Appetite Regulation, Growth Performances and Fish Quality Are Modulated by Alternative Dietary Protein Ingredients in Gilthead Sea Bream (Sparus aurata) Culture
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Experimental Diets
2.2. Fish Rearing and Sampling
2.3. Gene Expression Analyses
2.3.1. RNA Extraction and cDNA Synthesis
2.3.2. Real Time PCR
2.4. Physical and Chemical Analyses on Fillets
2.4.1. Marketable Indexes and Physical Analyses
2.4.2. Chemical Composition and Fatty Acid Profile
2.4.3. Fillet Oxidative Status
2.5. Statistical Analyses
3. Results
3.1. Growth Performance
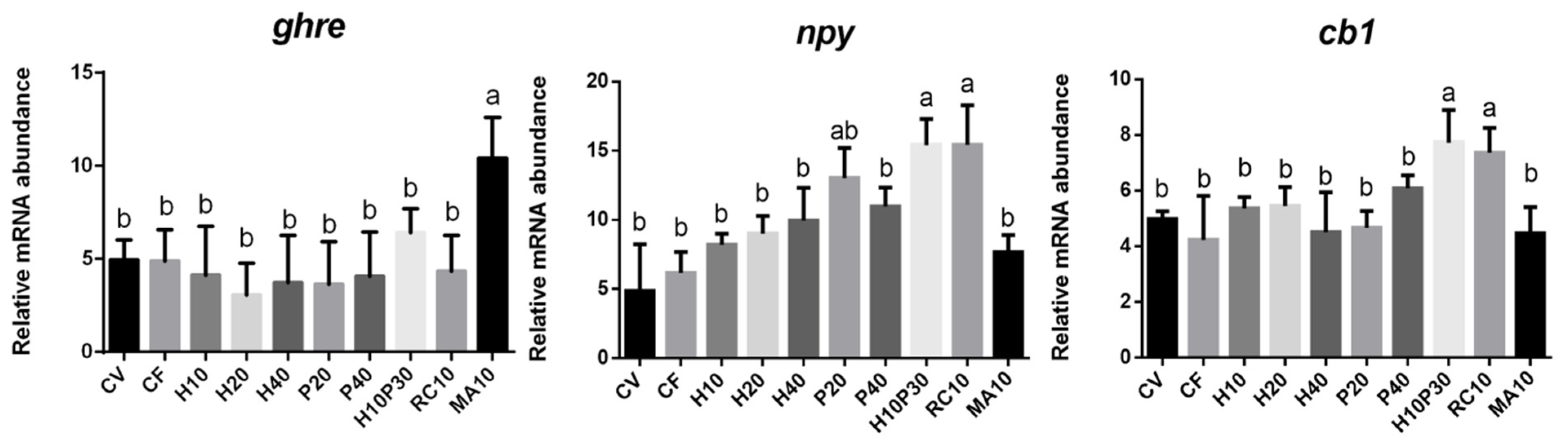
3.2. Gene Expression
3.3. Analyses on Fillets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals. Rome. Licence: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018; ISBN 978-92-5-130562-1. [Google Scholar]
- MacLeod, M.J.; Hasan, M.R.; Robb, D.H.F.; Mamun-Ur-Rashid, M. Quantifying greenhouse gas emissions from global aquaculture. Sci. Rep. 2020, 10, 11679. [Google Scholar] [CrossRef]
- Gasco, L.; Acuti, G.; Bani, P.; Zotte, A.D.; Danieli, P.P.; De Angelis, A.; Fortina, R.; Marino, R.; Parisi, G.; Piccolo, G.; et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Zarantoniello, M.; Zimbelli, A.; Randazzo, B.; Compagni, M.D.; Truzzi, C.; Antonucci, M.; Riolo, P.; Loreto, N.; Osimani, A.; Milanović, V.; et al. Black Soldier Fly (Hermetia illucens) reared on roasted coffee by-product and Schizochytrium sp. as a sustainable terrestrial ingredient for aquafeeds production. Aquaculture 2020, 518, 734659. [Google Scholar] [CrossRef]
- Maiolo, S.; Parisi, G.; Biondi, N.; Lunelli, F.; Tibaldi, E.; Pastres, R. Fishmeal partial substitution within aquafeed formulations: Life cycle assessment of four alternative protein sources. Int. J. Life Cycle Assess. 2020, 25, 1455–1471. [Google Scholar] [CrossRef]
- Randazzo, B.; Zarantoniello, M.; Gioacchini, G.; Cardinaletti, G.; Belloni, A.; Giorgini, E.; Faccenda, F.; Cerri, R.; Tibaldi, E.; Olivotto, I. Physiological response of rainbow trout (Oncorhynchus mykiss) to graded levels of Hermetia illucens or poultry by-product meals as single or combined substitute ingredients to dietary plant proteins. Aquaculture 2021, 538, 736550. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Chowdhury, M.A.K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Parisi, G.; Tulli, F.; Fortina, R.; Marino, R.; Bani, P.; Zotte, A.D.; De Angelis, A.; Piccolo, G.; Pinotti, L.; Schiavone, A.; et al. Protein hunger of the feed sector: The alternatives offered by the plant world. Ital. J. Anim. Sci. 2020, 19, 1204–1225. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Tibaldi, E.; Zittelli, G.C.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth performance and quality traits of European sea bass (D. labrax) fed diets including increasing levels of freeze-dried Isochrysis sp. (T-ISO) biomass as a source of protein and n-3 long chain PUFA in partial substitution of fish derivatives. Aquaculture 2015, 440, 60–68. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Fact. 2012, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Cardinaletti, G.; Messina, M.; Bruno, M.; Tulli, F.; Poli, B.M.; Giorgi, G.; Chini-Zittelli, G.; Tredici, M.; Tibaldi, E. Effects of graded levels of a blend of Tisochrysis lutea and Tetraselmis suecica dried biomass on growth and muscle tissue composition of European sea bass (Dicentrarchus labrax) fed diets low in fish meal and oil. Aquaculture 2018, 485, 173–182. [Google Scholar] [CrossRef]
