Copper Oxide Nanoparticles Alter Serum Biochemical Indices, Induce Histopathological Alterations, and Modulate Transcription of Cytokines, HSP70, and Oxidative Stress Genes in Oreochromis niloticus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Nanoparticles
2.2. Fish Acclimation, Maintenance, and Rearing Conditions
2.3. Ethical Approval
2.4. Preparation of CuONPs Stock Solution
2.5. Experimental Setup and CuONPs Exposure
2.5.1. The 96-h Acute Toxicity Test
2.5.2. Sub-Acute Toxicity Test
2.6. Sample Collection
2.6.1. Serum Samples
2.6.2. Tissue Samples
2.7. Serum Biochemical Indices
2.8. Gene Transcription
2.9. Histopathological Studies
2.10. Statistical Analysis
3. Results
3.1. Characterization of CuONPs
3.2. Serum Biochemical Indices
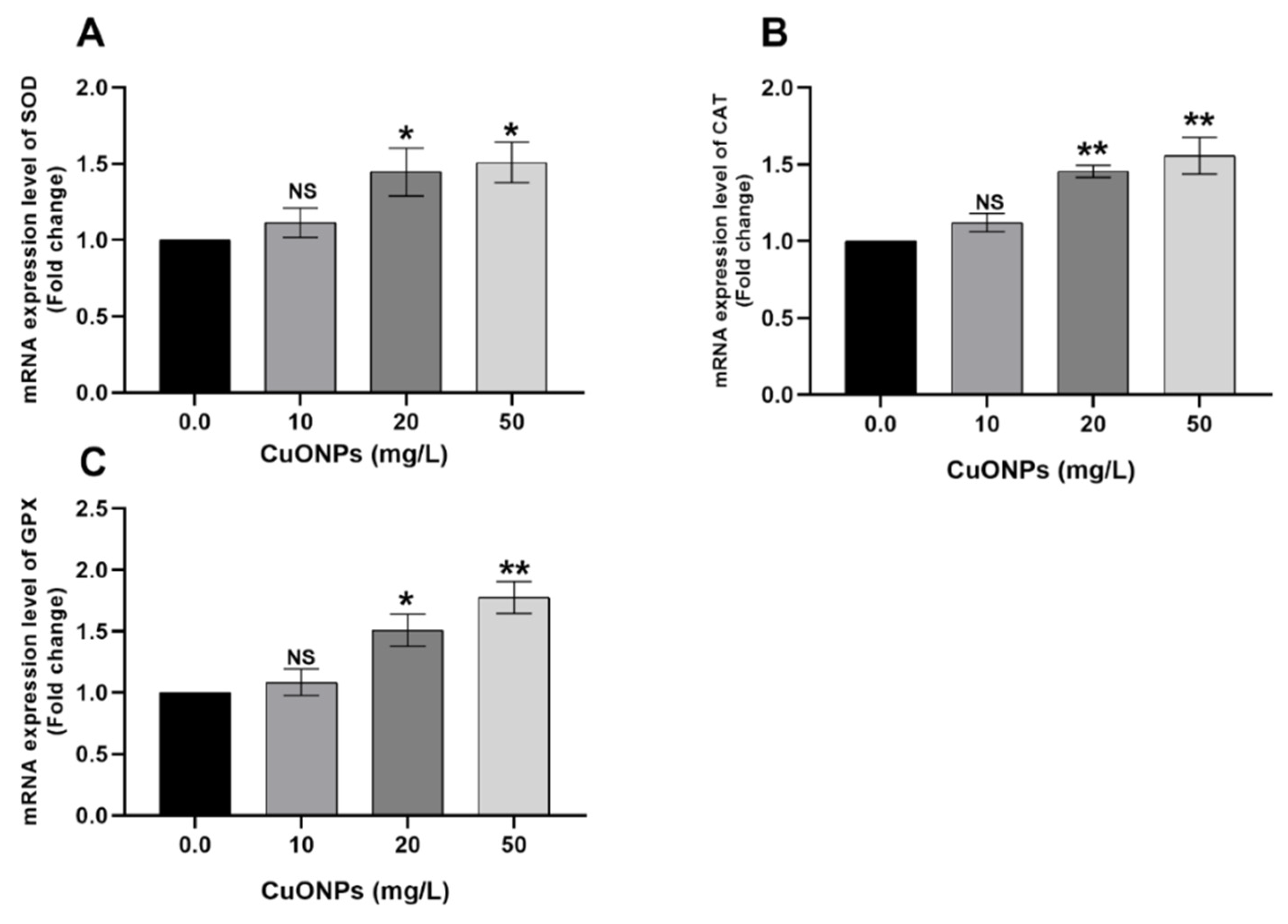
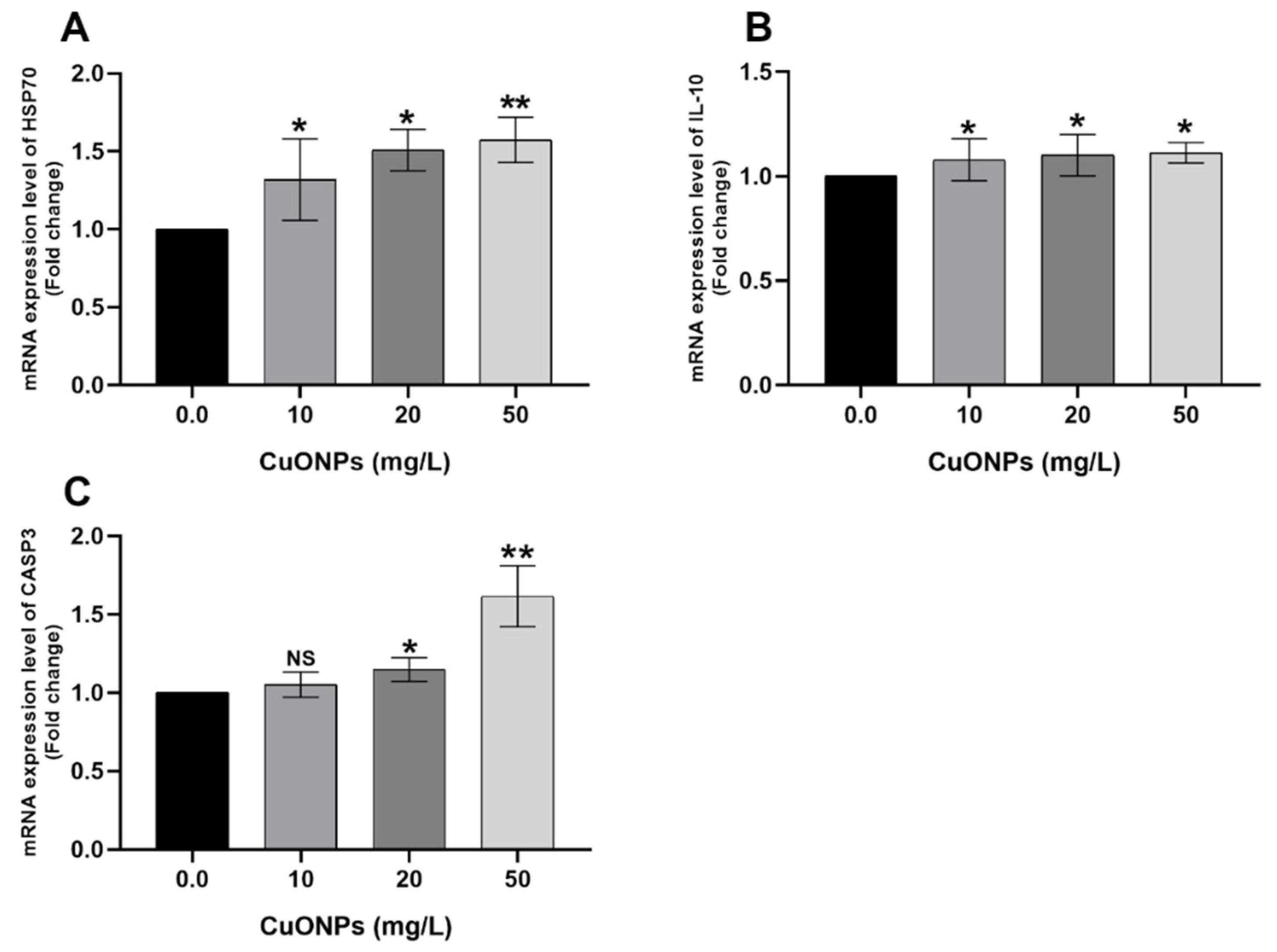
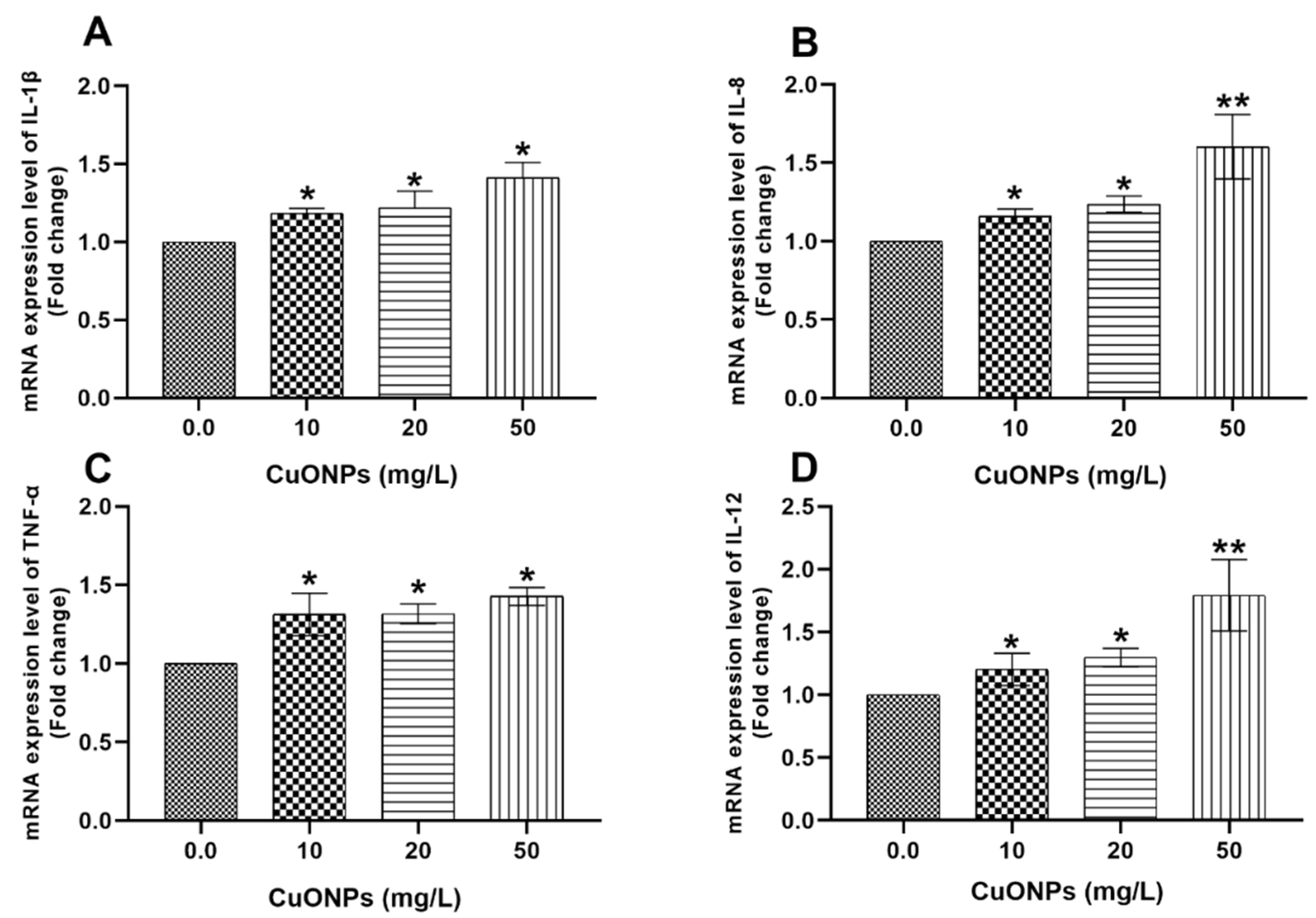
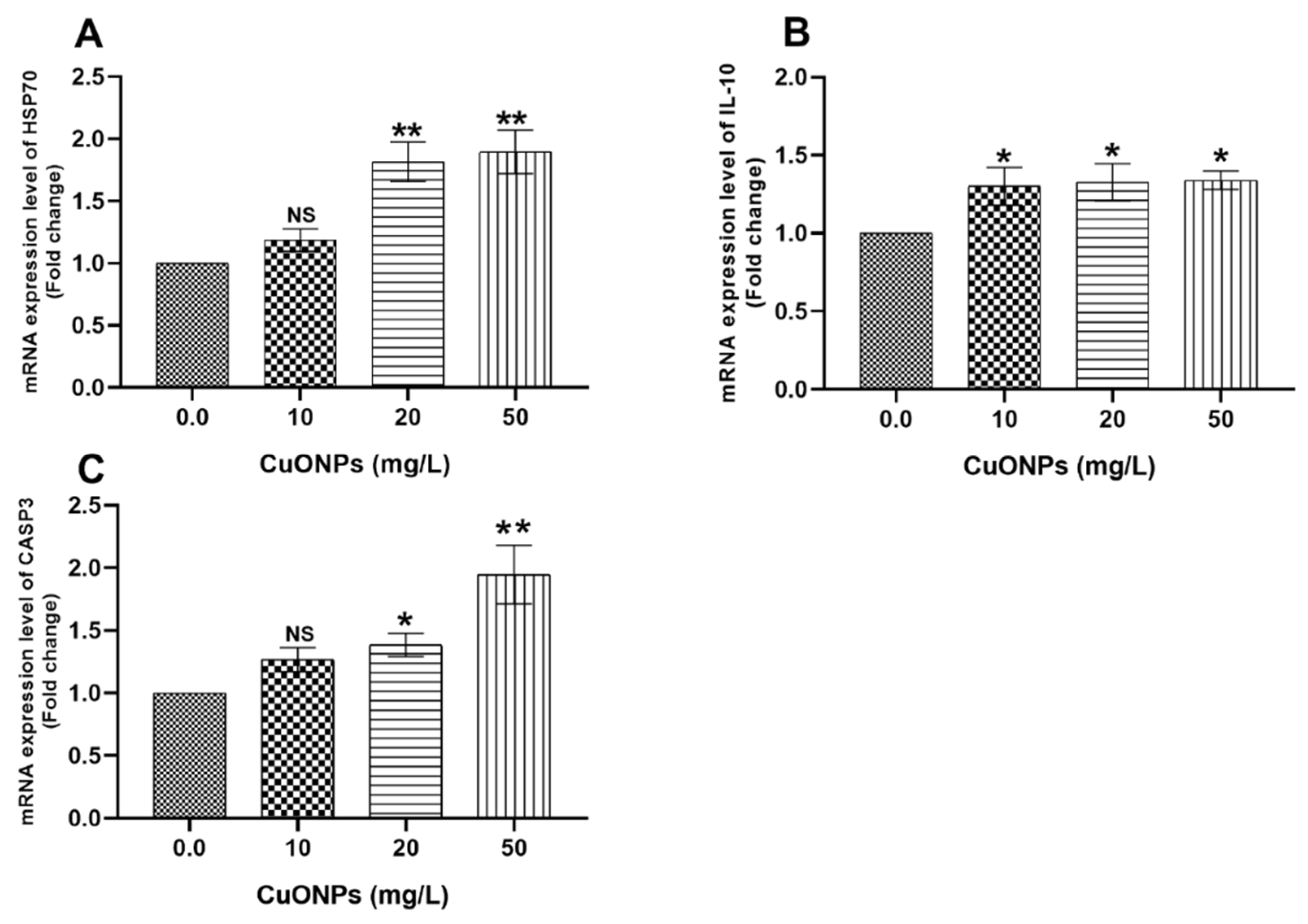
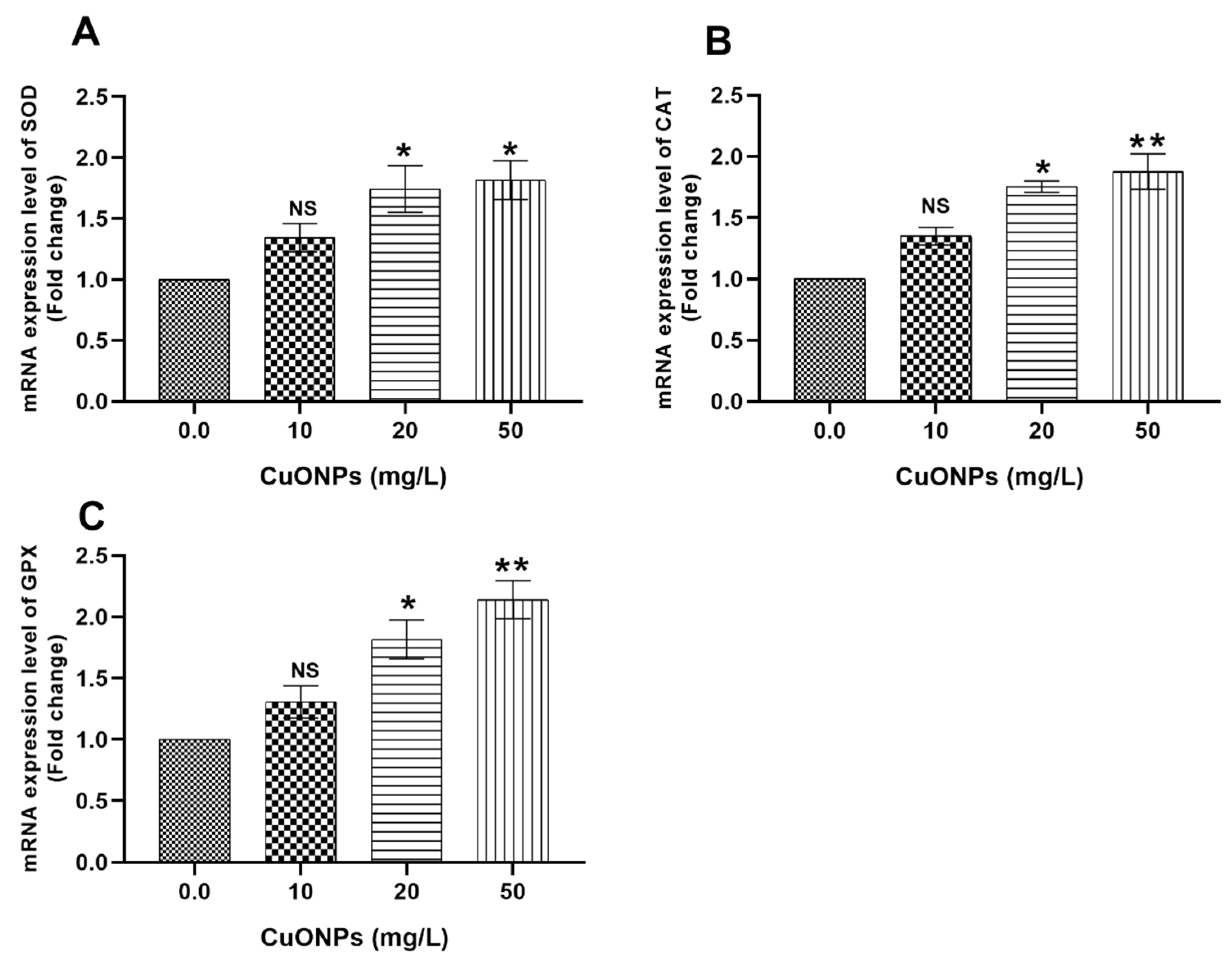
3.3. Gene Transcription
3.3.1. Gill Tissues
3.3.2. Hepatic Tissues
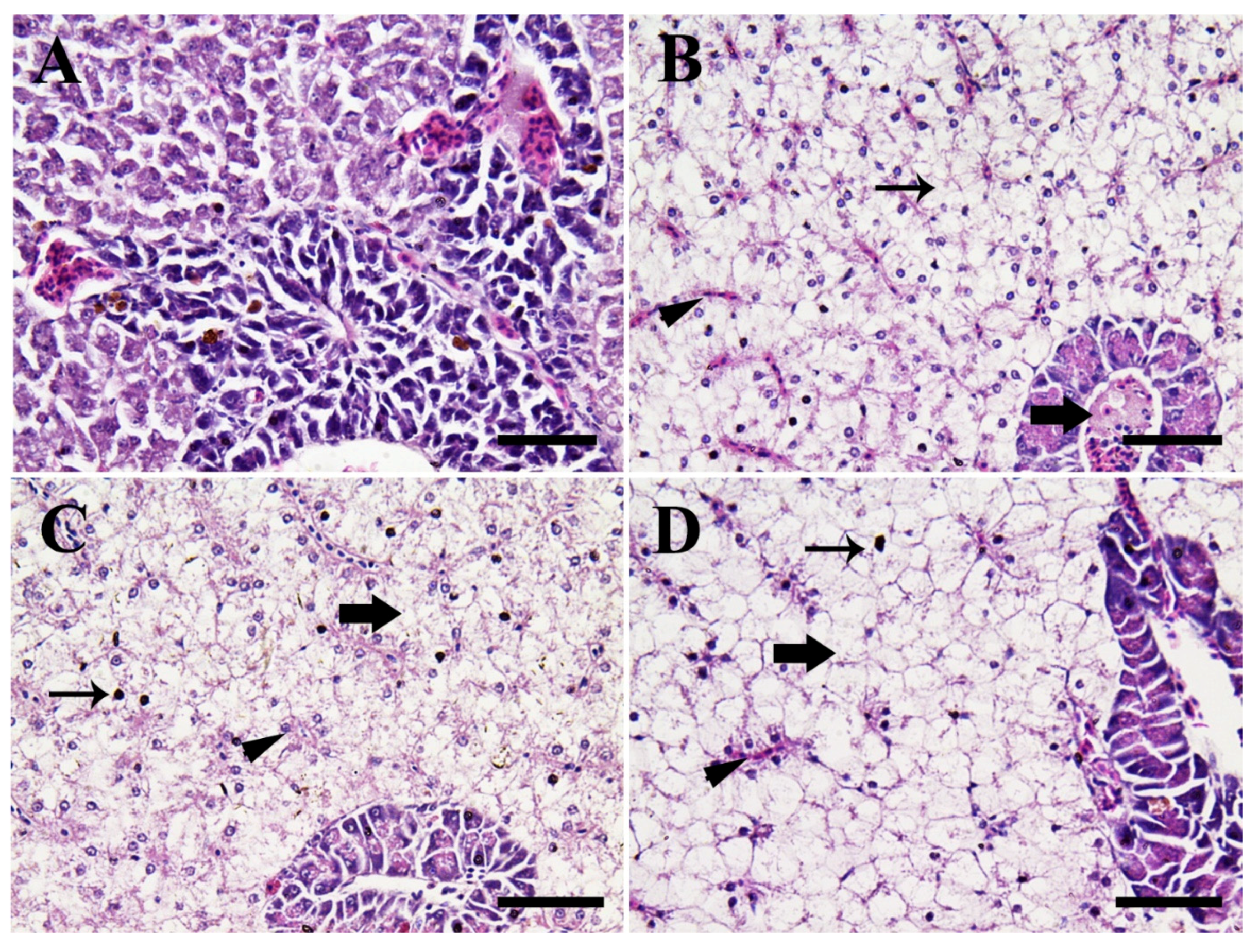
3.4. Histopathological Alterations
3.4.1. Gills
3.4.2. Hepatopancreatic Tissues
3.4.3. Kidneys
4. Discussion
4.1. Serum Biochemical Indices
4.2. Gene Transcriptions
4.2.1. Cytokines
4.2.2. Oxidative Stress-Related Genes
4.2.3. HSP70
4.2.4. CASP3
4.3. Histopathological Alterations
4.3.1. Gills
4.3.2. Hepatopancreatic Tissues
4.3.3. Posterior Kidneys
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lines, M. Nanomaterials for practical functional uses. J. Alloys Compd. 2008, 449, 242–245. [Google Scholar] [CrossRef]
- Radad, K.; Al-Shraim, M.; Moldzio, R.; Rausch, W.-D. Recent advances in benefits and hazards of engineered nanoparticles. Environ. Toxicol. Pharmacol. 2012, 34, 661–672. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Zeng, J.; Jing, L.; Qiao, R.; Liu, C.; Jiao, M.; Li, Z.; Gao, M. Aqueous synthesis of PEGylated copper sulfide nanoparticles for photoacoustic imaging of tumors. Nanoscale 2015, 7, 11075–11081. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.; Wang, R.; Xu, B.; Li, Y. Synthesis, characterization and catalytic properties of CuO nanocrystals with various shapes. Nanotechnology 2006, 17, 3939–3943. [Google Scholar] [CrossRef]
- Kalatehjari, P.; Yousefian, M.; Khalilzadeh, M.A. Assessment of antifungal effects of copper nanoparticles on the growth of the fungus Saprolegnia sp. on white fish (Rutilus frisii kutum) eggs. Egypt. J. Aquat. Res. 2015, 41, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Pantidos, N.; Edmundson, M.C.; Horsfall, L. Room temperature bioproduction, isolation and anti-microbial properties of stable elemental copper nanoparticles. New Biotechnol. 