Pain, Pathophysiological Mechanisms, and New Therapeutic Options for Alternative Analgesic Agents in Sheep: A Review and Investigation
Abstract
Simple Summary
Abstract
1. Introduction
2. Historical Outline
3. Definitions of Pain
4. Types of Pain
5. Neuropharmacological Basics of Pain
Consequences of Nociceptive Sensations and Their Therapeutic Implications
6. Pain as a Stress Factor
7. Pain Conduction Pathways
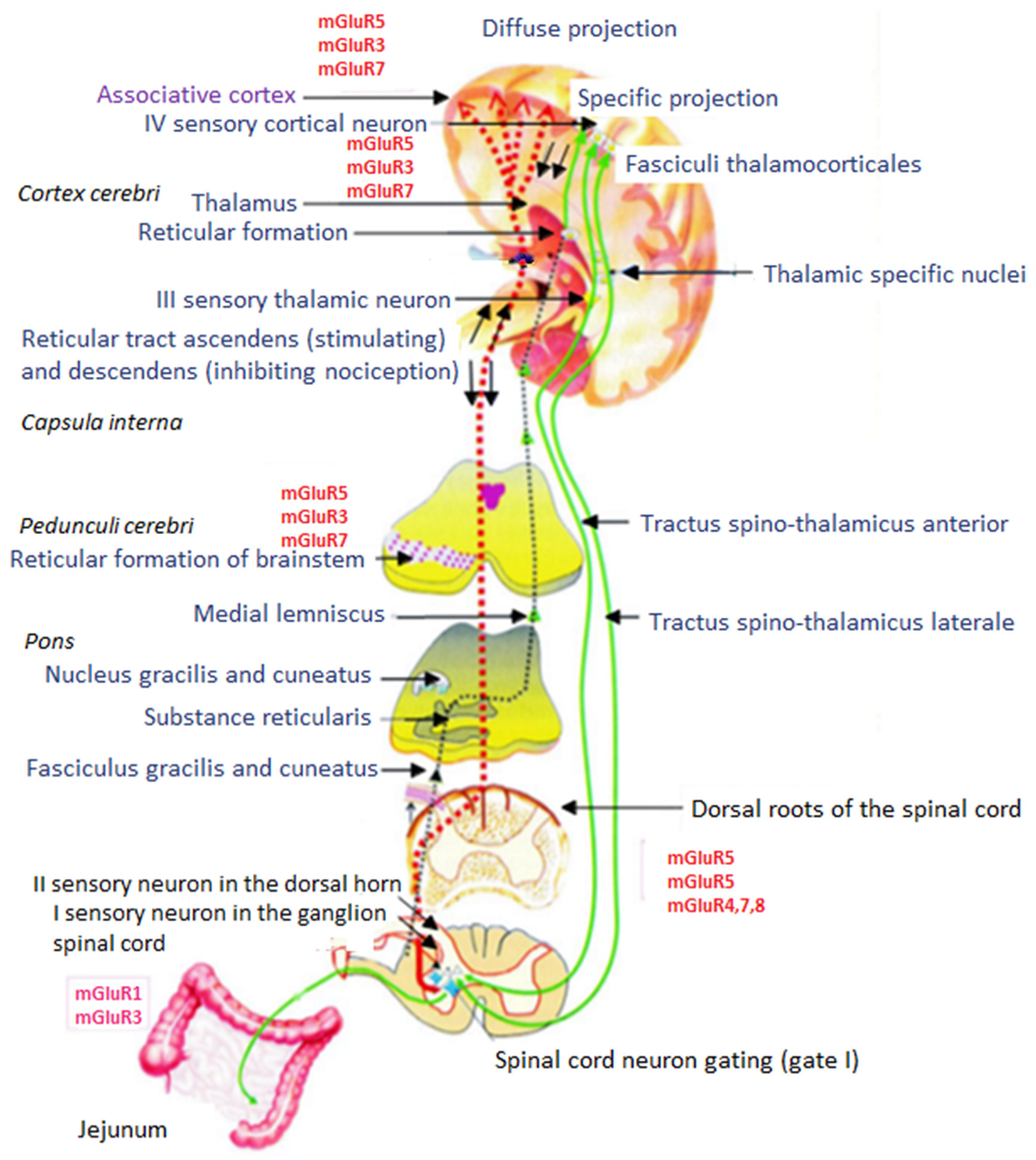
7.1. Perception of Stimuli and Nociceptive Pathways
7.2. Afferent Conduction Pathways of Nociception
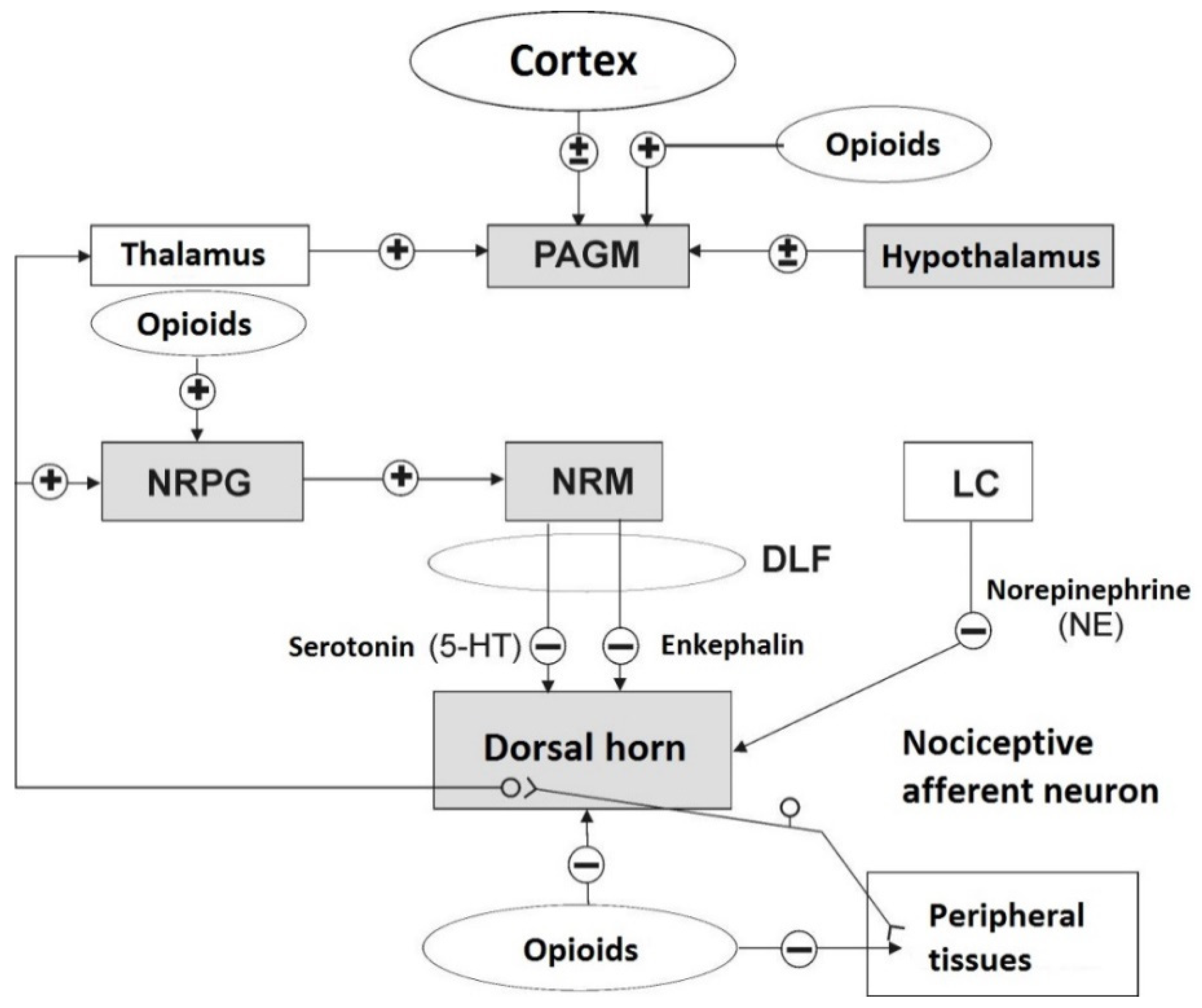
7.3. Efferent Inhibitory Pathways of Nociception
8. Opioid Receptors
9. Endogenous Opioid Peptides (EOPs)
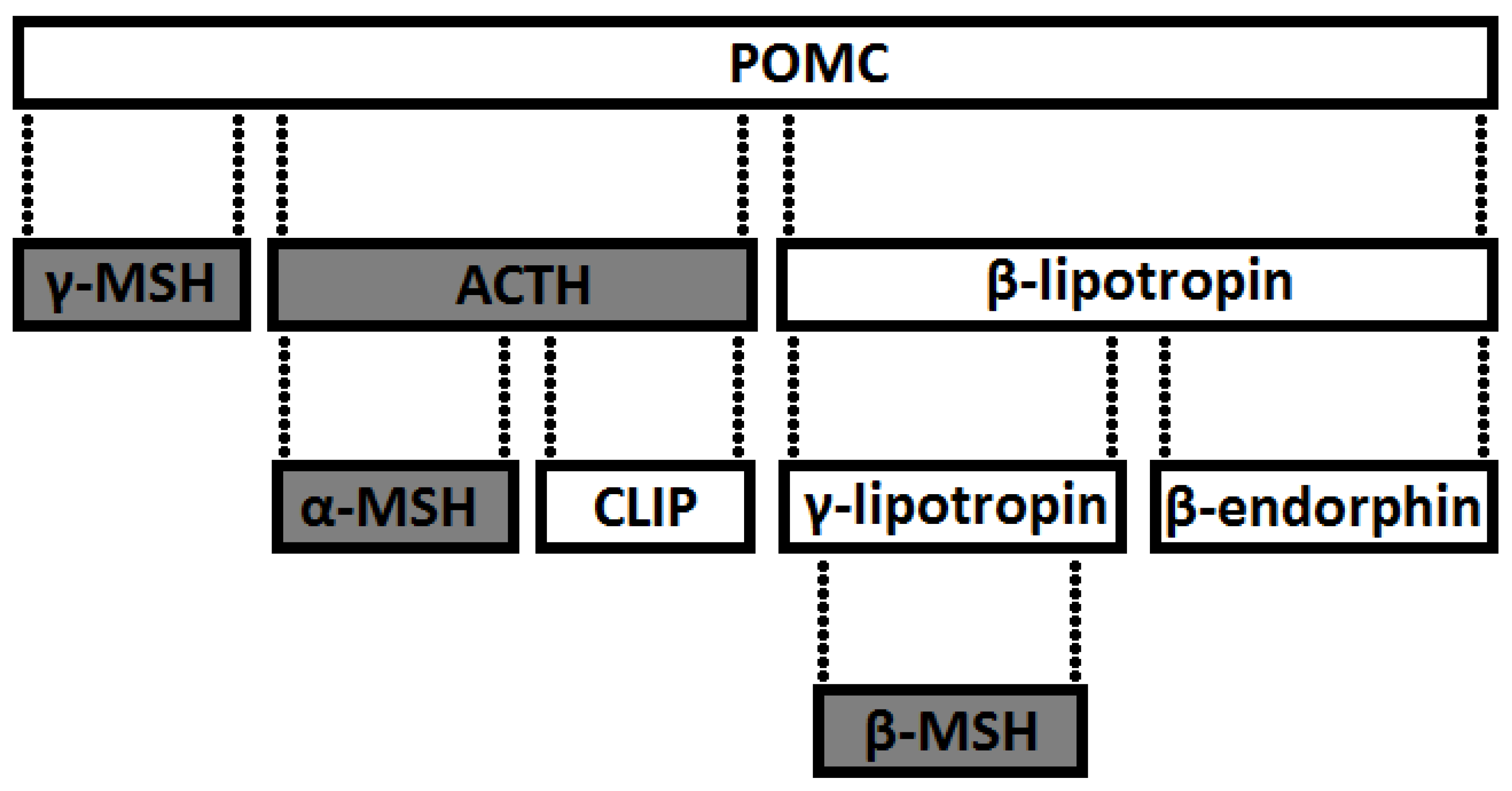
9.1. Classification
- derivatives of POMC—precursor of an ACTH-releasing factor (POMC1-39) and β-lipotropin (β-LPH), which is part of the POMC1-91 chain. Endorphins are formed from β-LPH1-91 (β = β-LPH61-91, γ = β-LPH65-77 and endorphin α = βLPH61-76),
- preproencephalin A derivatives (Leu- and Met-ENK),
- hypothalamic preprodynorphine derivatives (dynorphin A and B, l-neo-endorphin, rimorphin),
- exorphins that are also endorphins (milk casomorphin),
- endomorphins (endomorphin-1 and endomorphin-2),
- nociceptin (orphanin).
