Hermetia illucens and Poultry by-Product Meals as Alternatives to Plant Protein Sources in Gilthead Seabream (Sparus aurata) Diet: A Multidisciplinary Study on Fish Gut Status
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics
2.2. Experimental Diets
2.3. Fish Rearing Conditions, Calculation and Sampling
2.4. Histology, Morphometric Analysis, and Histopathological Indexes of Enteritis in Intestine and Evaluation of Fat Fraction in the Liver (PFF)
2.5. Fourier Transform Infrared Imaging (FTIRI) Spectroscopy Measurements and Data Analysis
2.5.1. Distal and Medium Intestine
2.5.2. Liver
2.6. RNA Extraction and cDNA Synthesis
2.7. Real Time PCR
2.8. Statistical Analysis
3. Results
3.1. Growth Performances
3.2. Intestine and Liver Histology
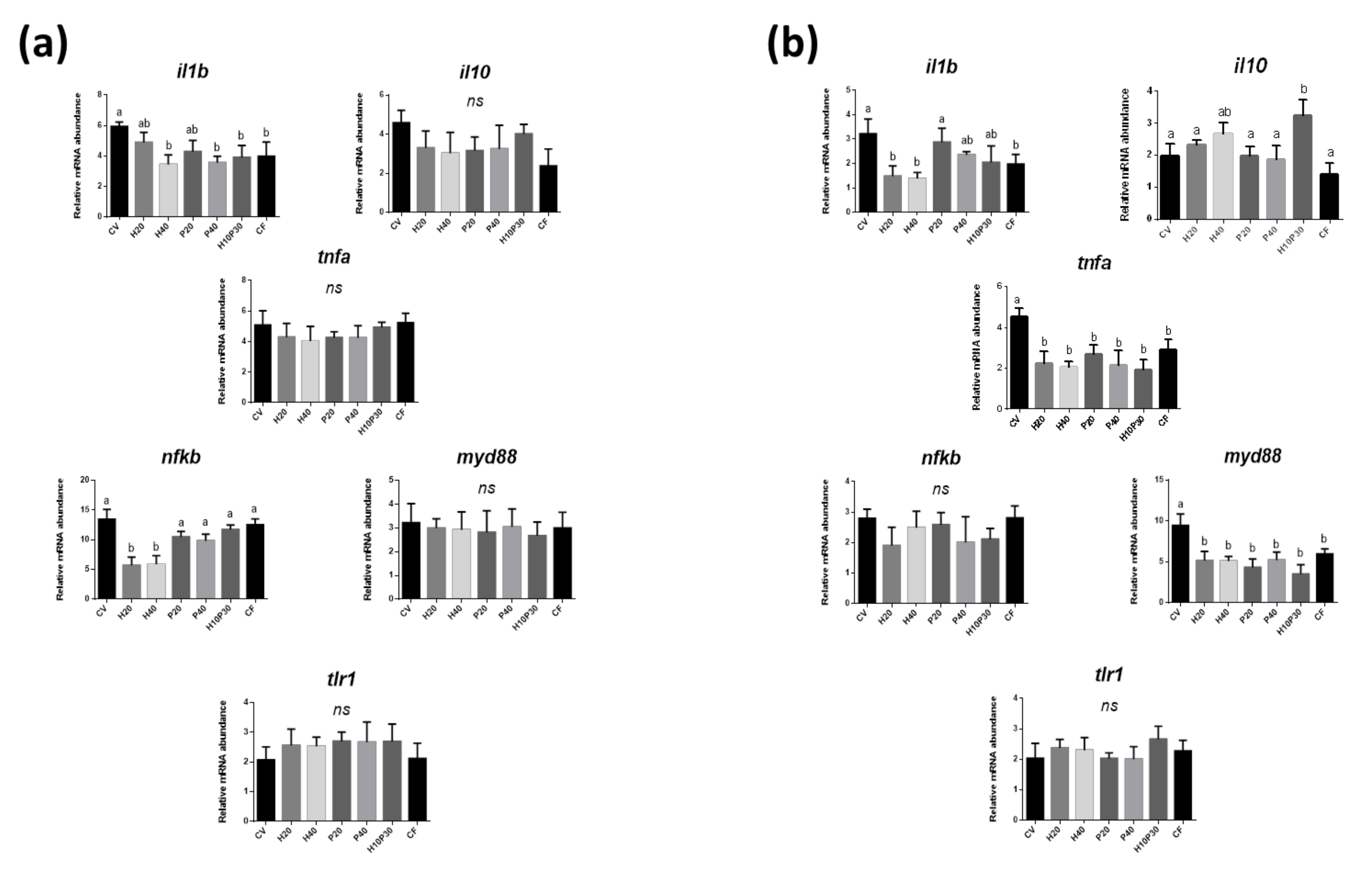
3.3. Gene Expression
3.4. FTIRI Analysis
3.4.1. Distal and Medium Intestine
3.4.2. Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodar, A.R.; Vasava, R.; Mahavadiya, D.R.; Joshi, N.H. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. J. Exp. Zool. India 2020, 23, 13–21. [Google Scholar]
- Ray, A.K.; Ringø, E. The gastrointestinal tract of fish. Aquac. Nutr. 2014, 41, 1–13. [Google Scholar] [CrossRef]
- Minghetti, M.; Drieschner, C.; Bramaz, N.; Schug, H.; Schirmer, K. A fish intestinal epithelial barrier model established from the rainbow trout (Oncorhynchus mykiss) cell line, RTgutGC. Cell Biol. Toxicol. 2017, 33, 539–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novriadi, R.; Rhodes, M.; Powell, M.; Hanson, T.; Davis, D. Effects of soybean meal replacement with fermented soybean meal on growth, serum biochemistry and morphological condition of liver and distal intestine of Florida pompano Trachinotus carolinus. Aquac. Nutr. 2018, 24, 1066–1075. [Google Scholar] [CrossRef]
- Vargas, A.; Randazzo, B.; Riolo, P.; Truzzi, C.; Gioacchini, G.; Giorgini, E.; Loreto, N.; Ruschioni, S.; Zarantoniello, M.; Antonucci, M.; et al. Rearing zebrafish on black soldier fly (Hermetia illucens): Biometric, histological, spectroscopic, biochemical, and molecular implications. Zebrafish 2018, 15, 404–419. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Randazzo, B.; Messina, M.; Zarantoniello, M.; Giorgini, E.; Zimbelli, A.; Bruni, L.; Parisi, G.; Olivotto, I.; Tulli, F. Effects of graded dietary inclusion level of full-fat hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss). Animals 2019, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Bruni, L.; Randazzo, B.; Cardinaletti, G.; Zarantoniello, M.; Mina, F.; Secci, G.; Tulli, F.; Olivotto, I.; Parisi, G. Dietary inclusion of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss): Lipid metabolism and fillet quality investigations. Aquaculture 2020, 529, 735678. [Google Scholar] [CrossRef]
- Wu, N.; Song, Y.-L.; Wang, B.; Zhang, X.-Y.; Zhang, X.-J.; Wang, Y.-L.; Cheng, Y.-Y.; Chen, D.-D.; Xia, X.-Q.; Lu, Y.-S.; et al. Fish gut-liver immunity during homeostasis or inflammation revealed by integrative transcriptome and proteome studies. Sci. Rep. 2016, 6, 36048. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Rahimnejad, S.; Wang, Y.-R.; Lu, K.; Song, K.; Wang, L.; Mai, K. Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus ): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture 2018, 483, 173–182. [Google Scholar] [CrossRef]
- Robaina, L.; Izquierdo, M.; Moyano, F.; Socorro, J.; Vergara, J.; Montero, D.; Fernández-Palacios, H. Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): Nutritional and histological implications. Aquaculture 1995, 130, 219–233. [Google Scholar] [CrossRef]
- Li, Y.; Kortner, T.M.; Chikwati, E.M.; Munang’Andu, H.M.; Lock, E.-J.; Krogdahl, Å. Gut health and vaccination response in pre-smolt Atlantic salmon (Salmo salar) fed black soldier fly (Hermetia illucens) larvae meal. Fish Shellfish Immunol. 2019, 86, 1106–1113. [Google Scholar] [CrossRef]
