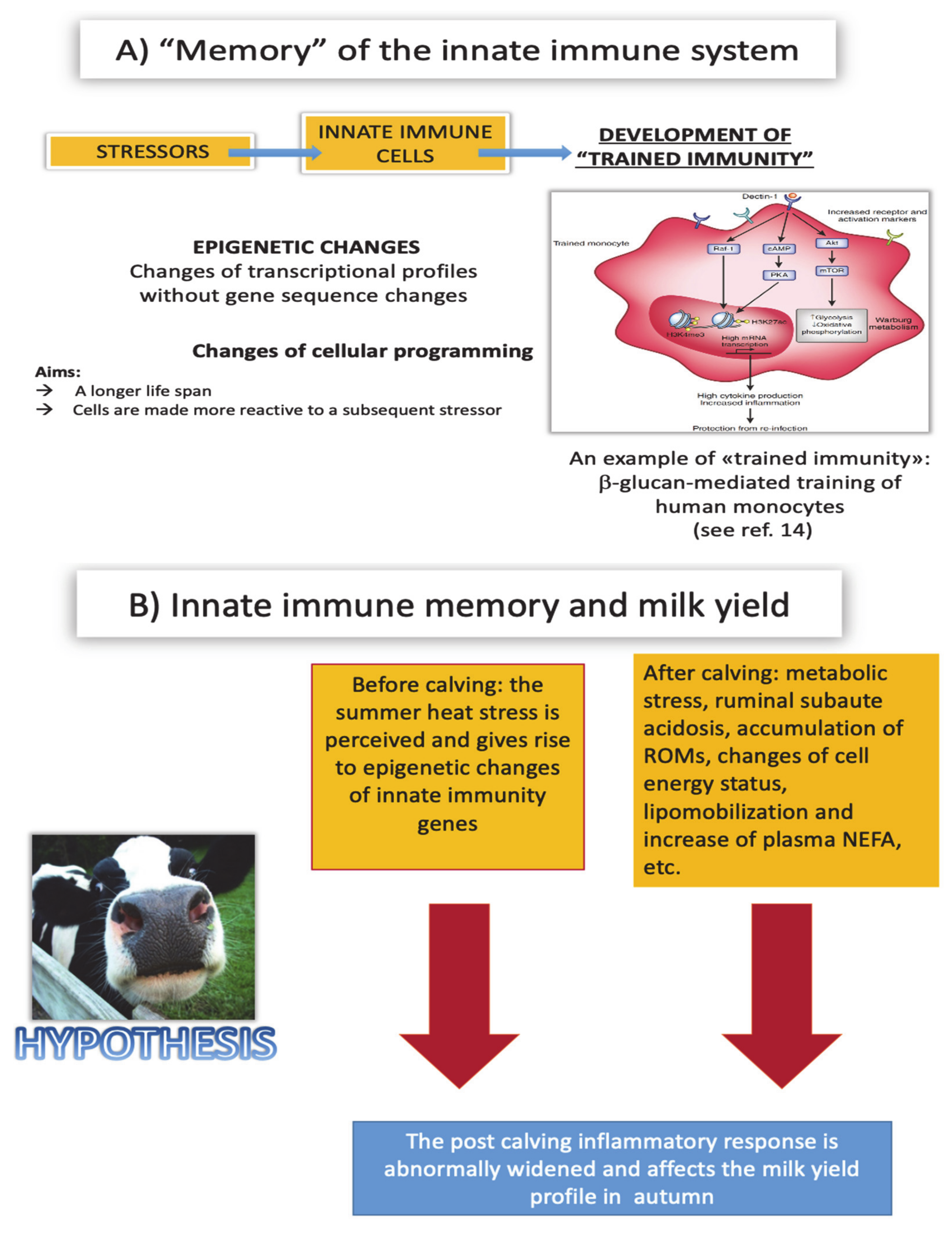
7. Overall Conceptual Framework
7.1. Persistence
7.2. Damaging Effects of Trained Immunity
7.3. Metabolic Shift
8. Why Does “Trained Immunity” Provide a Better Explanation of the Low Milk Yield Syndrome?
- (1)
- The autumn syndrome cannot be entirely accounted for by direct damages on the involuting mammary gland in summer.