2018, 40, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, F.; Lei, X.; Mang, L.; Cheng, S.; Song, J. Fluorescent copper nanoparticles: Recent advances in synthesis and applications for sensing metal ions. Nanoscale 2016, 8, 4852–4863. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Oranu, E.A.; Tao, M.; Keller, A.A. Release and detection of nanosized copper from a commercial antifouling paint. Water Res. 2016, 102, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Rahman, K.; Lu, Z.; Kwon, K.-S. Green laser sintering of copper oxide (CuO) nano particle (NP) film to form Cu conductive lines. AIP Adv. 2018, 8, 095008. [Google Scholar] [CrossRef] [Green Version]
- Horie, M.; Fujita, K. Chapter four—Toxicity of metal oxides nanoparticles. In Advances in Molecular Toxicology; Fishbein, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 5, pp. 145–178. [Google Scholar]
- Abdel-Daim, M.M.; Eissa, I.A.; Abdeen, A.; Abdel-Latif, H.M.; Ismail, M.; Dawood, M.A.; Hassan, A.M. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2019, 69, 44–50. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Dawood, M.A.; Menanteau-Ledouble, S.; El-Matbouli, M. Environmental transformation of n-TiO2 in the aquatic systems and their ecotoxicity in bivalve mollusks: A systematic review. Ecotoxicol. Environ. Saf. 2020, 200, 110776. [Google Scholar] [CrossRef]
- Malhotra, N.; Ger, T.-R.; Uapipatanakul, B.; Huang, J.-C.; Chen, K.H.-C.; Hsiao, C.-D. Review of copper and copper nanoparticle toxicity in fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef]
- Hua, J.; Vijver, M.G.; Ahmad, F.; Richardson, M.K.; Peijnenburg, W.J. Toxicity of different-sized copper nano- and submicron particles and their shed copper ions to zebrafish embryos. Environ. Toxicol. Chem. 2014, 33, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Adeleye, A.S.; Conway, J.R.; Garner, K.L.; Zhao, L.; Cherr, G.N.; Hong, J.; Gardea-Torresdey, J.L.; Godwin, H.A.; Hanna, S.; et al. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 2017, 7, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, S.; Thirumurthi, N.A.; Raghunath, A.; Vijayakumar, S.; Perumal, E. Acute and sub-lethal exposure to copper oxide nanoparticles causes oxidative stress and teratogenicity in zebrafish embryos. J. Appl. Toxicol. 2015, 36, 554–567. [Google Scholar] [CrossRef]
- Kaviani, E.; Naeemi, A.; Salehzadeh, A. Influence of copper oxide nanoparticle on hematology and plasma bio-chemistry of Caspian trout (Salmo trutta caspius), following acute and chronic exposure. Pollution 2019, 5, 225–234. [Google Scholar]
