Contactless Video-Based Heart Rate Monitoring of a Resting and an Anesthetized Pig
Abstract
:Simple Summary
Abstract
1. Introduction
- Investigate the combination of bandpass filtering and short-time Fourier transform (STFT) with sliding windows for extracting HR from noisy input data in a continuous fashion;
- Explore different regions of interests (ROI) from different anatomical parts of the pig’s body to find the most suitable ROI for signal extraction of cardiac activity;
- Optimize the different heart rate extraction processing steps to minimize the computational complexity of the algorithm for implementation of real-time monitoring applications.
2. Materials and Methods
2.1. Experimental Setup
2.1.1. Experiment on the Anesthetized Pig
2.1.2. Experiment on the Resting Pig
2.2. Algorithm
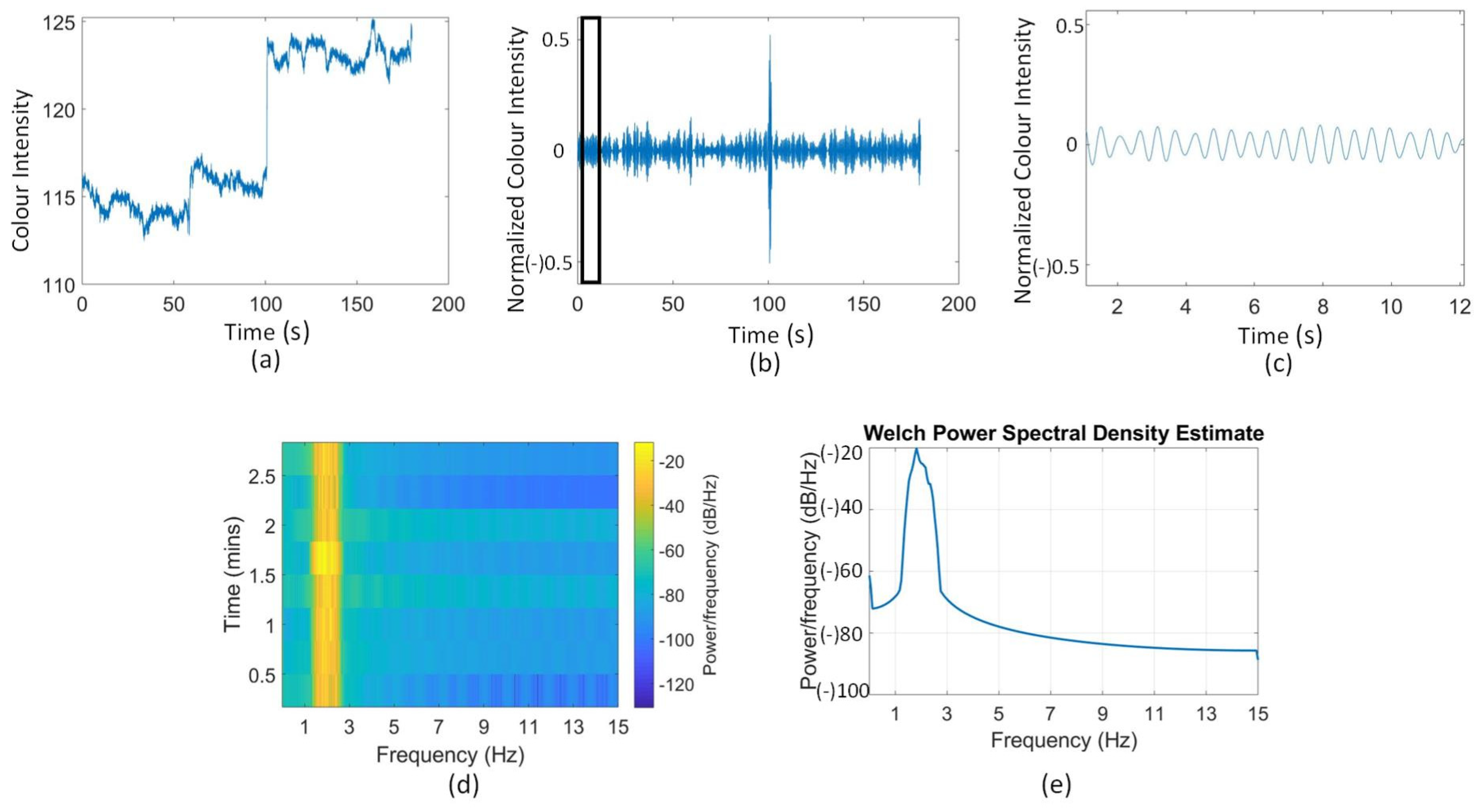
2.2.1. Region of Interest Selection
2.2.2. Noise Removal
2.2.3. Heart Rate Extraction
| Algorithms 1 Computation details of HR extraction | |
| Input: single colour signal ; sampling rate r; window size w; window function wf; overlap window size wn (here wn=3*w/4); minimum frequency f1 and maximum frequency f2. | |
| Output: Estimated heart rate hr | |
| 1: | |
| 2: | |
| 3: | For i in [1, 2, …, K, K= ⌊N/(w-wn) ⌋ ] do |
| 4: | |
| 5: | end for |
| 6: | For k in [1,2,…K] do |
| 7: | |
| 8: | |
| 9: | |
| 10: | |
| 11: | |
| 12: | end for |
2.2.4. Validation
2.2.5. Channel Selection
2.2.6. Algorithm Comparison
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Borell, E.; Langbein, J.; Després, G.; Hansen, S.; Leterrier, C.; Marchant-Forde, J.; Marchant-Forde, R.; Minero, M.; Mohr, E.; Prunier, A.; et al. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Von Borell, E.; Dobson, H.; Prunier, A. Stress, behaviour and reproductive performance in female cattle and pigs. Horm. Behav. 2007, 52, 130–138. [Google Scholar] [CrossRef]
- Schouten, W.G.; Wiegant, V.M. Individual responses to acute and chronic stress in pigs. Acta Physiol. Scand. Suppl. 1997, 640, 88–91. [Google Scholar]
- Joosen, P.; Norton, T.; Marchant-Forde, J.; Berckmans, D. Animal welfare monitoring by real-time physiological signals. In Proceedings of the 9th European Conference on Precision Livestock Farming, Cork, Ireland, 26–29 August 2019; pp. 337–344. [Google Scholar]
- Braga, V.A.; Burmeister, M.A. Applications of Telemetry in Small Laboratory Animals for Studying Cardiovascular Diseases; INTECH Open Access Publisher: London, UK, 2011; ISBN 9533074159. [Google Scholar]