- Seierstad, S.L.; Haugland, Ø.; Larsen, S.; Waagbø, R.; Evensen, Ø. Pro-inflammatory cytokine expression and respiratory burst activity following replacement of fish oil with rapeseed oil in the feed for Atlantic salmon (Salmo salar L.). Aquaculture 2009, 289, 212–218. [Google Scholar] [CrossRef]
- Marjara, I.S.; Chikwati, E.M.; Valen, E.C.; Krogdahl, Å.; Bakke, A.M. Transcriptional regulation of IL-17A and other inflammatory markers during the development of soybean meal-induced enteropathy in the distal intestine of Atlantic salmon (Salmo salar L.). Cytokine 2012, 60, 186–196. [Google Scholar] [CrossRef]
- Sahlmann, C.; Sutherland, B.J.; Kortner, T.M.; Koop, B.F.; Krogdahl, Å.; Bakke, A.M. Early response of gene expression in the distal intestine of Atlantic salmon (Salmo salar L.) during the development of soybean meal induced enteritis. Fish Shellfish Immunol. 2013, 34, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Fehrmann-Cartes, K.; Coronado, M.; Hernández, A.; Allende, M.; Feijoo, C. Anti-inflammatory effects of aloe vera on soy meal-induced intestinal inflammation in zebrafish. Fish Shellfish Immunol. 2019, 95, 564–573. [Google Scholar] [CrossRef]
- Randazzo, B.; Rolla, L.; Ofelio, C.; Planas, M.; Gioacchini, G.; Vargas, A.; Giorgini, E.; Olivotto, I. The influence of diet on the early development of two seahorse species (H. guttulatus and H. reidi): Traditional and innovative approaches. Aquaculture 2018, 490, 75–90. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Nozzi, V.; Truzzi, C.; Giorgini, E.; Cardinaletti, G.; Freddi, L.; Ratti, S.; Girolametti, F.; Osimani, A.; et al. Physiological responses of Siberian sturgeon (Acipenser baerii) juveniles fed on full-fat insect-based diet in an aquaponic system. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fisheries and Aquaculture Department FAO. The State of the World Fisheries and Aquaculture; FAO Fisheries and Aquaculture Department; Food and Agriculture Organization of the United Nations: Rome, Italy, 2000; p. 218. [Google Scholar]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Lim, S. Effects of dehulled soybean meal as a fish meal replacer in diets for fingerling and growing Korean rockfish Sebastes schlegeli. Aquaculture 2004, 231, 457–468. [Google Scholar] [CrossRef]
- Zhou, Q.-C.; Tan, B.-P.; Mai, K.-S.; Liu, Y.-J. Apparent digestibility of selected feed ingredients for juvenile cobia Rachycentron canadum. Aquaculture 2004, 241, 441–451. [Google Scholar] [CrossRef]
- Yasothai, R. Antinutritional factors in soybean meal and its deactivation. Int. J. Sci. Environ. Technol. 2016, 2, 3793–3797. [Google Scholar]
- Basto-Silva, C.; Guerreiro, I.; Oliva-Teles, A.; Neto, B. Life cycle assessment of diets for gilthead seabream (Sparus aurata) with different protein/carbohydrate ratios and fishmeal or plant feedstuffs as main protein sources. Int. J. Life Cycle Assess. 2019, 24, 2023–2034. [Google Scholar] [CrossRef]
- Zhou, Z.; Ringø, E.; Olsen, R.; Song, S. Dietary effects of soybean products on gut microbiota and immunity of aquatic animals: A review. Aquac. Nutr. 2018, 24, 644–665. [Google Scholar] [CrossRef]
- Gai, F.; Gasco, L.; Daprà, F.; Palmegiano, G.B.; Sicuro, B. Enzymatic and histological evaluations of gut and liver in rainbow trout, oncorhynchus mykiss, fed with rice protein concentrate-based diets. J. World Aquac. Soc. 2012, 43, 218–229. [Google Scholar] [CrossRef]
- Collins, S.A. Antinutritional Factors in Modelling Plant-Based Rainbow Trout. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2014; p. 215. [Google Scholar]