- Tuncsoy, M.; Duran, S.; Ay, O.; Cicik, B.; Erdem, C. Effects of copper oxide nanoparticles on antioxidant enzyme activities in liver tissue of Clarias gariepinus. Toxicol. Lett. 2017, 280, S186. [Google Scholar] [CrossRef]
- Duran, S.; Tuncsoy, M.; Ay, O.; Cicik, B.; Erdem, C. Accumulation of copper oxide nanoparticles in gill, liver and muscle tissues of Clarias gariepinus. Toxicol. Lett. 2017, 280, S186. [Google Scholar] [CrossRef]
- Al-Bairuty, G.A.; Shaw, B.J.; Handy, R.D.; Henry, T.B. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2013, 126, 104–115. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Hedayati, A.; Mirghaed, A.T.; Ghelichpour, M. Toxic effects of copper sulfate and copper nanoparticles on minerals, enzymes, thyroid hormones and protein fractions of plasma and histopathology in common carp Cyprinus carpio. Exp. Toxicol. Pathol. 2016, 68, 493–503. [Google Scholar] [CrossRef]
- Vajargah, M.F.; Yalsuyi, A.M.; Hedayati, A.; Faggio, C. Histopathological lesions and toxicity in common carp (Cyprinus carpio L. 1758) induced by copper nanoparticles. Microsc. Res. Tech. 2018, 81, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, B.; Rahmani, R.; Azadi, N.A.; Davari, B.; Johari, S.A.; Sobhani, P. Effect of waterborne copper oxide nanoparticles and copper ions on guppy (Poecilia reticulata): Bioaccumulation and histopathology. J. Adv. Environ. Health Res. 2015, 3, 215–223. [Google Scholar]
- Tesser, M.E.; de Paula, A.A.; Risso, W.E.; Monteiro, R.A.; do Espirito Santo Pereira, A.; Fraceto, L.F.; Bueno dos Reis Martinez, C. Sublethal effects of waterborne copper and copper nanoparticles on the freshwater Neotropical teleost Prochilodus lineatus: A comparative approach. Sci. Total Environ. 2020, 704, 135332. [Google Scholar] [CrossRef]
- Wang, T.; Long, X.; Liu, Z.; Cheng, Y.; Yan, S. Effect of copper nanoparticles and copper sulphate on oxidation stress, cell apoptosis and immune responses in the intestines of juvenile Epinephelus coioides. Fish Shellfish. Immunol. 2015, 44, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.R.; Sellegounder, D.; Kannan, M.; Deepa, S.; Senthilkumaran, B.; Basavaraju, Y. Effect of copper nanoparticles exposure in the physiology of the common carp (Cyprinus carpio): Biochemical, histological and proteomic approaches. Aquac. Fish. 2016, 1, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Aksakal, F.I.; Ciltas, A. Impact of copper oxide nanoparticles (CuO NPs) exposure on embryo development and expression of genes related to the innate immune system of zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 223, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wen, X.; Hu, Y.; Zhang, X.; Wang, D.; Yin, S. Copper nanoparticles induced oxidation stress, cell apoptosis and immune response in the liver of juvenile Takifugu fasciatus. Fish Shellfish. Immunol. 