- Pereira, C.B.; Kunczik, J.; Bleich, A.; Haeger, C.; Kiessling, F.; Thum, T.; Tolba, R.; Lindauer, U.; Treue, S.; Czaplik, M. Perspective review of optical imaging in welfare assessment in animal-based research. J. Biomed. Opt. 2019, 24, 070601. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.; Brugarolas, R.; Walker, K.; Mealin, S.; Cleghern, Z.; Yuschak, S.; Condit, J.; Adin, D.; Russenberger, J.; Gruen, M. Preliminary Evaluation of a Wearable Sensor System for Heart Rate Assessment in Guide Dog Puppies. IEEE Sens. J. 2020, 20, 9449–9459. [Google Scholar] [CrossRef]
- Duda, N.; Barthule, A.; Ripperger, S.; Mayer, F.; Weigel, R.; Koelpin, A. Non-Invasive Low Power ECG for Heart Beat Detection of Bats. In Proceedings of the 2019 IEEE Topical Conference on Wireless Sensors and Sensor Networks (WiSNet), Orlando, FL, USA, 20–23 January 2019; pp. 1–4. [Google Scholar]
- Youssef, A.; Peña Fernández, A.; Wassermann, L.; Biernot, S.; Wittauer, E.-M.; Bleich, A.; Hartung, J.; Berckmans, D.; Norton, T. An Approach towards Motion-Tolerant PPG-Based Algorithm for Real-Time Heart Rate Monitoring of Moving Pigs. Sensors 2020, 20, 4251. [Google Scholar] [CrossRef]
- Poh, M.-Z.; McDuff, D.J.; Picard, R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express 2010, 18, 10762. [Google Scholar] [CrossRef]
- Li, M.H.; Yadollahi, A.; Taati, B. Noncontact Vision-Based Cardiopulmonary Monitoring in Different Sleeping Positions. IEEE J. Biomed. Health Inform. 2017, 21, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- De Haan, G.; Jeanne, V. Robust pulse rate from chrominance-based rPPG. IEEE Trans. Biomed. Eng. 2013, 60, 2878–2886. [Google Scholar] [CrossRef]
- Wang, W.; den Brinker, A.C.; Stuijk, S.; de Haan, G. Algorithmic principles of remote PPG. IEEE Trans. Biomed. Eng. 2016, 64, 1479–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinstein, M.; Wu, H.-Y.; Durand, F.; Shih, E.; Freeman, W.; Guttag, J. Eulerian video magnification for revealing subtle changes in the world. ACM Trans. Graph. 2012, 31, 1–8. [Google Scholar]
- Alghoul, K.; Alharthi, S.; Al Osman, H.; El Saddik, A. Heart rate variability extraction from videos signals: ICA vs. EVM comparison. IEEE Access 2017, 5, 4711–4719. [Google Scholar] [CrossRef]
- Jorquera-Chavez, M.; Fuentes, S.; Dunshea, F.R.; Warner, R.D.; Poblete, T.; Jongman, E.C. Modelling and validation of computer vision techniques to assess heart rate, eye temperature, ear-base temperature and respiration rate in cattle. Animals 2019, 9, 1089. [Google Scholar] [CrossRef] [Green Version]
- Unakafov, A.M.; Möller, S.; Kagan, I.; Gail, A.; Treue, S.; Wolf, F. Using imaging photoplethysmography for heart rate estimation in non-human primates. PLoS ONE 2018, 13, e0202581. [Google Scholar] [CrossRef] [Green Version]