- Tulli, F.; Zittelli, G.C.; Giorgi, G.; Poli, B.M.; Tibaldi, E.; Tredici, M.R. Effect of the inclusion of dried Tetraselmis suecica on growth, feed utilization, and fillet composition of European sea bass Juveniles fed organic diets. J. Aquat. Food Prod. Technol. 2012, 21, 188–197. [Google Scholar] [CrossRef]
- Messina, M.; Bulfon, C.; Beraldo, P.; Tibaldi, E.; Cardinaletti, G. Intestinal morpho-physiology and innate immune status of European sea bass (Dicentrarchus labrax) in response to diets including a blend of two marine microalgae, Tisochrysis lutea and Tetraselmis suecica. Aquaculture 2019, 500, 660–669. [Google Scholar] [CrossRef]
- Loureiro, T.G.; Anastácio, P.M.S.G.; Araujo, P.B.; Souty-Grosset, C.; Almerão, M.P. Red swamp crayfish: Biology, ecology and invasion—An overview. Nauplius 2015, 23, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Negro, J.J.; Garrido-Fernández, J. Astaxanthin is the major carotenoid in tissues of white storks (Ciconia ciconia) feeding on introduced crayfish (Procambarus clarkii). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000, 126, 347–352. [Google Scholar] [CrossRef]
- Pulcini, D.; Capoccioni, F.; Franceschini, S.; Martinoli, M.; Tibaldi, E. Skin pigmentation in gilthead seabream (Sparus aurata L.) fed conventional and novel protein sources in diets deprived of fish meal. Animals 2020, 10, 2138. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, M.H.A.G.; Flik, G.; Huising, M.O. Peptides and proteins regulating food intake: A comparative view. Anim. Biol. 2006, 56, 447–473. [Google Scholar]
- White, S.L.; Volkoff, H.; Devlin, R.H. Regulation of feeding behavior and food intake by appetite-regulating peptides in wild-type and growth hormone-transgenic coho salmon. Horm. Behav. 2016, 84, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Velasco, C.; Librán-Pérez, M.; Otero-Rodiño, C.; López-Patiño, M.A.; Míguez, J.M.; Cerdá-Reverter, J.M.; Soengas, J.L. Ghrelin modulates hypothalamic fatty acid-sensing and control of food intake in rainbow trout. J. Endocrinol. 2016, 228, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkoff, H. Fish as models for understanding the vertebrate endocrine regulation of feeding and weight. Mol. Cell. Endocrinol. 2019, 497, 110437. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Bertucci, J.I.; Delgado, M.J.; Valenciano, A.I.; Unniappan, S. Tissue-specific expression of ghrelinergic and NUCB2/nesfatin-1 systems in goldfish (Carassius auratus) is modulated by macronutrient composition of diets. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 195, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Campos, V.F.; Robaldo, R.B.; Deschamps, J.C.; Seixas, F.K.; McBride, A.J.A.; Marins, L.F.; Okamoto, M.; Sampaio, L.A.; Collares, T. Neuropeptide Y gene expression around meal time in the Brazilian flounder Paralichthys orbignyanus. J. Biosci. 2012, 37, 227–232. [Google Scholar] [CrossRef]