2019, 84, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Naeemi, A.S.; Elmi, F.; Vaezi, G.; Ghorbankhah, M. Copper oxide nanoparticles induce oxidative stress mediated apoptosis in carp (Cyprinus carpio) larva. Gene Rep. 2020, 19, 100676. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Menanteau-Ledouble, S.; El-Matbouli, M. The nature and consequences of co-infections in tilapia: A review. J. Fish Dis. 2020, 43, 651–664. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.-F.M. Tilapia Culture, 2nd ed.; Elsevier: Oxford, UK; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Abdel-Daim, M.M.; Dawood, M.A.; AlKahtane, A.A.; Abdeen, A.; Abdel-Latif, H.M.; Senousy, H.H.; Aleya, L.; Alkahtani, S. Spirulina platensis mediated the biochemical indices and antioxidative function of Nile tilapia (Oreochromis niloticus) intoxicated with aflatoxin B1. Toxicon 2020, 184, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Metwally, A.E.-S.; Elkomy, A.H.; Gewaily, M.S.; Abdo, S.E.; Abdel-Razek, M.A.; Soliman, A.A.; Amer, A.A.; Abdel-Razik, N.I.; Abdel-Latif, H.M.; et al. The impact of menthol essential oil against inflammation, immunosuppression, and histopathological alterations induced by chlorpyrifos in Nile tilapia. Fish Shellfish. Immunol. 2020, 102, 316–325. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.A.; Kadry, M.A.; Badran, S.R.; Marie, M.-A.S. Comparative toxicity of copper oxide bulk and nano particles in Nile Tilapia; Oreochromis niloticus: Biochemical and oxidative stress. J. Basic Appl. Zool. 2015, 72, 43–57. [Google Scholar] [CrossRef] [Green Version]
- Tuncsoy, M.; Duran, S.; Ay, O.; Cicik, B.; Erdem, C. Effects of copper oxide nanoparticles on antioxidant enzyme activities in gill and liver tissues of Oreochromis niloticus. Toxicol. Lett. 2016, 258, S269. [Google Scholar] [CrossRef]
- Abdel-Khalek, A.A.; Badran, S.R.; Marie, M.-A.S. Toxicity evaluation of copper oxide bulk and nanoparticles in Nile tilapia, Oreochromis niloticus, using hematological, bioaccumulation and histological biomarkers. Fish Physiol. Biochem. 2016, 42, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Tunçsoy, M.; Duran, S.; Ay, Ö.; Cicik, B.; Erdem, C. Effects of copper oxide nanoparticles on antioxidant enzyme activities and on tissue accumulation of Oreochromis niloticus. Bull. Environ. Contam. Toxicol. 2017, 99, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Tunçsoy, M.; Erdem, C. Copper accumulation in tissues of Oreochromis niloticus exposed to copper oxide nanoparticles and copper sulphate with their effect on antioxidant enzyme activities in liver. Water Air Soil Pollut. 2018, 229, 1725–1737. [Google Scholar] [CrossRef]