- (2)
- A mechanism of temporal expansion of the primary stress is needed to provide a credible explanation of the observed phenomena. In this respect, epigenetic regulation of innate immunity genes (DNA methylation, histone modifications, nucleosome remodelling, non-coding RNAs, RNA modifications) [72] can be a robust explanation of the long-lasting effects of summer heat conditions. These could be investigated on proper tissue samples collected from the same cows before dry-off and after calving, respectively, by comparative analyses of e.g., chromatin compaction around innate immunity genes [48] and histon-modifying enzymes [47]. Also, the inclusion of cooled and control cows in the same herd would allow for a better focus of such cohort studies.
- (3)
- Accordingly, “trained immunity” is a robust conceptual framework to compose the above puzzle, which might be sided by analogous epigenetic changes on mammary gland precursors in the fetal period [39].
- (4)
- The “memory” of past stressors and the persistent inflammatory response defines the scenario in which the low milk yield autumn syndrome should be reasonably set and interpreted.
9. Intervention Strategies
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bronzo, V.; Lopreiato, V.; Riva, F.; Amadori, M.; Curone, G.; Addis, M.F.; Cremonesi, P.; Moroni, P.; Trevisi, E.; Castiglioni, B. The Role of Innate Immune Response and Microbiome in Resilience of Dairy Cattle to Disease: The Mastitis Model. Animals 2020, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 7, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oltenacu, P.A.; Broom, D.M. The impact of genetic selection for increased milk yield on the welfare of dairy cows. Anim. Welf. 2010, 19, 39–49. [Google Scholar]
- Fey, P.D.; Safranek, T.J.; Rupp, M.E.; Dunne, E.F.; Ribot, E.; Iwen, P.C.; Bradford, P.A.; Angulo, F.J.; Hinrichs, S.H. Ceftriaxone-resistant salmonella infection acquired by a child from cattle. N. Engl. J. Med. 2000, 342, 1242–1249. [Google Scholar] [CrossRef] [Green Version]
- De Vries, A.; Marcondes, M.I. Review: Overview of factors affecting productive lifespan of dairy cows. Animal 2020, 14, s155–s164. [Google Scholar] [CrossRef] [Green Version]
- Sumner, C.L.; von Keyserlingk, M.A.G.; Weary, D.M. Perspectives of farmers and veterinarians concerning dairy cattle welfare. Anim. Front. 2018, 8, 8–13. [Google Scholar] [CrossRef]
- Garcia-Llorente, M.; Rubio-Olivar, R.; Gutierrez-Briceno, I. Farming for Life Quality and Sustainability: A Literature Review of Green Care Research Trends in Europe. Int. J. Environ. Res. Public Health 2018, 15, 1282. [Google Scholar] [CrossRef] [Green Version]
- Lacetera, N. Metabolic stress, Heat Shock Proteins, and Innate Immune Response. In The Innate Immune Response to Noninfectious Stressors; Amadori, M., Ed.; Academic Press: London, UK, 2016; pp. 107–131. [Google Scholar]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Gallucci, S. An Overview of the Innate Immune Response to Infectious and Noninfectious Stressors. In The Innate Immune Response to Noninfectious Stressors; Amadori, M., Ed.; Academic Press: London, UK, 2016; pp. 1–24. [Google Scholar]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef]
- Amadori, M. Preface. In The Innate Immune Response to Noninfectious Stressors: Human and Animal Models; Academic Press: London, UK, 2016. [Google Scholar]
- Bordon, Y. Macrophages: Innate memory training. Nat. Rev. Immunol. 2014, 14, 713. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Nusrat, J.; Bertoni, G.; Ferrari, A.; Minuti, A. Pro-Inflammatory Cytokine Profile in Dairy Cows: Consequences for New Lactation. Ital. J. Anim. Sci. 2015, 14, 285–292. [Google Scholar] [CrossRef]