- Kuz’mina, V.V. The regulatory mechanisms of feeding behavior in fish. J. Evol. Biochem. Physiol. 2019, 55, 1–13. [Google Scholar] [CrossRef]
- Olivotto, I.; Di Stefano, M.; Rosetti, S.; Cossignani, L.; Pugnaloni, A.; Giantomassi, F.; Carnevali, O. Live prey enrichment, with particular emphasis on HUFAs, as limiting factor in false percula clownfish (Amphiprion ocellaris, Pomacentridae) larval development and metamorphosis: Molecular and biochemical implications. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 159, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Maradonna, F.; Gioacchini, G.; Falcinelli, S.; Bertotto, D.; Radaelli, G.; Olivotto, I.; Carnevali, O. Probiotic supplementation promotes calcification in Danio rerio larvae: A molecular study. PLoS ONE 2013, 8, e83155. [Google Scholar] [CrossRef] [Green Version]
- Piccinetti, C.C.; Montis, C.; Bonini, M.; Laurà, R.; Guerrera, M.C.; Radaelli, G.; Vianello, F.; Santinelli, V.; Maradonna, F.; Nozzi, V.; et al. Transfer of silica-coated magnetic (Fe3O4) nanoparticles through food: A molecular and morphological study in zebrafish. Zebrafish 2014, 11, 567–579. [Google Scholar] [CrossRef]
- Vargas-Abúndez, A.J.; Randazzo, B.; Foddai, M.; Sanchini, L.; Truzzi, C.; Giorgini, E.; Gasco, L.; Olivotto, I. Insect meal based diets for clownfish: Biometric, histological, spectroscopic, biochemical and molecular implications. Aquaculture 2019, 498, 1–11. [Google Scholar] [CrossRef]
- CIE. Recommendations on Uniform Colour Spaces-Colour Difference Equations, Psychometric Colour Terms; CIE Publ. No. 15; Commission Internationale de l’Éclairage: Paris, France, 1976. [Google Scholar]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analysis Chemists International: Washington, DC, USA, 2012; ISBN 0-935584-83-8. [Google Scholar]
- Folch, J.; Less, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoridea-methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Secci, G.; Borgogno, M.; Lupi, P.; Rossi, S.; Paci, G.; Mancini, S.; Bonelli, A.; Parisi, G. Effect of mechanical separation process on lipid oxidation in European aquacultured sea bass, gilthead sea bream, and rainbow trout products. Food Control 2016, 67, 75–81. [Google Scholar] [CrossRef]
- Srinivasan, S.; Xiong, Y.L.; Decker, E.A. Inhibition of protein and lipid oxidation in beef heart surimi-like material by antioxidants and combinations of pH, NaCl, and buffer type in the washing media. J. Agric. Food Chem. 1996, 44, 119–125. [Google Scholar] [CrossRef]
- SAS. SAS/STAT Software, Release 9.4; SAS Institute, Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Randazzo, B.; Zarantoniello, M.; Cardinaletti, G.; Cerri, R.; Giorgini, E.; Belloni, A.; Contò, M.; Tibaldi, E.; Olivotto, I. Hermetia illucens and poultry by-product meals as alternatives to plant protein sources in gilthead seabream (Sparus aurata) diet: A multidisciplinary study on fish gut status. Animals 2021, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Karapanagiotidis, I.T.; Psofakis, P.; Mente, E.; Malandrakis, E.; Golomazou, E. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquac. Nutr. 2019, 25, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, M.; Schiavone, R.; Brizzi, G.; Sicuro, B.; Zilli, L.; Vilella, S. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture 2019, 511, 734220. [Google Scholar] [CrossRef]