- Canli, E.G.; Dogan, A.; Canli, M. Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environ. Toxicol. Pharmacol. 2018, 62, 181–187. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2016, 6, 21–26. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Nutrient Requirements of Fish; National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer: Boston, MA, USA, 1998; ISBN 1461554071. [Google Scholar]
- Finney, D.J. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve; Cambridge University Press: Cambridge, UK, 1952. [Google Scholar]
- Coulombe, J.J.; Favreau, L. A new simple semimicro method for colorimetric determination of urea. Clin. Chem. 1963, 9, 102–108. [Google Scholar] [CrossRef]
- Larsen, K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta 1972, 41, 209–217. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A Colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Tietz, N.W.; Burtis, C.A.; Duncan, P.; Ervin, K.; Petitclerc, C.J.; Rinker, A.D.; Shuey, D.; Zygowicz, E.R. A reference method for measurement of alkaline phosphatase activity in human serum. Clin. Chem. 1983, 29, 751–761. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. The Hematoxylin and eosin. In Theory and Practice of Histological Techniques, 7th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Churchill Livingstone: Edinburgh, UK; New York, NY, USA, 2013; pp. 179–220. [Google Scholar]
- Khafaga, A.F.; Naiel, M.A.; Dawood, M.A.; Abdel-Latif, H.M. Dietary Origanum vulgare essential oil attenuates cypermethrin-induced biochemical changes, oxidative stress, histopathological alterations, apoptosis, and reduces DNA damage in Common carp (Cyprinus carpio). Aquat. Toxicol. 2020, 228, 105624. [Google Scholar] [CrossRef]
- Vaglio, A.; Landriscina, C. Changes in liver enzyme activity in the teleost Sparus aurata in response to cadmium intoxication. Ecotoxicol. Environ. Saf. 1999, 43, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Abdel-Tawwab, M.; Abdel-Latif, H.M. Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in fish. Rev. Aquac. 2020, 12, 2511–2526. [Google Scholar] [CrossRef]
- Teles, M.; Reyes-López, F.; Fierro-Castro, C.; Tort, L.; Soares, A.; Oliveira, M. Modulation of immune genes mRNA levels in mucosal tissues and DNA damage in red blood cells of Sparus aurata by gold nanoparticles. Mar. Pollut. Bull. 2018, 133, 428–435. [Google Scholar] [CrossRef]
- Goetz, F.W.; Planas, J.V.; MacKenzie, S. Tumor necrosis factors. Dev. Comp. Immunol. 2004, 28, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.; Hardie, L.; Daniels, G. Cytokines in fish: An update. Fish Shellfish. Immunol. 1996, 6, 291–304. [Google Scholar] [CrossRef]