- Krogdahl, Å.; Bakke-McKellep, A.; Baeverfjord, G. Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2003, 9, 361–371. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, Å.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Alexis, M.N.; Nengas, I. Current State of Knowledge Concerning the use of Soy Products in Diets for Feeding Sea Bass and Sea Bream Needs For Future Research; American Soybean Association: Luxembourg, Brussels, 2001; p. 32. [Google Scholar]
- Venou, B.; Alexis, M.N.; Fountoulaki, E.; Nengas, I.; Apostolopoulou, M.; Castritsi-Cathariou, I. Effect of extrusion of wheat and corn on gilthead seabream (Sparus aurata) growth, nutrient utilization efficiency, rates of gastric evacuation and digestive enzyme activities. Aquaculture 2003, 225, 207–223. [Google Scholar] [CrossRef]
- Bonaldo, A.; Roem, A.J.; Fagioli, P.; Pecchini, A.; Cipollini, I.; Gatta, P.P. Influence of dietary levels of soybean meal on the performance and gut histology of gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.). Aquac. Res. 2008, 39, 970–978. [Google Scholar] [CrossRef]
- Kokou, F.; Rigos, G.; Henry, M.; Kentouri, M.; Alexis, M. Growth performance, feed utilization and non-specific immune response of gilthead sea bream (Sparus aurata L.) fed graded levels of a bioprocessed soybean meal. Aquaculture 2012, 364, 74–81. [Google Scholar] [CrossRef]
- Kokou, F.; Sarropoulou, E.; Cotou, E.; Kentouri, M.; Alexis, M.; Rigos, G. Effects of graded dietary levels of soy protein concentrate supplemented with methionine and phosphate on the immune and antioxidant responses of gilthead sea bream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 64, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yao, W.; Ye, B.; Wu, X.; Li, X.; Dong, Y. Effects of replacing fishmeal protein with poultry by-product meal protein and soybean meal protein on growth, feed intake, feed utilization, gut and liver histology of hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Aquaculture 2020, 516, 734503. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Fishmeal Alternative Protein Sources for Aquaculture Feeds. In Food Traceability in Jordan; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–28. [Google Scholar]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed. Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.; Wilson, A.E.; Davis, D.A. Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: A meta-analysis. Rev. Aquac. 2019, 12, 1624–1636. [Google Scholar] [CrossRef]
- Irm, M.; Taj, S.; Jin, M.; Luo, J.; Andriamialinirina, H.J.T.; Zhou, Q. Effects of replacement of fish meal by poultry by-product meal on growth performance and gene expression involved in protein metabolism for juvenile black sea bream (Acanthoparus schlegelii). Aquaculture 2020, 528, 735544. [Google Scholar] [CrossRef]
- Bureau, D.; Harris, A.; Cho, C. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture 1999, 180, 345–358. [Google Scholar] [CrossRef]
- González-Rodríguez, Á.; Celada, J.D.; Carral, J.M.; Sáez-Royuela, M.; García, V.; Fuertes, J.B. Evaluation of poultry by-product meal as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca L.). Aquac. Res. 2014, 47, 1612–1621. [Google Scholar] [CrossRef]
- Zhou, Q.-C.; Zhao, J.; Li, P.; Wang, H.-L.; Wang, L.-G. Evaluation of poultry by-product meal in commercial diets for juvenile cobia (Rachycentron canadum). Aquaculture 2011, 322, 122–127. [Google Scholar] [CrossRef]