- Soengas, J.L.; Cerdá-Reverter, J.M.; Delgado, M.J. Central regulation of food intake in fish: An evolutionary perspective. J. Mol. Endocrinol. 2018, 60, R171–R199. [Google Scholar] [CrossRef]
- van de Pol, I.; Flik, G.; Gorissen, M. Comparative physiology of energy metabolism: Fishing for endocrine signals in the early vertebrate pool. Front. Endocrinol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.; Guterres, H.A.D.S.; Huntley, M.; Sørensen, M.; Kiron, V. Digestibility of the defatted microalgae Nannochloropsis sp. and Desmodesmus sp. when fed to Atlantic salmon, Salmo salar. Aquac. Nutr. 2018, 24, 56–64. [Google Scholar] [CrossRef]
- Batista, S.; Pintado, M.; Marques, A.; Abreu, H.; Silva, J.L.; Jessen, F.; Tulli, F.; Valente, L.M.P. Use of technological processing of seaweed and microalgae as strategy to improve their apparent digestibility coefficients in European seabass (Dicentrarchus labrax) juveniles. J. Appl. Phycol. 2021, 530, 7359142. [Google Scholar] [CrossRef]
- Skrede, A.; Mydland, L.T.; Ahlstrem, O.; Reitan, K.I.; Gislered, H.R.; Overland, M. Evaluation of microalgae as sources of digestible nutrients for monogastric animals. J. Anim. Feed Sci. 2011, 20, 131–142. [Google Scholar] [CrossRef]
- Sørensen, M.; Berge, G.M.; Reitan, K.I.; Ruyter, B. Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar) -Effect on nutrient digestibility, growth and utilization of feed. Aquaculture 2016, 460, 116–123. [Google Scholar] [CrossRef]
- Norambuena, F.; Hermon, K.; Skrzypczyk, V.; Emery, J.A.; Sharon, Y.; Beard, A.; Turchini, G.M. Algae in fish feed: Performances and fatty acid metabolism in juvenile Atlantic salmon. PLoS ONE 2015, 10, e0124042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiron, V.; Phromkunthong, W.; Huntley, M.; Archibald, I.; De Scheemaker, G. Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac. Nutr. 2012, 18, 521–531. [Google Scholar] [CrossRef]
- Gouveia, L.; Choubert, G.; Pereira, N.; Santinha, J.; Empis, J.; Gomes, E. Pigmentation of gilthead seabream, Sparus aurata (L. 1875), using Chlorella vulgaris (Chlorophyta, Volvocales) microalga. Aquac. Res. 2002, 33, 987–993. [Google Scholar] [CrossRef]
- Galafat, A.; Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Jérez-Cepa, I.; Mancera, J.M.; Alarcón, F.J. Evaluation of Arthrospira sp. enzyme hydrolysate as dietary additive in gilthead seabream (Sparus aurata) juveniles. J. Appl. Phycol. 2020, 32, 3089–3100. [Google Scholar] [CrossRef]
- Gomes, E.; Dias, J.; Silva, P.; Valente, L.; Empis, J.; Gouveia, L.; Bowen, J.; Young, A. Utilization of natural and synthetic sources of carotenoids in the skin pigmentation of gilthead seabream (Sparus aurata). Eur. Food Res. Technol. 2002, 214, 287–293. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Gonçalves, A.; Barbeiro, M.; Bandarra, N.; Nunes, M.L.; Carvalho, M.L.; Silva, J.; Navalho, J.; Dinis, M.T.; Silva, T.; et al. Phaeodactylum tricornutum in finishing diets for gilthead seabream: Effects on skin pigmentation, sensory properties and nutritional value. J. Appl. Phycol. 2017, 29, 1945–1956. [Google Scholar] [CrossRef]