- Savan, R.; Sakai, M. Genomics of fish cytokines. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Cerpa, S.; Maisey, K.; Reyes-López, F.; Toro-Ascuy, D.; Sandino, A.M.; Imarai, M. Fish cytokines and immune response. New Adv. Contrib. Fish Biol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, X.; Zhu, R.; Luo, Z.; Ren, B. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquat. Toxicol. 2016, 180, 56–70. [Google Scholar] [CrossRef]
- Saddick, S.; Afifi, M.; Abu Zinada, O.A. Effect of Zinc nanoparticles on oxidative stress-related genes and antioxidant enzymes activity in the brain of Oreochromis niloticus and Tilapia zillii. Saudi J. Biol. Sci. 2017, 24, 1672–1678. [Google Scholar] [CrossRef]
- Mahjoubiyan, M.; Naeemi, A.S.; Sheykhan, M. Toxicological effects of Ag2O and Ag2CO3 doped TiO2 nanoparticles and pure TiO2 particles on Zebrafish (Danio rerio). Chemosphere 2021, 263, 128182. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Todgham, A.; Ackerman, P.; Bibeau, M.; Nakano, K.; Schulte, P.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Iwama, G.K.; Thomas, P.T.; Forsyth, R.B.; Vijayan, M.M. Heat shock protein expression in fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Iwama, G.K.; Vijayan, M.M.; Forsyth, R.B.; Ackerman, P.A. Heat shock proteins and physiological stress in fish. Am. Zool. 1999, 39, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-N.; Tian, H.-Y.; Li, X.-F.; Zhu, J.; Cai, D.-S.; Xu, C.; Wang, F.; Zhang, D.-D.; Liu, W.-B. The effects of fructooligosaccharide on the immune response, antioxidant capability and HSP70 and HSP90 expressions in blunt snout bream (Megalobrama amblycephala Yih) under high heat stress. Aquaculture 2014, 433, 458–466. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Choi, Y.C.; Kim, R.; Lee, S.K. Multiwall carbon nanotube-induced apoptosis and antioxidant gene expression in the gills, liver, and intestine of Oryzias latipes. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ostaszewska, T.; Chojnacki, M.; Kamaszewski, M.; Sawosz-Chwalibóg, E. Histopathological effects of silver and copper nanoparticles on the epidermis, gills, and liver of Siberian sturgeon. Environ. Sci. Pollut. Res. 2016, 23, 1621–1633. [Google Scholar] [CrossRef] [Green Version]
- Handy, R.D.; Maunder, R.J. The biological roles of mucus: Importance for osmoregulation and osmoregulatory disorders of fish health. In Osmoregulation and Ion Transport: Integrating Physiological, Molecular and Environmental Aspects. Essential Reviews in Experimental Biology; Handy, R.D., Bury, N.R., Flik, G., Eds.; Society for Experimental Biology Press: London, UK, 2009; Volume 1, pp. 203–235. [Google Scholar]