- Kunczik, J.; Pereira, C.B.; Zieglowski, L.; Tolba, R.; Wassermann, L.; Häger, C.; Bleich, A.; Janssen, H.; Thum, T.; Czaplik, M. Remote vitals monitoring in rodents using video recordings. Biomed. Opt. Express 2019, 10, 4422–4436. [Google Scholar] [CrossRef]
- Costa, A.; Borgonovo, F.; Leroy, T.; Berckmans, D.; Guarino, M. Dust concentration variation in relation to animal activity in a pig barn. Biosyst. Eng. 2009, 104, 118–124. [Google Scholar] [CrossRef]
- Oczak, M.; Ismayilova, G.; Costa, A.; Viazzi, S.; Sonoda, L.T.; Fels, M.; Bahr, C.; Hartung, J.; Guarino, M.; Berckmans, D. Analysis of aggressive behaviours of pigs by automatic video recordings. Comput. Electron. Agric. 2013, 99, 209–217. [Google Scholar] [CrossRef]
- Kashiha, M.; Bahr, C.; Haredasht, S.A.; Ott, S.; Moons, C.P.H.; Niewold, T.A.; Ödberg, F.O.; Berckmans, D. The automatic monitoring of pigs water use by cameras. Comput. Electron. Agric. 2013, 90, 164–169. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, W.; Liu, D.; Steibel, J.; Siegford, J.; Wurtz, K.; Han, J.; Norton, T. Detection of aggressive behaviours in pigs using a RealSence depth sensor. Comput. Electron. Agric. 2019, 166, 105003. [Google Scholar] [CrossRef]
- Yang, A.; Huang, H.; Zhu, X.; Yang, X.; Chen, P.; Li, S.; Xue, Y. Automatic recognition of sow nursing behaviour using deep learning-based segmentation and spatial and temporal features. Biosyst. Eng. 2018, 175, 133–145. [Google Scholar] [CrossRef]
- Zhao, F.; Li, M.; Qian, Y.; Tsien, J.Z. Remote Measurements of Heart and Respiration Rates for Telemedicine. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, C.B.; Dohmeier, H.; Kunczik, J.; Hochhausen, N.; Tolba, R.; Czaplik, M. Contactless monitoring of heart and respiratory rate in anesthetized pigs using infrared thermography. PLoS ONE 2019, 14, e0224747. [Google Scholar]
- Blanik, N.; Pereira, C.; Czaplik, M.; Blazek, V.; Leonhardt, S. Remote Photopletysmographic Imaging of Dermal Perfusion in a porcine animal model. In Proceedings of the 15th International Conference on Biomedical Engineering, Singapore, 4–7 December 2013; Springer: Berlin/Heidelberg, Germany, 2014; pp. 92–95. [Google Scholar]
- Addison, P.S.; Jacquel, D.; Foo, D.M.H.; Antunes, A.; Borg, U.R. Video-Based Physiologic Monitoring during an Acute Hypoxic Challenge: Heart Rate, Respiratory Rate, and Oxygen Saturation. Anesth. Analg. 2017, 125, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Haugse, C.N.; Dinusson, W.E.; Erickson, D.O.; Johnson, J.N.; Buchanan, M.L. A day in the life of a pig. North Dakota Farm Res. 1965, 23, 18–23. [Google Scholar]