- Sales, R.; Galafat, A.; Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Cerón-García, M.C.; Navarro-López, E.; Tsuzuki, M.Y.; Acién-Fernández, F.G.; Molina-Grima, E.; et al. Effects of dietary use of two lipid extracts from the microalga Nannochloropsis gaditana (Lubián, 1982) alone and in combination on growth and muscle composition in juvenile gilthead seabream, Sparus aurata. Algal Res. 2021, 53, 102162. [Google Scholar] [CrossRef]
- Wassef, E.A.; Chatzifotis, S.; Sakr, E.M.; Saleh, N.E. Effect of two natural carotenoid sources in diets for gilthead seabream, Sparus aurata, on growth and skin coloration. J. Appl. Aquac. 2010, 22, 216–229. [Google Scholar] [CrossRef]
- Sánchez-Moya, A.; García-Meilán, I.; Riera-Heredia, N.; Vélez, E.J.; Lutfi, E.; Fontanillas, R.; Gutiérrez, J.; Capilla, E.; Navarro, I. Effects of different dietary vegetable oils on growth and intestinal performance, lipid metabolism and flesh quality in gilthead sea bream. Aquaculture 2020, 519, 734881. [Google Scholar] [CrossRef]
- García, J.R.; Kalinowski, C.T.H.; Izquierdo, M.S.L.; Robaina, L.E.R. Marine and freshwater crab meals in diets for red porgy (Pagrus pagrus): Effect on growth, fish composition and skin colour. Aquac. Res. 2010, 41, 1759–1769. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Bruni, L.; Randazzo, B.; Cardinaletti, G.; Zarantoniello, M.; Mina, F.; Secci, G.; Tulli, F.; Olivotto, I.; Parisi, G. Dietary inclusion of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss): Lipid metabolism and fillet quality investigations. Aquaculture 2020, 529, 735678. [Google Scholar] [CrossRef]
- Ng, W.-K.; Gibon, V. Palm Oil and Saturated Fatty Acid-Rich Vegetable Oils. In Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press: Boca Raton, FL, USA, 2010; pp. 99–132. [Google Scholar]
- Bruni, L.; Belghit, I.; Lock, E.; Secci, G.; Taiti, C.; Parisi, G. Total replacement of dietary fish meal with black soldier fly (Hermetia illucens) larvae does not impair physical, chemical or volatile composition of farmed Atlantic salmon (Salmo salar L.). J. Sci. Food Agric. 2020, 100, 1038–1047. [Google Scholar] [CrossRef]
- Mancini, S.; Medina, I.; Iaconisi, V.; Gai, F.; Basto, A.; Parisi, G. Impact of black soldier fly larvae meal on the chemical and nutritional characteristics of rainbow trout fillets. Animal 2018, 12, 1672–1681. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Romarheim, O.H.; Skrede, A.; Penn, M.; Mydland, L.T.; Krogdahl, Å.; Storebakken, T. Lipid digestibility, bile drainage and development of morphological intestinal changes in rainbow trout (Oncorhynchus mykiss) fed diets containing defatted soybean meal. Aquaculture 2008, 274, 329–338. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Moñino, A.V.; Vidal, A.T.; Salvador, V.J.M.; Pla Torres, M.; Cerdá, M.J. Soybean meal as a protein source in gilthead sea bream (Sparus aurata L.) diets: Effects on growth and nutrient utilization. Aquac. Res. 2007, 38, 82–90. [Google Scholar] [CrossRef]
- Hua, K.; Bureau, D.P. Development of a model to estimate digestible lipid content of salmonid fish feeds. Aquaculture 2009, 286, 271–276. [Google Scholar] [CrossRef]