- Rossi, W.; Davis, D.A. Replacement of fishmeal with poultry by-product meal in the diet of Florida pompano Trachinotus carolinus L. Aquaculture 2012, 338–341, 160–166. [Google Scholar] [CrossRef]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect meals in fish nutrition. Rev. Aquac. 2018, 11, 1080–1103. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Zimbelli, A.; Randazzo, B.; Compagni, M.D.; Truzzi, C.; Antonucci, M.; Riolo, P.; Loreto, N.; Osimani, A.; Milanović, V.; et al. Black soldier fly (Hermetia illucens) reared on roasted coffee by-product and Schizochytrium sp. as a sustainable terrestrial ingredient for aquafeeds production. Aquaculture 2020, 518, 734659. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Gioacchini, G.; Truzzi, C.; Giorgini, E.; Riolo, P.; Gioia, G.; Bertolucci, C.; Osimani, A.; Cardinaletti, G.; et al. Zebrafish (Danio rerio) physiological and behavioural responses to insect-based diets: A multidisciplinary approach. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Karlsen, Ø.; He, S.; Olsen, R.E.; Yao, B.; Ringø, E. The effect of dietary chitin on the autochthonous gut bacteria of Atlantic cod (Gadus morhua L.). Aquac. Res. 2012, 44, 1889–1900. [Google Scholar] [CrossRef]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; De Clercq, P.; De Smet, S. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 33–42. [Google Scholar] [CrossRef]
- Nogueira, N.; Cordeiro, N.; Andrade, C.; Aires, T. Inclusion of low levels of blood and feathermeal in practical diets for gilthead seabream (Sparus aurata). Turk. J. Fish. Aquat. Sci. 2012, 12, 641–650. [Google Scholar] [CrossRef]
- Moutinho, S.; Martínez-Llorens, S.; Tomás-Vidal, A.; Jover-Cerdá, M.; Oliva-Teles, A.; Peres, H. Meat and bone meal as partial replacement for fish meal in diets for gilthead seabream (Sparus aurata) juveniles: Growth, feed efficiency, amino acid utilization, and economic efficiency. Aquaculture 2017, 468, 271–277. [Google Scholar] [CrossRef]
- Al-Souti, A.; Gallardo, W.; Claereboudt, M.; Mahgoub, O. Attractability and palatability of formulated diets incorporated with chicken feather and algal meals for juvenile gilthead seabream, Sparus aurata. Aquac. Rep. 2019, 14, 100199. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Psofakis, P.; Mente, E.; Malandrakis, E.; Golomazou, E. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquac. Nutr. 2019, 25, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, M.; Schiavone, R.; Brizzi, G.; Sicuro, B.; Zilli, L.; Vilella, S. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture 2019, 511, 734220. [Google Scholar] [CrossRef]
- De Haro, C.; Bueno, R.P.R.; Barroso, F.G.; Muros, M.J.S.; Cervera, M.A.R.; Guil-Guerrero, J.L. Insect larvae as feed ingredient selectively increase arachidonic acid content in farmed gilthead sea bream (Sparus aurata L.). Aquac. Res. 2015, 47, 2881–2887. [Google Scholar] [CrossRef]
- Oliva-Teles, A. Recent advances in European sea bass and gilthead sea bream nutrition. Aquac. Int. 2000, 8, 477–492. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1975. [Google Scholar]
- Burja, A.M.; Armenta, R.E.; Radianingtyas, H.; Barrow, C.J. Evaluation of fatty acid extraction methods for thraustochytriumsp. ONC-Tj. Agric. Food Chem. 2007, 55, 4795–4801. [Google Scholar] [CrossRef] [PubMed]
- Urán, P.A.; Schrama, J.W.; Rombout, J.H.W.M.; Taverne-Thiele, J.J.; Obach, A.; Koppe, W.; Verreth, J.A.J. Time-related changes of the intestinal morphology of Atlantic salmon, Salmo salar L.: At two different soybean meal inclusion levels. J. Fish Dis. 2009, 32, 733–744. [Google Scholar] [CrossRef]
- Panettieri, V.; Chatzifotis, S.; Messina, C.M.; Olivotto, I.; Manuguerra, S.; Randazzo, B.; Ariano, A.; Bovera, F.; Santulli, A.; Severino, L.; et al. Honey bee pollen in meagre (Argyrosomus regius) juvenile diets: Effects on growth, diet digestibility, intestinal traits, and biochemical markers related to health and stress. Animals 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Api, M.; Notarstefano, V.; Olivotto, I.; Cellerino, A.; Carnevali, O. Breeders age affects reproductive success in Nothobranchius furzeri. Zebrafish 2018, 15, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Notarstefano, V.; Sabbatini, S.; Conti, C.; Pisani, M.; Astolfi, P.; Pro, C.; Rubini, C.; Vaccari, L.; Giorgini, E. Investigation of human pancreatic cancer tissues by Fourier Transform Infrared Hyperspectral Imaging. J. Biophotonics 2019, 13, e201960071. [Google Scholar] [CrossRef]