- Grosell, M.; Blanchard, J.; Brix, K.; Gerdes, R. Physiology is pivotal for interactions between salinity and acute copper toxicity to fish and invertebrates. Aquat. Toxicol. 2007, 84, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M. Copper. Fish Physiology: Homeostasis and Toxicology of Essential Metals; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: San Diego, CA, USA, 2012; pp. 54–112. [Google Scholar]
- Shaw, B.J.; Al-Bairuty, G.; Handy, R.D. Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchus mykiss): Physiology and accumulation. Aquat. Toxicol. 2012, 90–101. [Google Scholar] [CrossRef]
- Al-Bairuty, G.A.; Boyle, D.; Henry, T.B.; Handy, R.D. Sublethal effects of copper sulphate compared to copper nanoparticles in rainbow trout (Oncorhynchus mykiss) at low pH: Physiology and metal accumulation. Aquat. Toxicol. 2016, 174, 188–198. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Weil, R.; Hyndman, K.A.; Denslow, N.D.; Powers, K.; Taylor, D.; Barber, D.S. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ. Sci. Technol. 2007, 41, 8178–8186. [Google Scholar] [CrossRef]
- Velma, V.; Tchounwou, P.B. Chromium-induced biochemical, genotoxic and histopathologic effects in liver and kidney of goldfish, carassius auratus. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 698, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, B.J.; Handy, R.D. Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environ. Int. 2011, 37, 1083–1097. [Google Scholar] [CrossRef]
- Noureen, A.; Jabeen, F.; Tabish, T.A.; Yaqub, S.; Ali, M.; Chaudhry, A.S. Assessment of copper nanoparticles (Cu-NPs) and copper (II) oxide (CuO) induced hemato- and hepatotoxicity in Cyprinus carpio. Nanotechnology 2018, 29, 144003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef] [Green Version]
- El Euony, O.I.; Elblehi, S.S.; Abdel-Latif, H.M.; Abdel-Daim, M.M.; El-Sayed, Y.S. Modulatory role of dietary Thymus vulgaris essential oil and Bacillus subtilis against thiamethoxam-induced hepatorenal damage, oxidative stress, and immunotoxicity in African catfish (Clarias garipenus). Environ. Sci. Pollut. Res. 2020, 27, 23108–23128. [Google Scholar] [CrossRef] [PubMed]



| Target mRNA | Primer Sequences (5′–3′) (F: Forward and R: Reverse) | NCBI GenBank Accession No. |
|---|---|---|
| CASP3 | F-GGCTCTTCGTCTGCTTCTGT | GQ421464.1 |
| R-GGGAAATCGAGGCGGTATCT | ||
| HSP70 | F-CATCGCCTACGGTCTGGACAA | FJ207463.1 |
| R-GCCGTCTTCAATGGTCAGGAT | ||
| SOD | F-CCCTACGTCAGTGCAGAGAT | JF801727.1 |
| R-GTCACGTCTCCCTTTGCAAG | ||
| GPX | F-CGCCGAAGGTCTCGTTATTT | NM_001279711.1 |
| R-TCCCTGGACGGACATACTT | ||