- Carvalho, M.; Montero, D.; Torrecillas, S.; Castro, P.; Zamorano, M.J.; Izquierdo, M. Hepatic biochemical, morphological and molecular effects of feeding microalgae and poultry oils to gilthead sea bream (Sparus aurata). Aquaculture 2021, 532, 736073. [Google Scholar] [CrossRef]
- García-Romero, J.; Ginés, R.; Izquierdo, M.; Robaina, L. Marine and freshwater crab meals in diets for red porgy (Pagrus pagrus): Effect on fillet fatty acid profile and flesh quality parameters. Aquaculture 2014, 420, 231–239. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Kumar, B.R.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
| CV | CF | H10 | H20 | H40 | P20 | P40 | H10P30 | RC10 | MA10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ingredient composition | ||||||||||
| Fish meal 1 | 14.0 | |||||||||
| Fish meal (trimmings) 2 | 40.0 | |||||||||
| Feeding stimulants 3 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| Veg.-protein mix 4 | 69 | - | 60.5 | 52.6 | 36.6 | 52.5 | 35.4 | 35.4 | 58.8 | 58.3 |
| Hermetia meal 5 | - | - | 8.10 | 16.2 | 32.4 | - | - | 8.1 | - | - |
| PBM 6 | - | - | - | - | - | 13.8 | 27.5 | 20.6 | - | - |
| RC meal 7 | - | - | - | - | - | - | - | - | 10.1 | - |
| Microalgae mix 8 | - | - | - | - | - | - | - | - | - | 11.6 |
| Wheat meal * | 0.4 | 3.0 | 0.6 | 1.6 | 4.5 | 3.0 | 5.6 | 5.5 | 0.4 | - |
| Whole pea * | 3.0 | 20.5 | 4.8 | 5.8 | 6.0 | 6.2 | 9.0 | 8.8 | 4.1 | 4.0 |
| Fish oil 9 | 6.2 | 8.6 | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 | 6.2 |
| Veg. oil mix 10 | 11.4 | 6.5 | 10.0 | 8.4 | 5.4 | 9.8 | 8.2 | 7.4 | 10.8 | 10.5 |
| Vit. & Min. Premix 11 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline HCL | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Sodium phosphate | 1.6 | - | 1.5 | 1.2 | 1.0 | 0.7 | 0.3 | 0.2 | 1.5 | 1.3 |
| L-Lysine 12 | 0.5 | - | 0.5 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 |
| DL-Methionine 13 | 0.5 | - | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.4 |
| Celite | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Chemical composition | ||||||||||
| Moisture | 6.5 | 8.2 | 4.2 | 6.0 | 4.5 | 7.1 | 7.1 | 8.6 | 6.1 | 3.5 |
| Crude protein (N × 6.25) | 45.1 | 45.4 | 45.5 | 45.3 | 45.2 | 45.1 | 45.1 | 45.2 | 45.4 | 45.2 |
| Total lipids | 20.4 | 20.3 | 20.2 | 20.2 | 20.4 | 20.5 | 20.3 | 20.4 | 20.1 | 20.4 |
| Ash | 5.8 | 12.4 | 6.7 | 6.6 | 6.6 | 7.1 | 7.8 | 7.7 | 8.9 | 6.9 |
| Chitin # | 0.02 | 0.02 | 0.40 | 0.76 | 1.51 | 0.02 | 0.02 | 0.39 | 0.73 | 0.02 |
| Gene Name | Primer Sequence | A.T. (°C) | Gene Bank ID | |
|---|---|---|---|---|
| Forward | Reverse | |||
| ghre | GGAAAGTCTTCCAGGGTCGG | CGCATAGTCCTCTTCTGTCATGGAG | 59 | MK089519.1 |
| cb1 | GCTGGGCTGGAACTGTAAAC | TTCCACAGGATGTATATGTAGGC | 60 | EF051620.1 |
| npy | GGAGCTGGCCAAGTACTACTCA | GAGACCAGCGTGTCCAGAAT | 60 | XM_030411288.1 |
| β-actin (hk) | TCCTGCGGAATCCATGAGA | GACGTCGCACTTCATGATGCT | 57 | X89920.1 |
| rps18 (hk) | AGGGTGTTGGCAGACGTTAC | CTTCTGCCTGTTGAGGAACC | 57 | AM490061.1 |
| CV | CF | H10 | H20 | H40 | P20 | P40 | H10P30 | RC10 | MA10 | p Value | RMSE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FW | 327.2 a | 327.5 a | 334.1 a | 349.7 a | 343.7 a | 335.6 a | 342.6 a | 349.8 a | 330.4 a | 302.8 b | 0.035 | 115.58 |