- Mezzetti, M.; Minuti, A.; Piccioli-Cappelli, F.; Amadori, M.; Bionaz, M.; Trevisi, E. The role of altered immune function during the dry period in promoting the development of subclinical ketosis in early lactation. J. Dairy Sci. 2019, 102, 9241–9258. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, E.; Nusrat, J.; Gubbiotti, A.; Bertoni, G. Effects of inflammation in peripartum dairy cows on milk yield, energy balance and efficiency. In Energy and Protein Metabolism and Nutrition; Ortigues-Marty, I., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2007; pp. 395–396. [Google Scholar]
- Morris, D.G.; Waters, S.M.; McCarthy, S.D.; Patton, J.; Earley, B.; Fitzpatrick, R.; Murphy, J.J.; Diskin, M.G.; Kenny, D.A.; Brass, A.; et al. Pleiotropic effects of negative energy balance in the postpartum dairy cow on splenic gene expression: Repercussions for innate and adaptive immunity. Physiol. Genom. 2009, 39, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Salzano, S.; Checconi, P.; Hanschmann, E.M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, D.E.; Halbach, T.J.; Armstrong, D.V. Season and lactation number effects on milk production and reproduction of dairy cattle in Arizona. J. Dairy Sci. 1992, 75, 2976–2983. [Google Scholar] [CrossRef]
- Wakayo, B.U.; Brar, P.S.; Prabhakar, S. Review on mechanisms of dairy summer infertility and implications for hormonal intervention. Open Vet. J. 2015, 5, 6–10. [Google Scholar]
- Garcia, S.C.; Holmes, C.W. Effects of time of calving on the productivity of pasture-based dairy systems: A review. N. Z. J. Agric. Res. 1999, 42, 347–362. [Google Scholar] [CrossRef]
- Mulligan, F.J.; Doherty, M.L. Production diseases of the transition cow. Vet. J. 2008, 176, 3–9. [Google Scholar] [CrossRef]
- Loiselle, M.C.; Ster, C.; Talbot, B.G.; Zhao, X.; Wagner, G.F.; Boisclair, Y.R.; Lacasse, P. Impact of postpartum milking frequency on the immune system and the blood metabolite concentration of dairy cows. J. Dairy Sci. 2009, 92, 1900–1912. [Google Scholar] [CrossRef]
- Suriyasathaporn, W.; Heuer, C.; Noordhuizen-Stassen, E.N.; Schukken, Y.H. Hyperketonemia and the impairment of udder defense: A review. Vet. Res. 2000, 31, 397–412. [Google Scholar] [CrossRef] [Green Version]
- Grinberg, N.; Elazar, S.; Rosenshine, I.; Shpigel, N.Y. Beta-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia coli. Infect. Immun. 2008, 76, 2802–2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, K.; Kutyavin, V.I.; Chawla, A. Tissue Immunometabolism: Development, Physiology, and Pathobiology. Cell Metab. 2017, 25, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, R.C.; Forsyth, L.E.; Richman, P.I.; Wells, C.; Spencer, J.; MacDonald, T.T. Changes in the rate of crypt epithelial cell proliferation and mucosal morphology induced by a T-cell-mediated response in human small intestine. Gastroenterology 1990, 98, 1255–1263. [Google Scholar] [CrossRef]
- Hoytema van Konijnenburg, D.P.; Reis, B.S.; Pedicord, V.A.; Farache, J.; Victora, G.D.; Mucida, D. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell 2017, 171, 783–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosteli, A.; Sugaru, E.; Haemmerle, G.; Martin, J.F.; Lei, J.; Zechner, R.; Ferrante, A.W. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J. Clin. Investig. 2010, 120, 3466–3479. [Google Scholar] [CrossRef] [Green Version]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef]