- Randazzo, B.; Zarantoniello, M.; Gioacchini, G.; Giorgini, E.; Truzzi, C.; Notarstefano, V.; Cardinaletti, G.; Huyen, K.T.; Carnevali, O.; Olivotto, I.; et al. Can insect-based diets affect zebrafish (Danio rerio) reproduction? A multidisciplinary study. Zebrafish 2020, 17, 287–304. [Google Scholar] [CrossRef]
- Olivotto, I.; Yasumasu, S.; Gioacchini, G.; Maradonna, F.; Cionna, C.; Carnevali, O. Cloning and expression of high choriolytic enzyme, a component of the hatching enzyme system, during embryonic development of the marine ornamental fish Chrysiptera parasema. Mar. Biol. 2004, 145, 1235–1241. [Google Scholar] [CrossRef]
- Olivotto, I.; Di Stefano, M.; Rosetti, S.; Cossignani, L.; Pugnaloni, A.; Giantomassi, F.; Carnevali, O. Live prey enrichment, with particular emphasis on HUFAs, as limiting factor in false percula clownfish (Amphiprion ocellaris, Pomacentridae) larval development and metamorphosis: Molecular and biochemical implications. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2011, 159, 207–218. [Google Scholar] [CrossRef]
- Piccinetti, C.C.; Donati, M.; Radaelli, G.; Caporale, G.; Mosconi, G.; Palermo, F.A.; Cossignani, L.; Salvatori, R.; Lopez, R.P.; Olivotto, I.; et al. The effects of starving and feeding on Dover sole (Solea solea, Soleidae, Linnaeus, 1758) stress response and early larval development. Aquac. Res. 2015, 46, 2512–2526. [Google Scholar] [CrossRef]
- Robaina, L.; Moyano, F.; Izquierdo, M.; Socorro, J.; Vergara, J.; Montero, D. Corn gluten and meat and bone meals as protein sources in diets for gilthead seabream (Sparus aurata): Nutritional and histological implications. Aquaculture 1997, 157, 347–359. [Google Scholar] [CrossRef]
- Monge-Ortiz, R.; Martínez-Llorens, S.; Márquez, L.; Moyano, F.J.; Jover-Cerdá, M.; Tomás-Vidal, A. Potential use of high levels of vegetal proteins in diets for market-sized gilthead sea bream (Sparus aurata). Arch. Anim. Nutr. 2016, 70, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Sitjà-Bobadilla, A.; Peña-Llopis, S.; Gómez-Requeni, P.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J.; Sitjà-Bobadilla, A.; Peña-Llopis, S.; Gómez-Requeni, P.; Médale, F.; et al. Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparus aurata). Aquaculture 2005, 249, 387–400. [Google Scholar] [CrossRef] [Green Version]
- Estruch, G.; Collado, M.C.; Monge-Ortiz, R.; Tomás-Vidal, A.; Jover-Cerdá, M.; Peñaranda, D.S.; Martínez, G.P.; Martínez-Llorens, S. Long-term feeding with high plant protein based diets in gilthead seabream (Sparus aurata, L.) leads to changes in the inflammatory and immune related gene expression at intestinal level. BMC Veter. Res. 2018, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Diógenes, A.F.; Basto, A.; Estevão-Rodrigues, T.T.; Moutinho, D.; Aires, T.; Oliva-Teles, A.; Peres, H. Soybean meal replacement by corn distillers dried grains with solubles (DDGS) and exogenous non-starch polysaccharidases supplementation in diets for gilthead seabream (Sparus aurata) juveniles. Aquaculture 2019, 500, 435–442. [Google Scholar] [CrossRef]
- Piccolo, G.; Iaconisi, V.; Marono, S.; Gasco, L.; Loponte, R.; Nizza, S.; Bovera, F.; Parisi, G. Effect of Tenebrio molitor larvae meal on growth performance, In Vivo nutrients digestibility, somatic and marketable indexes of gilthead sea bream (Sparus aurata). Anim. Feed Sci. Technol. 2017, 226, 12–20. [Google Scholar] [CrossRef]
- Lecrenier, M.C.; Marien, A.; Berben, G.; Fumière, O.; Veys, P.; Baeten, V. Survey of animal by-products in feeding stuffs before the reintroduction of processed animal proteins in aquafeed. BASE 2019, 23, 218–225. [Google Scholar] [CrossRef]