| FI | 11.7 bc | 12.2 b | 11.3 c | 11.5 c | 11.7 bc | 11.9 bc | 11.7 bc | 11.6 c | 12.1 b | 13.6 a | 0.006 | 0.07 |
| SGR | 1.32 ab | 1.31 b | 1.33 ab | 1.36 a | 1.35 a | 1.33 ab | 1.34 ab | 1.36 a | 1.32 ab | 1.26 c | 0.009 | 0.0003 |
| FCR | 1.18 a | 1.25 b | 1.15 a | 1.16 a | 1.15 a | 1.15 a | 1.16 a | 1.14 a | 1.24 b | 1.39 c | <0.001 | 0.0003 |
| CV | CF | H10 | H20 | H40 | P20 | P40 | H10P30 | RC10 | MA10 | p Value | RMSE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TL, cm | 25.13 | 25.41 | 25.96 | 26.09 | 25.94 | 25.52 | 25.77 | 26.30 | 25.67 | 25.20 | NS | 0.92 |
| K, % | 1.99 | 2.00 | 1.94 | 2.02 | 2.02 | 2.04 | 1.97 | 2.01 | 2.00 | 1.89 | NS | 0.11 |
| FY, % | 54.33 | 52.88 | 52.86 | 54.02 | 55.20 | 54.28 | 54.69 | 54.08 | 52.56 | 53.25 | NS | 3.09 |
| HSI, % | 0.95 | 1.04 | 0.92 | 1.04 | 1.02 | 1.00 | 0.88 | 1.08 | 0.96 | 0.87 | NS | 0.23 |
| pH | 6.17 | 6.19 | 6.16 | 6.15 | 6.21 | 6.14 | 6.13 | 6.24 | 6.20 | 6.21 | NS | 0.09 |
| Texture, N | 44.52 | 42.43 | 43.17 | 46.97 | 46.03 | 39.64 | 45.39 | 45.33 | 51.69 | 44.62 | NS | 10.65 |
| Skin colour | ||||||||||||
| L* | 75.50 ab | 74.41 ab | 74.66 ab | 71.68 ab | 75.82 ab | 69.60 b | 73.99 ab | 73.01 ab | 70.87 ab | 76.41 a | 0.015 | 4.53 |
| a* | −2.69 | −2.95 | −2.81 | −2.58 | −2.78 | −2.62 | −2.32 | −2.81 | −2.55 | −2.95 | NS | 0.55 |
| b* | −1.11 ab | −3.85 b | −0.35 ab | −0.29 ab | 1.28 a | −0.06 ab | −1.86 b | −1.46 b | −1.27 ab | −1.19 ab | <0.0001 | 1.89 |
| Fillet colour | ||||||||||||
| L* | 49.85 | 49.45 | 48.97 | 49.75 | 50.08 | 49.31 | 49.59 | 49.06 | 50.25 | 51.70 | NS | 2.15 |
| a* | 0.30 | 0.31 | −0.14 | 0.08 | 0.20 | −0.11 | 0.06 | 0.29 | 0.10 | −0.38 | NS | 0.68 |
| b* | −0.14 | −0.97 | −0.52 | −2.14 | −1.31 | −0.62 | −0.49 | −0.49 | −0.04 | 0.00 | NS | 1.92 |
| CV | CF | H10 | H20 | H40 | P20 | P40 | H10P30 | RC10 | MA10 | p Value | RMSE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | 69.31 | 69.66 | 69.85 | 68.87 | 69.36 | 69.23 | 69.99 | 69.27 | 69.13 | 70.00 | NS | 1.26 |
| Ash | 1.41 | 1.38 | 1.41 | 1.40 | 1.46 | 1.44 | 1.44 | 1.39 | 1.37 | 1.34 | NS | 0.11 |
| Crude protein | 19.74 | 19.85 | 19.93 | 19.91 | 19.78 | 20.22 | 20.16 | 20.30 | 20.29 | 19.58 | NS | 0.52 |
| Total lipids | 9.15 | 8.78 | 8.46 | 9.32 | 8.88 | 8.60 | 7.89 | 8.63 | 8.84 | 8.46 | NS | 1.46 |
| Fatty acids | ||||||||||||
| C12:0 | 0.07 c | 0.29 bc | 0.59 b | 1.25 a | 1.61 a | 0.08 c | 0.12 c | 0.68 b | 0.14 c | 0.06 c | <0.0001 | 0.28 |
| C14:0 | 2.28 d | 3.32 a | 2.72 c | 2.92 bc | 3.07 ab | 2.24 d | 2.39 d | 2.65 cd | 2.46 cd | 2.31 d | <0.0001 | 0.21 |
| C16:0 | 14.61 b | 16.40 a | 14.82 b | 14.84 b | 14.76 b | 15.20 b | 15.17 b | 15.59 ab | 14.97 b | 14.54 b | <0.0001 | 0.62 |
| C16:1-n7 | 3.59 c | 5.35 a | 4.08 bc | 4.00 bc | 4.00 bc | 4.06 bc | 4.38 b | 4.51 b | 4.06 bc | 3.69 c | <0.0001 | 0.36 |
| C18:0 | 3.38 ab | 3.62 a | 3.24 ab | 3.11 b | 3.20 b | 3.51 ab | 3.53 ab | 3.46 ab | 3.34 ab | 3.20 b | <0.0001 | 1.18 |
| C18:1n-9 | 32.17 a | 27.22 b | 30.69 a | 31.26 a | 31.62 a | 32.14 a | 31.90 a | 31.49 a | 31.80 a | 30.79 a | <0.0001 | 0.09 |
| C18:1n-7 | 2.44 bc | 2.80 a | 2.49 bc | 2.42 c | 2.44 bc | 2.50 bc | 2.55 b | 2.51 bc | 2.54 bc | 2.52 bc | <0.0001 | 1.28 |