- Teng, G.; Yu, Q. Pig behavior research and its application in breeding-landrace pigs as an example. Biomed. Res. 2017, 28, 111–117. [Google Scholar]
- Warriss, P.D.; Brown, S.N.; Franklin, J.G.; Kestin, S.C. The thickness and quality of backfat in various pig breeds and their relationship to intramuscular fat and the setting of joints from the carcasses. Meat Sci. 1990, 28, 21–29. [Google Scholar] [CrossRef]
- Unakafov, A.M. Pulse rate estimation using imaging photoplethysmography: Generic framework and comparison of methods on a publicly available dataset. Biomed. Phys. Eng. Express 2018, 4, 45001. [Google Scholar] [CrossRef]
- Strauch, J.T.; Lauten, A.; Zhang, N.; Wahlers, T.; Griepp, R.B. Anatomy of Spinal Cord Blood Supply in the Pig. Ann. Thorac. Surg. 2007, 83, 2130–2134. [Google Scholar] [CrossRef]
- Litwin, L. FIR and IIR digital filters. IEEE Potentials 2000, 19, 28–31. [Google Scholar] [CrossRef]
- Youssef, A.; Pena Fernandez, A.; Wasserman, L.; Biernot, S.; Bleich, A.; Hartung, J.; Norton, T. Heart rate monitoring in pigs using photo pethysmography (PPG) technology. In Proceedings of the 9th European Conference on Precision Livestock Farming, Cork, Ireland, 26–29 August 2019; pp. 842–850. [Google Scholar]
- Hülsbusch, M. An Image-Based Functional Method for Opto-Electronic Detection of Skin-Perfusion; RWTH Aachen: Aachen, Germany, 2008. [Google Scholar]
- Feng, L.; Po, L.-M.; Xu, X.; Li, Y.; Ma, R. Motion-resistant remote imaging photoplethysmography based on the optical properties of skin. IEEE Trans. Circuits Syst. Video Technol. 2014, 25, 879–891. [Google Scholar] [CrossRef]
- Fortin, A. Development of backfat and individual fat layers in the pig and its relationship with carcass lean. Meat Sci. 1986, 18, 255–270. [Google Scholar] [CrossRef]
- Antink, C.H.; Pirhonen, M.; Väätäjä, H.; Somppi, S.; Törnqvist, H.; Cardó, A.V.; Teichmann, D.; Vainio, O.; Surakka, V.; Vehkaoja, A. Sensor Fusion for Unobtrusive Respiratory Rate Estimation in Dogs. IEEE Sens. J. 2019, 19, 7072–7081. [Google Scholar] [CrossRef]





| R Channel | G Channel | B Channel | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Window Size (s) | Window Function | MAE | RMSE | PE3.5 | MAE | RMSE | PE3.5 | MAE | RMSE | PE3.5 |
| 8.53 | rect | 5.41 | 6.41 | 37% | 4.66 | 5.25 | 22% | 6.11 | 6.94 | 31% |
| 8.53 | hamming | 5.67 | 6.62 | 31% | 4.74 | 5.37 | 22% | 6.45 | 7.31 | 30% |
| 8.53 | hanning | 5.85 | 6.80 | 30% | 4.81 | 5.46 | 22% | 6.72 | 7.55 | 27% |
| 8.53 | blackman | 6.17 | 7.11 | 26% | 5.17 | 5.90 | 20% | 6.63 | 7.49 | 28% |
| 17.07 | rect | 3.86 | 5.44 | 54% | 3.11 | 4.61 | 54% | 6.59 | 7.66 | 23% |
| 17.07 | hamming | 4.39 | 5.94 | 49% | 3.26 | 4.80 | 54% | 7.55 | 8.69 | 23% |