- Nengas, I.; Alexis, M.N.; Davies, S.J. High inclusion levels of poultry meals and related byproducts in diets for gilthead seabream Sparus aurata L. Aquaculture 1999, 179, 13–23. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.; Daskalopoulou, E.; Vogiatzis, I.; Christos, R.; Eleni, M.; Christos, A. Substitution of Fishmeal by Fly Hermetia illucens Prepupae Meal in the Diet of Gilthead Seabream (Sparus aurata). In Proceedings of the HydroMedit, Volos, Greece, 13–15 November 2014. [Google Scholar]
- De Francesco, M.; Parisi, G.; Pérez-Sánchez, J.; Gómez-Requeni, P.; Medale, F.; Kaushik, S.; Mecatti, M.; Poli, B. Effect of high-level fish meal replacement by plant proteins in gilthead sea bream (Sparus aurata) on growth and body/fillet quality traits. Aquac. Nutr. 2007, 13, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, J.G.; Oliva-Teles, A. Apparent digestibility coefficients of feedstuffs in seabass (Dicentrarchus labrax) juveniles. Aquat. Living Resour. 1998, 11, 187–191. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Torrecillas, S.; Caballero, M.J.; Mompel, D.; Montero, D.; Zamorano, M.; Robaina, L.; Rivero-Ramírez, F.; Karalazos, V.; Kaushik, S.; Izquierdo, M. Disease resistance and response against Vibrio anguillarum intestinal infection in European seabass (Dicentrarchus labrax) fed low fish meal and fish oil diets. Fish Shellfish Immunol. 2017, 67, 302–311. [Google Scholar] [CrossRef]
- Kumar, V.; Hossain, S.; Ragaza, J.A.; Benito, M.R. The potential impacts of soy protein on fish gut health. Soybean Hum. Consum. Anim. Feed 2020. [Google Scholar] [CrossRef]
- Gasco, L.; Józefiak, A.; Henry, M. Beyond the protein concept: Health aspects of using edible insects on animals. J. Insects Food Feed 2020, 1–28. [Google Scholar] [CrossRef]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Roncolini, A.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N.; Franciosi, E.; Tuohy, K.; Olivotto, I.; et al. Hermetia illucens in diets for zebrafish (Danio rerio): A study of bacterial diversity by using PCR-DGGE and metagenomic sequencing. PLoS ONE 2019, 14, e0225956. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; Salem, M.E. Effects of dietary chitosan supplementation on farmed fish; a review. Rev. Aquac. 2019, 12, 438–452. [Google Scholar] [CrossRef]
- Cuesta, A.; Esteban, M.; Meseguer, J. In Vitro effect of chitin particles on the innate cellular immune system of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2003, 15, 1–11. [Google Scholar] [CrossRef]
- Skrivanova, E.; Marounek, M.; Dlouha, G.; Kanka, J. Susceptibility of Clostridium perfringens to C2–C18 fatty acids. Lett. Appl. Microbiol. 2005, 41, 77–81. [Google Scholar] [CrossRef]
- Skrivanova, E.; Marounek, M.; Benda, V.; Brezina, P. Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringensto organic acids and monolaurin. Veterinární Med. 2012, 51, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Giorgini, E.; Randazzo, B.; Gioacchini, G.; Cardinaletti, G.; Vaccari, L.; Tibaldi, E.; Olivotto, I. New insights on the macromolecular building of rainbow trout (O. mykiss) intestine: FTIR Imaging and histological correlative study. Aquaculture 2018, 497, 1–9. [Google Scholar] [CrossRef]
- Olli, J.J.; Hjelmeland, K.; Krogdahl, Å. Soybean trypsin inhibitors in diets for Atlantic salmon (Salmo salar, L): Effects on nutrient digestibilities and trypsin in pyloric caeca homogenate and intestinal content. Comp. Biochem. Physiol. Part. A Physiol. 1994, 109, 923–928. [Google Scholar] [CrossRef]