- Huzzey, J.M.; Mann, S.; Nydam, D.V.; Grant, R.J.; Overton, T.R. Associations of peripartum markers of stress and inflammation with milk yield and reproductive performance in Holstein dairy cows. Prev. Vet. Med. 2015, 120, 291–297. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Contreras, G.A.; Sordillo, L.M. Lipid mobilization and inflammatory responses during the transition period of dairy cows. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Vitali, A.; Segnalini, M.; Bertocchi, L.; Bernabucci, U.; Nardone, A.; Lacetera, N. Seasonal pattern of mortality and relationships between mortality and temperature-humidity index in dairy cows. J. Dairy Sci. 2009, 92, 3781–3790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.; Dahl, G.E. Invited review: Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef]
- Dahl, G.E.; Tao, S.; Monteiro, A.P.A. Effects of late-gestation heat stress on immunity and performance of calves. J. Dairy Sci. 2016, 99, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Lacetera, N.; Bernabucci, U.; Scalia, D.; Ronchi, B.; Kuzminsky, G.; Nardone, A. Lymphocyte functions in dairy cows in hot environment. Int. J. Biometeorol. 2005, 50, 105–110. [Google Scholar] [CrossRef]
- Do Amaral, B.C.; Connor, E.E.; Tao, S.; Hayen, M.J.; Bubolz, J.W.; Dahl, G.E. Heat stress abatement during the dry period influences metabolic gene expression and improves immune status in the transition period of dairy cows. J. Dairy Sci. 2011, 94, 86–96. [Google Scholar] [CrossRef]
- Skibiel, A.L.; Zachut, M.; do Amaral, B.C.; Levin, Y.; Dahl, G.E. Liver proteomic analysis of postpartum Holstein cows exposed to heat stress or cooling conditions during the dry period. J. Dairy Sci. 2018, 101, 705–716. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Tao, S.; Bubolz, J.W.; do Amaral, B.C.; Thompson, I.M.; Hayen, M.J.; Johnson, S.E.; Dahl, G.E. Effect of heat stress during the dry period on mammary gland development. J. Dairy Sci. 2011, 94, 5976–5986. [Google Scholar] [CrossRef]
- Butler, W.R. Fisiopatologia delle bovine da latte in transizione. Large Anim. Rev. 2010, 16, 295–298. (In Italian) [Google Scholar]
- Huzzey, J.M.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A. Prepartum behavior and dry matter intake identify dairy cows at risk for metritis. J. Dairy Sci. 2007, 90, 3220–3233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, R.; O’Farrelly, C.; Meade, K.G. Epigenetic regulation of the innate immune response to LPS in bovine peripheral blood mononuclear cells (PBMC). Vet. Immunol. Immunopathol. 2013, 154, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Petzl, W.; Vanselow, J.; Gunther, J.; Shen, X.; Seyfert, H.M. Epigenetic mechanisms contribute to enhanced expression of immune response genes in the liver of cows after experimentally induced Escherichia coli mastitis. Vet. J. 2015, 203, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.A.; Loving, C.L.; McGill, J.L. Innate Immunomodulation in Food Animals: Evidence for Trained Immunity? Front. Immunol. 2020, 11, 1099. [Google Scholar] [CrossRef]
- Emam, M.; Livernois, A.; Paibomesai, M.; Atalla, H.; Mallard, B. Genetic and Epigenetic Regulation of Immune Response and Resistance to Infectious Diseases in Domestic Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 405–429. [Google Scholar] [CrossRef]
- Detilleux, J. Tolerance to bovine clinical mastitis: Total, direct, and indirect milk losses. J. Dairy Sci. 2018, 101, 3334–3343. [Google Scholar] [CrossRef] [Green Version]
- Watkins, A.J.; Dias, I.; Tsuro, H.; Allen, D.; Emes, R.D.; Moreton, J.; Wilson, R.; Ingram, R.J.M.; Sinclair, K.D. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10064–10069. [Google Scholar] [CrossRef] [Green Version]