| C18:2n-6 | 16.33 ab | 10.22 c | 14.98 b | 15.09 b | 14.97 b | 15.39 b | 15.19 b | 14.68 b | 14.89 b | 17.99 a | <0.0001 | 0.02 |
| C18:3n-3 | 8.46 a | 7.63 b | 7.89 b | 7.92 b | 7.09 c | 7.43 bc | 6.72 c | 6.81 c | 7.96 ab | 8.37 a | <0.0001 | 0.36 |
| C20:1n-9 | 0.90 bc | 1.32 a | 1.02 b | 1.01 b | 0.95 bc | 0.93 bc | 0.96 bc | 0.93 bc | 0.95 bc | 0.86 c | <0.0001 | 0.10 |
| C20:5n-3 | 3.45 bc | 4.82 a | 3.87 b | 3.58 bc | 3.74 b | 3.64 bc | 3.76 b | 3.76 b | 3.79 b | 3.25 c | <0.0001 | 0.33 |
| C22:5n-3 | 1.21 c | 1.73 a | 1.42 b | 1.38 bc | 1.29 bc | 1.32 bc | 1.37 bc | 1.30 bc | 1.32 bc | 1.03 c | <0.0001 | 0.13 |
| C22:6n-3 | 5.17 bc | 7.72a | 6.06 b | 5.41 bc | 5.51 bc | 5.52 bc | 5.75 bc | 5.48 bc | 5.67 bc | 4.94 c | <0.0001 | 0.64 |
| ƩSFA | 21.18 c | 24.79 a | 22.26 bc | 22.92 bc | 23.46 ab | 21.86 bc | 22.04 bc | 23.22 b | 21.79 c | 20.89 c | <0.0001 | 0.97 |
| ƩMUFA | 40.22 ab | 38.33 c | 39.47 b | 39.84 ab | 40.14 ab | 40.74 a | 40.94 a | 40.58 ab | 40.52 ab | 39.43 bc | <0.0001 | 0.79 |
| Ʃn-6 PUFA | 17.70 ab | 11.57 c | 16.23 b | 16.26 b | 16.13 b | 16.87 b | 16.78 b | 16.19 b | 16.18 b | 19.34 a | <0.0001 | 1.25 |
| Ʃn-3 PUFA | 19.91 bc | 23.86 a | 20.89 b | 19.87 bc | 19.18 bc | 19.48 bc | 19.12 c | 18.88 c | 20.39 bc | 19.42 bc | <0.0001 | 1.24 |
| n-3 PUFA/n-6PUFA | 1.13 b | 2.16 a | 1.33 b | 1.22 b | 1.89 b | 1.15 b | 1.39 b | 1.17 b | 1.29 b | 1.00 b | <0.0001 | 0.23 |
| CV | CF | H10 | H20 | H40 | P20 | P40 | H10P30 | RC10 | MA10 | p Value | RMSE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD | 0.20 b | 0.27 a | 0.22 ab | 0.25 ab | 0.23 ab | 0.21 ab | 0.21 ab | 0.22 ab | 0.24 ab | 0.20 b | 0.014 | 0.04 |
| TBARS | 0.86 | 1.22 | 0.71 | 0.76 | 0.82 | 0.77 | 0.65 | 0.72 | 0.70 | 0.74 | NS | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulido-Rodriguez, L.F.; Cardinaletti, G.; Secci, G.; Randazzo, B.; Bruni, L.; Cerri, R.; Olivotto, I.; Tibaldi, E.; Parisi, G. Appetite Regulation, Growth Performances and Fish Quality Are Modulated by Alternative Dietary Protein Ingredients in Gilthead Sea Bream (Sparus aurata) Culture. Animals 2021, 11, 1919. https://doi.org/10.3390/ani11071919
Pulido-Rodriguez LF, Cardinaletti G, Secci G, Randazzo B, Bruni L, Cerri R, Olivotto I, Tibaldi E, Parisi G. Appetite Regulation, Growth Performances and Fish Quality Are Modulated by Alternative Dietary Protein Ingredients in Gilthead Sea Bream (Sparus aurata) Culture. Animals. 2021; 11(7):1919. https://doi.org/10.3390/ani11071919
Chicago/Turabian StylePulido-Rodriguez, Lina Fernanda, Gloriana Cardinaletti, Giulia Secci, Basilio Randazzo, Leonardo Bruni, Roberto Cerri, Ike Olivotto, Emilio Tibaldi, and Giuliana Parisi. 2021. "Appetite Regulation, Growth Performances and Fish Quality Are Modulated by Alternative Dietary Protein Ingredients in Gilthead Sea Bream (Sparus aurata) Culture" Animals 11, no. 7: 1919. https://doi.org/10.3390/ani11071919
APA StylePulido-Rodriguez, L. F., Cardinaletti, G., Secci, G., Randazzo, B., Bruni, L., Cerri, R., Olivotto, I., Tibaldi, E., & Parisi, G. (2021). Appetite Regulation, Growth Performances and Fish Quality Are Modulated by Alternative Dietary Protein Ingredients in Gilthead Sea Bream (Sparus aurata) Culture. Animals, 11(7), 1919. https://doi.org/10.3390/ani11071919