- Romarheim, O.H.; Skrede, A.; Penn, M.; Mydland, L.T.; Krogdahl, Å.; Storebakken, T. Lipid digestibility, bile drainage and development of morphological intestinal changes in rainbow trout (Oncorhynchus mykiss) fed diets containing defatted soybean meal. Aquaculture 2008, 274, 329–338. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Moñino, A.V.; Tomás Vidal, A.; Salvador, V.J.M.; Pla Torres, M.; Jover Cerdá, M. Soybean meal as a protein source in gilthead sea bream (Sparus aurata L.) diets: Effects on growth and nutrient utilization. Aquac. Res. 2007, 38, 82–90. [Google Scholar] [CrossRef]
- Hua, K.; Bureau, D.P. Development of a model to estimate digestible lipid content of salmonid fish feeds. Aquaculture 2009, 286, 271–276. [Google Scholar] [CrossRef]
- Diaz, J.P.; Guyot, E.; Vigierand, S.; Connes, R. First events in lipid absorption during post-embryonic development of the anterior intestine in gilt-head sea bream. J. Fish Biol. 1997, 51, 180–192. [Google Scholar] [CrossRef]
- Sánchez-Moya, A.; García-Meilán, I.; Riera-Heredia, N.; Vélez, E.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of different dietary vegetable oils on growth and intestinal performance, lipid metabolism and flesh quality in gilthead sea bream. Aquaculture 2020, 519, 734881. [Google Scholar] [CrossRef]
- Santigosa, E.; Pérez-Sánchez, J.; Médale, F.; Kaushik, S.; Gallardo, M.Á. Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 2008, 282, 68–74. [Google Scholar] [CrossRef]
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Moriñigo, M.Á.; Esteban, M.Á. Histological alterations and microbial ecology of the intestine in gilthead seabream (Sparus aurata L.) fed dietary probiotics and microalgae. Cell Tissue Res. 2012, 350, 477–489. [Google Scholar] [CrossRef]
- Krogdahl, A.; Hemre, G.-I.; Mommsen, T. Carbohydrates in fish nutrition: Digestion and absorption in postlarval stages. Aquac. Nutr. 2005, 11, 103–122. [Google Scholar] [CrossRef]
- El-Hack, M.E.A.; Shafi, M.E.; Alghamdi, W.Y.; Abdelnour, S.A.; Shehata, A.M.; Noreldin, A.E.; Ashour, E.A.; Swelum, A.A.; Al-Sagan, A.A.; Alkhateeb, M.; et al. Black soldier fly (Hermetia illucens) Meal as a promising feed ingredient for poultry: A comprehensive review. Agriculture 2020, 10, 339. [Google Scholar] [CrossRef]







| Ingredient Composition | CV | H20 | H40 | P20 | P40 | H10P30 | CF |
|---|---|---|---|---|---|---|---|
| Fish meals 1 | 54.0 | ||||||
| Vegetable-protein mix 2 | 69 | 52.6 | 36.6 | 52.5 | 35.4 | 35.4 | - |
| Hermetia meal 3 | - | 16.2 | 32.4 | - | - | 8.1 | - |
| PBM 4 | - | - | - | 13.8 | 27.5 | 20.6 | - |
| Feeding stimulants 5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| Wheat meal * | 0.4 | 1.6 | 4.5 | 3.0 | 5.6 | 5.5 | 3.0 |
| Whole pea * | 3.0 | 5.8 | 6.0 | 6.2 | 9.0 | 8.8 | 20.5 |
| Fish oil 6 | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 | 8.6 |
| Veg. oil mix 7 | 11.4 | 8.4 | 5.4 | 9.8 | 8.2 | 7.4 | 6.5 |
| Vit. & Min. Premix 8 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline HCL | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Sodium phosphate (NaH2PO4) | 1.6 | 1.2 | 1.0 | 0.7 | 0.3 | 0.2 | - |
| L-Lysine 9 | 0.5 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | - |
| DL-Methionine 10 | 0.5 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | - |
| Celite | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Proximate composition | |||||||
| Moisture | 6.7 | 6.1 | 4.7 | 7.1 | 7.2 | 8.7 | 8.2 |
| Protein (N × 6.25) | 45.0 | 45.2 | 45.2 | 45.1 | 45.1 | 45.1 | 45.4 |
| Total lipid | 20.4 | 20.1 | 20.4 | 20.4 | 20.2 | 20.4 | 20.3 |
| Ash | 5.8 | 6.6 | 6.5 | 7.1 | 7.9 | 7.6 | 12.4 |
| Chitin # | 0.02 | 0.76 | 1.51 | 0.02 | 0.02 | 0.39 | 0.02 |
| Score | Description | |
|---|---|---|
| Mucosal folds fusion (MF f) | + | 0–5 observation per section |
| ++ | 5–15 observation per section | |
| +++ | >15 observation per section | |
| Supranuclear vacuoles (SN v) | + | Scarce |
| ++ | Diffused in the enterocytes | |
| +++ | Abundantly filling enterocytes | |
| Sub-mucosa width (SM w) | + | 10–15 µm |