9.2. Other Opioid Mediators of Nociception
10. Exogenous Opioids (Opiates)
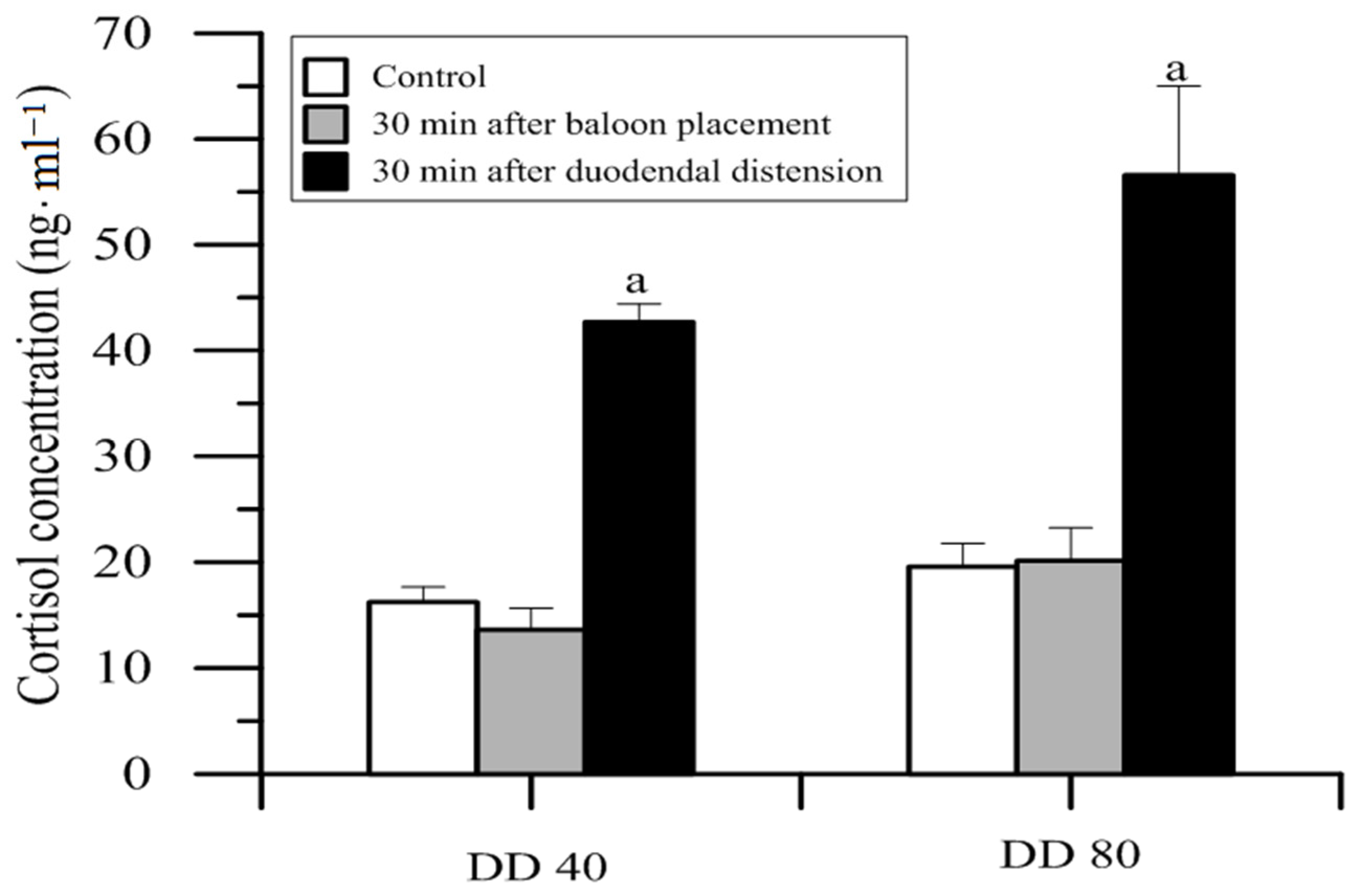
11. Visceral/Intestinal Pain