| CAT | F-CCCAGCTCTTCATCCAGAAAC | JF801726.1 |
| R-GCCTCCGCATTGTACTTCTT | ||
| IL-10 | F-CTGCTAGATCAGTCCGTCGAA | XM_003441366.2 |
| R-GCAGAACCGTGTCCAGGTAA | ||
| TNF-α | F-GAAGCAGCTCCACTCTGATGA | JF957373.1 |
| R-ACAGCGTGTCTCCTTCGTTCA | ||
| IL-1β | F-AAGGATGACGACAAGCCAACC | XM_003460625.2 |
| R-GCGGACAGACATGAGAGTGC | ||
| IL-8 | F-TCATTGTCAGCTCCATCGTG | NM_001279704.1 |
| R-CCTGTCCTTTTCAGTGTGGC | ||
| IL-12 | F-GGGTGCGAGTCAGCTATGAG | XM_003437924.4 |
| R-GGTTGTGGATTGGTTGCGTC | ||
| β-actin | F-CCACACAGTGCCCATCTACGA | EU887951.1 |
| R-CACGCTCTGTCAGGATCTTCA |
| Parameters | Copper Oxide Nanoparticles (CuONPs) (mg/L) | |||
|---|---|---|---|---|
| 0.0 | 10 | 20 | 50 | |
| Urea (mg/dL) | 7.85 ± 0.81 c | 9.65 ± 0.39 bc | 11.01 ± 0.84 b | 14.91 ± 0.88 a |
| Creatinine (mg%) | 0.64 ± 0.06 c | 0.99 ± 0.17 b | 1.11 ± 0.09 ab | 1.87 ± 0.25 a |
| AST (U/L) | 59.74 ± 2.89 c | 73.01 ± 4.02 b | 79.53 ± 4.29 b | 104.3 ± 0.98 a |
| ALT (U/L) | 13.88 ± 0.23 c | 19.86 s± 0.81 b | 21.96 ± 0.42 b | 29.83 ± 2.56 a |
| ALP (U/L) | 9.61 ± 0.31 c | 12.63 ± 0.92 b | 15.22 ± 0.18 b | 21.24 ± 1.53 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Latif, H.M.R.; Dawood, M.A.O.; Mahmoud, S.F.; Shukry, M.; Noreldin, A.E.; Ghetas, H.A.; Khallaf, M.A. Copper Oxide Nanoparticles Alter Serum Biochemical Indices, Induce Histopathological Alterations, and Modulate Transcription of Cytokines, HSP70, and Oxidative Stress Genes in Oreochromis niloticus. Animals 2021, 11, 652. https://doi.org/10.3390/ani11030652
Abdel-Latif HMR, Dawood MAO, Mahmoud SF, Shukry M, Noreldin AE, Ghetas HA, Khallaf MA. Copper Oxide Nanoparticles Alter Serum Biochemical Indices, Induce Histopathological Alterations, and Modulate Transcription of Cytokines, HSP70, and Oxidative Stress Genes in Oreochromis niloticus. Animals. 2021; 11(3):652. https://doi.org/10.3390/ani11030652
Chicago/Turabian StyleAbdel-Latif, Hany M. R., Mahmoud A. O. Dawood, Samy F. Mahmoud, Mustafa Shukry, Ahmed E. Noreldin, Hanan A. Ghetas, and Mohamed A. Khallaf. 2021. "Copper Oxide Nanoparticles Alter Serum Biochemical Indices, Induce Histopathological Alterations, and Modulate Transcription of Cytokines, HSP70, and Oxidative Stress Genes in Oreochromis niloticus" Animals 11, no. 3: 652. https://doi.org/10.3390/ani11030652
APA StyleAbdel-Latif, H. M. R., Dawood, M. A. O., Mahmoud, S. F., Shukry, M., Noreldin, A. E., Ghetas, H. A., & Khallaf, M. A. (2021). Copper Oxide Nanoparticles Alter Serum Biochemical Indices, Induce Histopathological Alterations, and Modulate Transcription of Cytokines, HSP70, and Oxidative Stress Genes in Oreochromis niloticus. Animals, 11(3), 652. https://doi.org/10.3390/ani11030652