- Sordillo, L.M.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Matzinger, P. An innate sense of danger. Ann. N. Y. Acad. Sci. 2002, 961, 341–342. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickerstaffe, R.; Annison, E.F.; Linzell, J.L. The metabolism of glucose, acetate, lipids and amino acids in lactating dairy cows. J. Agric. Sci. 1974, 82, 71–85. [Google Scholar] [CrossRef]
- Filkins, J.P. Phases of glucose dyshomeostasis in endotoxicosis. Circ. Shock 1978, 5, 347–355. [Google Scholar] [PubMed]
- McGuinness, O.P. The impact of infection on gluconeogenesis in the conscious dog. Shock 1994, 2, 336–343. [Google Scholar] [CrossRef]
- Michaeli, B.; Martinez, A.; Revelly, J.P.; Cayeux, M.C.; Chiolero, R.L.; Tappy, L.; Berger, M.M. Effects of endotoxin on lactate metabolism in humans. Crit. Care 2012, 16, R139. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C.; Dimitriadis, G.; Newsholme, P. Glucose metabolism in lymphoid and inflammatory cells and tissues. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 531–540. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; O’Neill, L.A. The Warburg effect then and now: From cancer to inflammatory diseases. Bioessays 2013, 35, 965–973. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Horst, E.A.; Abuajamieh, M.; Mayorga, E.J.; Fernandez, M.V.; Baumgard, L.H. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 2017, 100, 2360–2374. [Google Scholar] [CrossRef] [Green Version]
- Waldron, M.R.; Nishida, T.; Nonnecke, B.J.; Overton, T.R. Effect of lipopolysaccharide on indices of peripheral and hepatic metabolism in lactating cows. J. Dairy Sci. 2003, 86, 3447–3459. [Google Scholar] [CrossRef]
- Lang, C.H.; Dobrescu, C.; Meszaros, K. Insulin-mediated glucose uptake by individual tissues during sepsis. Metabolism 1990, 39, 1096–1107. [Google Scholar] [CrossRef]
- Song, M.J.; Kim, K.H.; Yoon, J.M.; Kim, J.B. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 2006, 346, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.; Sundrum, A. Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows. Animals 2020, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Dado-Senn, B.; Skibiel, A.L.; Fabris, T.F.; Dahl, G.E.; Laporta, J. Dry period heat stress induces microstructural changes in the lactating mammary gland. PLoS ONE 2019, 14, e0222120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.; Orellana, R.M.; Weng, X.; Marins, T.N.; Dahl, G.E.; Bernard, J.K. Symposium review: The influences of heat stress on bovine mammary gland function. J. Dairy Sci. 2018, 101, 5642–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curone, G.; Filipe, J.; Cremonesi, P.; Trevisi, E.; Amadori, M.; Pollera, C.; Castiglioni, B.; Turin, L.; Tedde, V.; Vigo, D.; et al. What we have lost: Mastitis resistance in Holstein Friesians and in a local cattle breed. Res. Vet. Sci. 2018, 116, 88–98. [Google Scholar] [CrossRef]
- Vitali, A.; Felici, A.; Lees, A.M.; Giacinti, G.; Maresca, C.; Bernabucci, U.; Gaughan, J.B.; Nardone, A.; Lacetera, N. Heat load increases the risk of clinical mastitis in dairy cattle. J. Dairy Sci. 2020, 103, 8378–8387. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, Y.; Zhao, X.; Wang, F.; Gao, S.; Bu, D. Heat stress induces proteomic changes in the liver and mammary tissue of dairy cows independent of feed intake: An iTRAQ study. PLoS ONE 2019, 14, e0209182. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, X. Epigenetic regulation of the innate immune response to infection. Nat. Rev. Immunol. 2019, 19, 417–432. [Google Scholar] [CrossRef]
- Nalon, E.; Stevenson, P. Protection of Dairy Cattle in the EU: State of Play and Directions for Policymaking from a Legal and Animal Advocacy Perspective. Animals 2019, 9, 1066. [Google Scholar] [CrossRef] [Green Version]