12. Non-Opioid Analgesics
12.1. Purpose of the Study
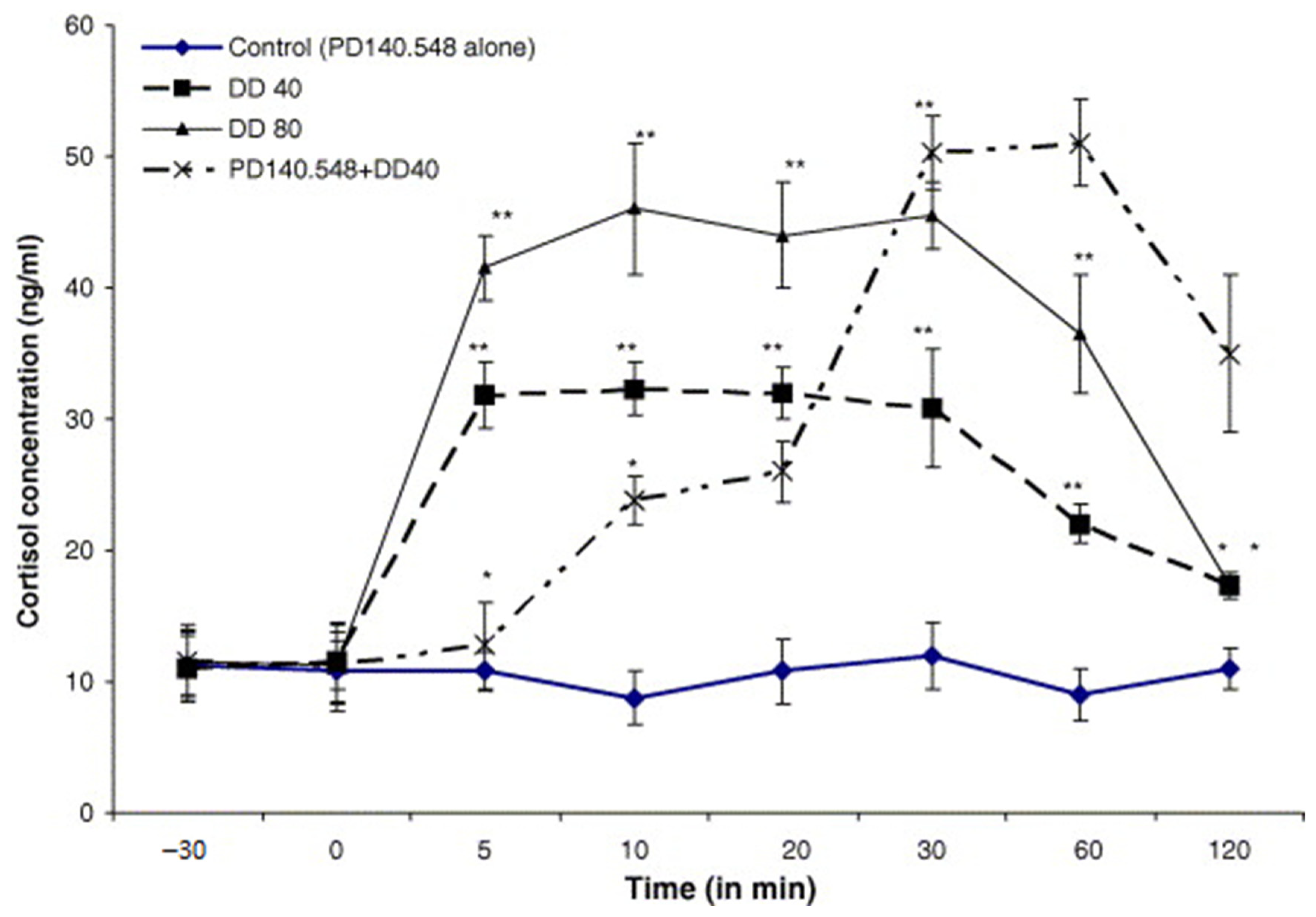
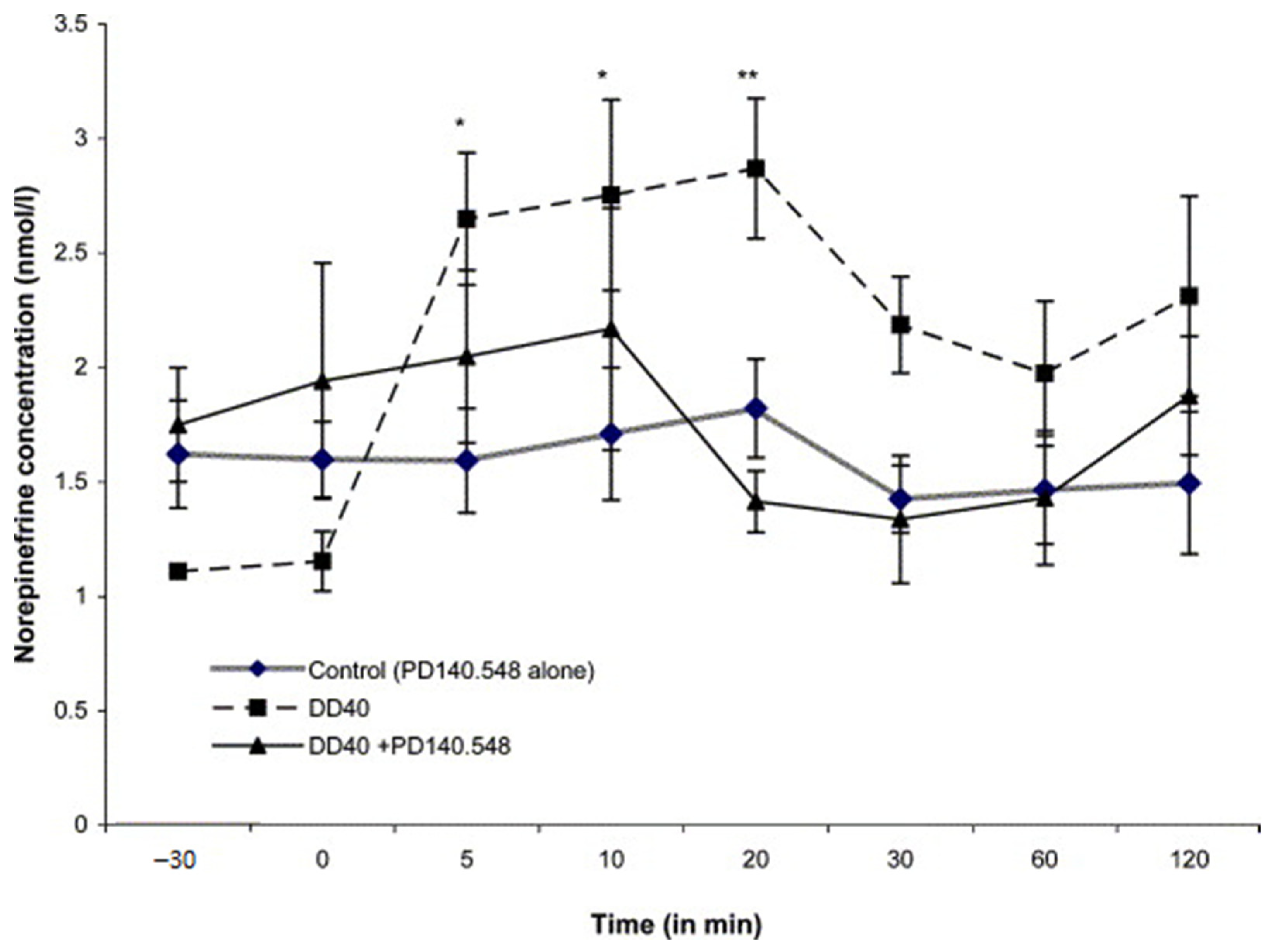