| ++ | 15–30 µm | |
| +++ | >30 µm |
| Gene Name | Primer Sequence | A.T. (°C) | Gene Bank ID | |
|---|---|---|---|---|
| Forward | Reverse | |||
| Il1b | ATCCAGCTGTCTTTCCCTCA | TTGCATGTCATCTCGGATTC | 59 | XM_030435228.1 |
| il10 | AACATCCTGGGCTTCTATCTG | TGTCCTCCGTCTCATCTG | 60 | JX976621.1 |
| tnfa | CTGTGGAGGGAAGAATCGAG | CTTTCTGGTCCACCTCACCT | 60 | XM_030412624.1 |
| nfkb | GTTTGTCGTGTCGTTGGGAG | CGAGTGGACAAGTGAGTGGA | 58 | XM_030403588.1 |
| myd88 | CCGTCGTCTGTGGCTAACAT | GTCCCACGCCTTTTTCAACC | 56 | XM_030399037.1 |
| tlr1 | CTTGTGCCCAGCAGTGTTTC | CGGTTTGTAGCACGGTCTTC | 60 | XM_030396315.1 |
| β-actin (hk) | TCCTGCGGAATCCATGAGA | GACGTCGCACTTCATGATGCT | 57 | X89920.1 |
| rps18 (hk) | AGGGTGTTGGCAGACGTTAC | CTTCTGCCTGTTGAGGAACC | 57 | AM490061.1 |
| Dietary Treatments | Final Weight g/Fish | SGR | RFI g/kg ABW/d | FCR |
|---|---|---|---|---|
| CV | 177.7 ± 2.10 c | 1.54 ± 0.01 c | 15.9 ± 0.21 b | 1.18 ± 0.05 b |
| H20 | 187.5 ± 3.60 b | 1.59 ± 0.02 b | 15.8 ± 0.16 b | 1.13 ± 0.04 a |
| H40 | 192.2 ± 0.80 a | 1.64 ± 0.02 a | 15.6 ± 0.07 b | 1.11 ± 0.04 a |
| P20 | 191.6 ± 1.47 a | 1.63 ± 0.01 a | 15.9 ± 0.33 b | 1.11 ± 0.03 a |
| P40 | 192.3 ± 0.36 a | 1.63 ± 0.01 a | 15.8 ± 0.38 b | 1.10 ± 0.02 a |
| H10P30 | 190.7 ± 1.32 ab | 1.62 ± 0.01 a | 15.7 ± 0.08 b | 1.11 ± 0.02 a |
| CF | 180.1 ± 1.41 c | 1.55 ± 0.02 c | 16.4 ± 0.08 a | 1.21 ± 0.02 b |
| ± esm | 2.27 | 0.017 | 0.22 | 0.022 |
| Groups | MF | MF f | SN v | SM w | Groups | MF | MF f | SN v | SM w |
|---|---|---|---|---|---|---|---|---|---|
| (µm) | (µm) | ||||||||
| CV | 853 ± 12 b | +++ | + | ++ | CV | 631 ± 79 b | +++ | +++ | +++ |
| H20 | 1003 ± 88 a | + | + | + | H20 | 672 ± 97 b | + | ++ | + |
| H40 | 1092 ± 88 a | + | ++ | + | H40 | 813 ± 15 a | + | + | + |
| P20 | 1010 ± 12 a | + | ++ | + | P20 | 965 ± 94 a | + | + | + |
| P40 | 1023 ± 11 a | + | ++ | + | P40 | 846 ± 59 a | + | + | + |
| H10P30 | 1073 ± 23 a | + | +++ | + | H10P30 | 948 ± 95 a | + | + | + |
| CF | 1100 ± 13 a | + | +++ | + | CF | 821 ± 80 a | + | + | + |
| (a) | (b) |
| Diets | PFF (%) |
|---|---|
| CV | 58.8 ± 1.8 |
| H20 | 63.5 ± 2.2 |
| H40 | 65.6 ± 2.2 |
| P20 | 61.7 ± 1.3 |
| P40 | 63.1 ± 2.3 |
| H10P30 | 62.9 ± 2.3 |
| CF | 62.4 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Randazzo, B.; Zarantoniello, M.; Cardinaletti, G.; Cerri, R.; Giorgini, E.; Belloni, A.; Contò, M.; Tibaldi, E.; Olivotto, I. Hermetia illucens and Poultry by-Product Meals as Alternatives to Plant Protein Sources in Gilthead Seabream (Sparus aurata) Diet: A Multidisciplinary Study on Fish Gut Status. Animals 2021, 11, 677. https://doi.org/10.3390/ani11030677
Randazzo B, Zarantoniello M, Cardinaletti G, Cerri R, Giorgini E, Belloni A, Contò M, Tibaldi E, Olivotto I. Hermetia illucens and Poultry by-Product Meals as Alternatives to Plant Protein Sources in Gilthead Seabream (Sparus aurata) Diet: A Multidisciplinary Study on Fish Gut Status. Animals. 2021; 11(3):677. https://doi.org/10.3390/ani11030677
Chicago/Turabian StyleRandazzo, Basilio, Matteo Zarantoniello, Gloriana Cardinaletti, Roberto Cerri, Elisabetta Giorgini, Alessia Belloni, Michela Contò, Emilio Tibaldi, and Ike Olivotto. 2021. "Hermetia illucens and Poultry by-Product Meals as Alternatives to Plant Protein Sources in Gilthead Seabream (Sparus aurata) Diet: A Multidisciplinary Study on Fish Gut Status" Animals 11, no. 3: 677. https://doi.org/10.3390/ani11030677
APA StyleRandazzo, B., Zarantoniello, M., Cardinaletti, G., Cerri, R., Giorgini, E., Belloni, A., Contò, M., Tibaldi, E., & Olivotto, I. (2021). Hermetia illucens and Poultry by-Product Meals as Alternatives to Plant Protein Sources in Gilthead Seabream (Sparus aurata) Diet: A Multidisciplinary Study on Fish Gut Status. Animals, 11(3), 677. https://doi.org/10.3390/ani11030677