| 17.07 | hanning | 4.75 | 6.40 | 46% | 3.26 | 4.80 | 54% | 7.55 | 8.69 | 23% |
| 17.07 | blackman | 4.83 | 6.42 | 46% | 3.43 | 4.96 | 51% | 7.55 | 8.52 | 18% |
| 34.13 | rect | 3.36 | 4.61 | 67% | 2.62 | 3.40 | 67% | 6.59 | 7.84 | 22% |
| 34.13 | hamming | 3.66 | 4.76 | 61% | 2.33 | 3.09 | 67% | 6.35 | 7.54 | 28% |
| 34.13 | hanning | 3.66 | 4.76 | 61% | 2.33 | 3.09 | 67% | 6.48 | 7.60 | 22% |
| 34.13 | blackman | 3.68 | 4.77 | 61% | 2.33 | 3.09 | 67% | 6.77 | 7.88 | 22% |
| 68.27 | rect | 2.96 | 3.96 | 57% | 2.03 | 2.76 | 59% | 7.21 | 8.19 | 14% |
| 68.27 | hamming | 3.34 | 4.30 | 57% | 2.03 | 2.76 | 59% | 8.21 | 9.57 | 14% |
| 68.27 | hanning | 3.34 | 4.30 | 57% | 2.03 | 2.76 | 59% | 8.21 | 9.57 | 14% |
| 68.27 | blackman | 3.46 | 4.35 | 57% | 2.03 | 2.76 | 59% | 6.58 | 8.45 | 29% |
| G | GRD | aGRD | CHROM | POS | ICA | |
|---|---|---|---|---|---|---|
| MAE | 2.33 | 2.79 | 2.76 | 4.66 | 4.32 | 3.04 |
| RMSE | 3.09 | 3.74 | 3.64 | 6.07 | 5.79 | 4.12 |
| PE3.5 | 67% | 53% | 49% | 33% | 41% | 54% |
| Face | Front Leg | Abdomen | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Window Size (s) | MAE | RMSE | PE3.5 | MAE | RMSE | PE3.5 | MAE | RMSE | PE3.5 |
| 34.14 | 5.48 | 6.98 | 45% | 7.04 | 8.47 | 34% | 5.24 | 7.07 | 53% |
| 68.27 | 5.64 | 6.52 | 29% | 6.27 | 7.52 | 32% | 4.69 | 6.43 | 57% |
| MAE | RMSE | PE3.5 | |
|---|---|---|---|
| Pig (current study) | 4.69 | 6.43 | 57% |
| Primate [17] | 4.4 | / | 56% |
| Human [31] | 1.99 | 3.25 | 87% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Youssef, A.; Larsen, M.; Rault, J.-L.; Berckmans, D.; Marchant-Forde, J.N.; Hartung, J.; Bleich, A.; Lu, M.; Norton, T. Contactless Video-Based Heart Rate Monitoring of a Resting and an Anesthetized Pig. Animals 2021, 11, 442. https://doi.org/10.3390/ani11020442
Wang M, Youssef A, Larsen M, Rault J-L, Berckmans D, Marchant-Forde JN, Hartung J, Bleich A, Lu M, Norton T. Contactless Video-Based Heart Rate Monitoring of a Resting and an Anesthetized Pig. Animals. 2021; 11(2):442. https://doi.org/10.3390/ani11020442
Chicago/Turabian StyleWang, Meiqing, Ali Youssef, Mona Larsen, Jean-Loup Rault, Daniel Berckmans, Jeremy N. Marchant-Forde, Joerg Hartung, André Bleich, Mingzhou Lu, and Tomas Norton. 2021. "Contactless Video-Based Heart Rate Monitoring of a Resting and an Anesthetized Pig" Animals 11, no. 2: 442. https://doi.org/10.3390/ani11020442
APA StyleWang, M., Youssef, A., Larsen, M., Rault, J.-L., Berckmans, D., Marchant-Forde, J. N., Hartung, J., Bleich, A., Lu, M., & Norton, T. (2021). Contactless Video-Based Heart Rate Monitoring of a Resting and an Anesthetized Pig. Animals, 11(2), 442. https://doi.org/10.3390/ani11020442