- Mezzetti, M.; Minuti, A.; Piccioli-Cappelli, F.; Trevisi, E. Inflammatory status and metabolic changes at dry-off in high-yield dairy cows. Ital. J. Anim. Sci. 2020, 19, 51–65. [Google Scholar] [CrossRef]
- Rajala-Schultz, P.J.; Hogan, J.S.; Smith, K.L. Short communication: Association between milk yield at dry-off and probability of intramammary infections at calving. J. Dairy Sci. 2005, 88, 577–579. [Google Scholar] [CrossRef]

| STRENGTH | WEAKNESS |
|---|---|
| Multi-disciplinary, comprehensive approach, combining field and experimental evidence. | Conceptual complexity of the experimental approach. |
| Clear time connection between event and previous exposure to summer heat. | Need for integration of widely different expertise sectors. |
| The syndrome is not seen in autochtonous cattle breeds with no summer anestrus and more regular distribution of calvings all over the year. | Lack of a validated trained immunity model in cattle. |
| Previous evidence of a major impact of stressors in the dry period on milk yield. | No studies about epigenetic changes in innate immunity genes of affected cows. |
| Previous evidence of life-long reduced milk yield in Friesian cows born from dams with summer dry period. | Overlapping of diverse stressors may be a serious confounding element. |
| Evidence in cattle of epigenetic changes of genes involved in innate immunity. | A cause/effect relationship can be hardly defined. |
| Reduction of heat stress in summer causes lesser drop of milk yield in autumn. | Experimental studies present utmost complexity and several operational constraints. |
| OPPORTUNITIES | THREATS |
| Establishment of useful investigation models into the crucial issues of adaptation physiology and sterile inflammation. | Continuous evolution of the dairy farming sector. |
| A wide scope for translation of the main scientific findings into working protocols in the animal health and welfare sectors. | Poor awareness of the real costs incurred as a result of the autumn low yield syndrome. |
| Establishment of new strategies for better profitability of dairy farming activities. | Poor awareness of the role of innate immunity in the response to non-infectious stressors. |
| Poor awareness of long-term impact of heat stress | |
| Establishment of adequate investigation tools for an effective monitoring of cattle herds. | Poor awareness of the crucial links between heat stress and quality of primary production. |
| A modern concept of the requirements to be met by welfare-friendly farms. | Farmers and veterinary practitioners often think of the syndrome as an unavoidable cost of farming activities. |
| Poor awareness of the crucial links between heat stress and quality of primary production. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amadori, M.; Spelta, C. The Autumn Low Milk Yield Syndrome in High Genetic Merit Dairy Cattle: The Possible Role of a Dysregulated Innate Immune Response. Animals 2021, 11, 388. https://doi.org/10.3390/ani11020388
Amadori M, Spelta C. The Autumn Low Milk Yield Syndrome in High Genetic Merit Dairy Cattle: The Possible Role of a Dysregulated Innate Immune Response. Animals. 2021; 11(2):388. https://doi.org/10.3390/ani11020388
Chicago/Turabian StyleAmadori, Massimo, and Chiara Spelta. 2021. "The Autumn Low Milk Yield Syndrome in High Genetic Merit Dairy Cattle: The Possible Role of a Dysregulated Innate Immune Response" Animals 11, no. 2: 388. https://doi.org/10.3390/ani11020388
APA StyleAmadori, M., & Spelta, C. (2021). The Autumn Low Milk Yield Syndrome in High Genetic Merit Dairy Cattle: The Possible Role of a Dysregulated Innate Immune Response. Animals, 11(2), 388. https://doi.org/10.3390/ani11020388