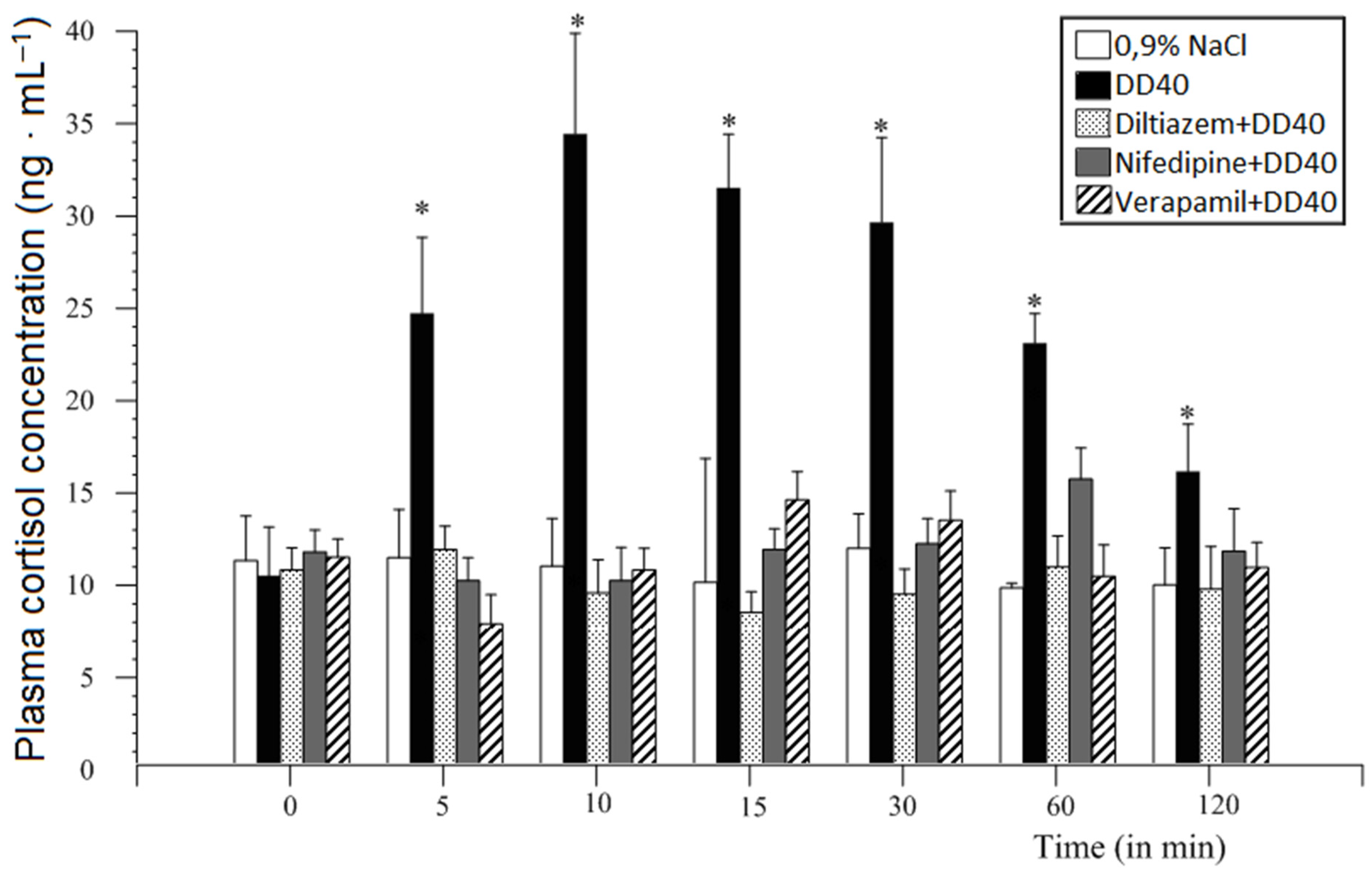
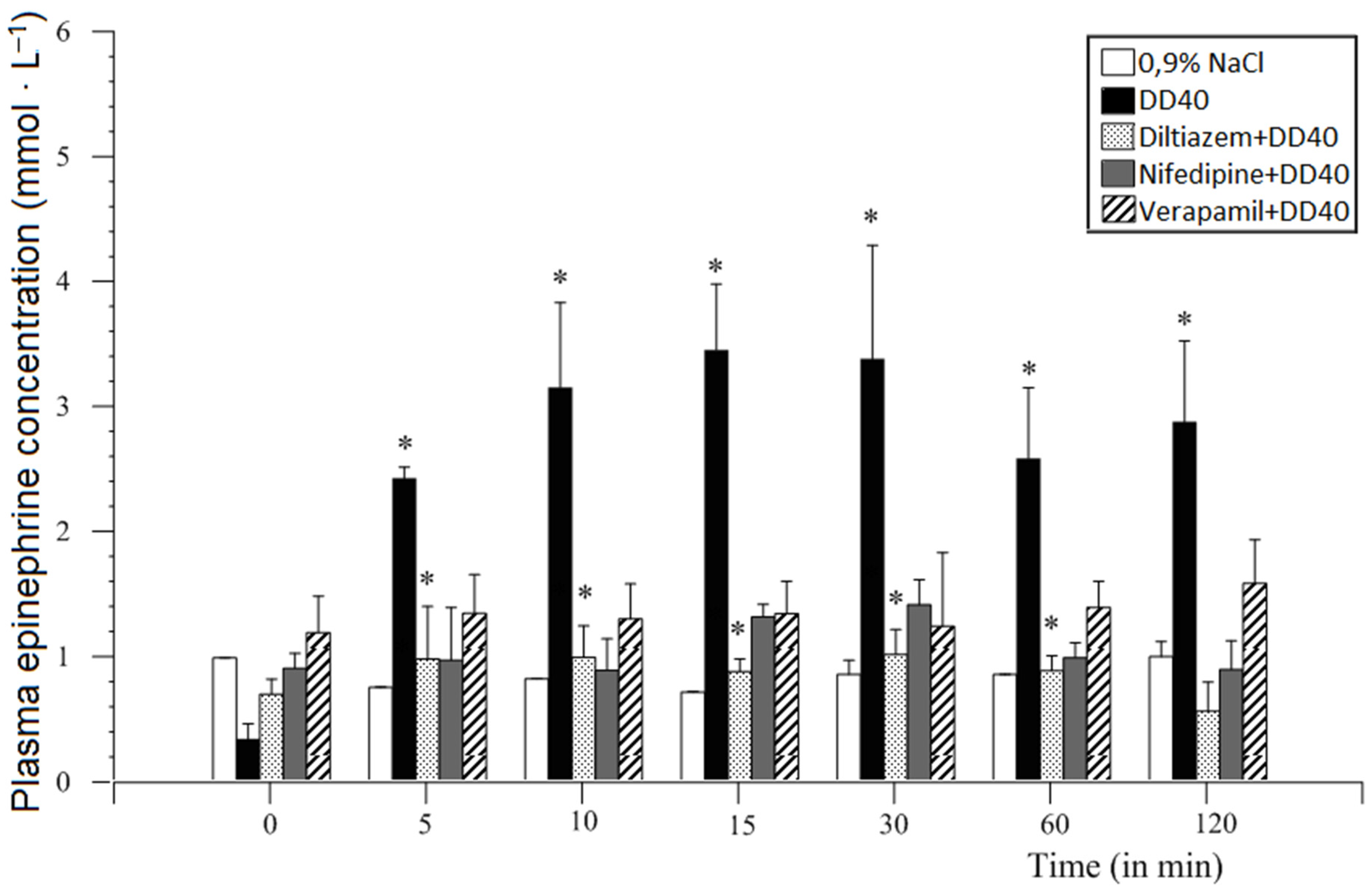
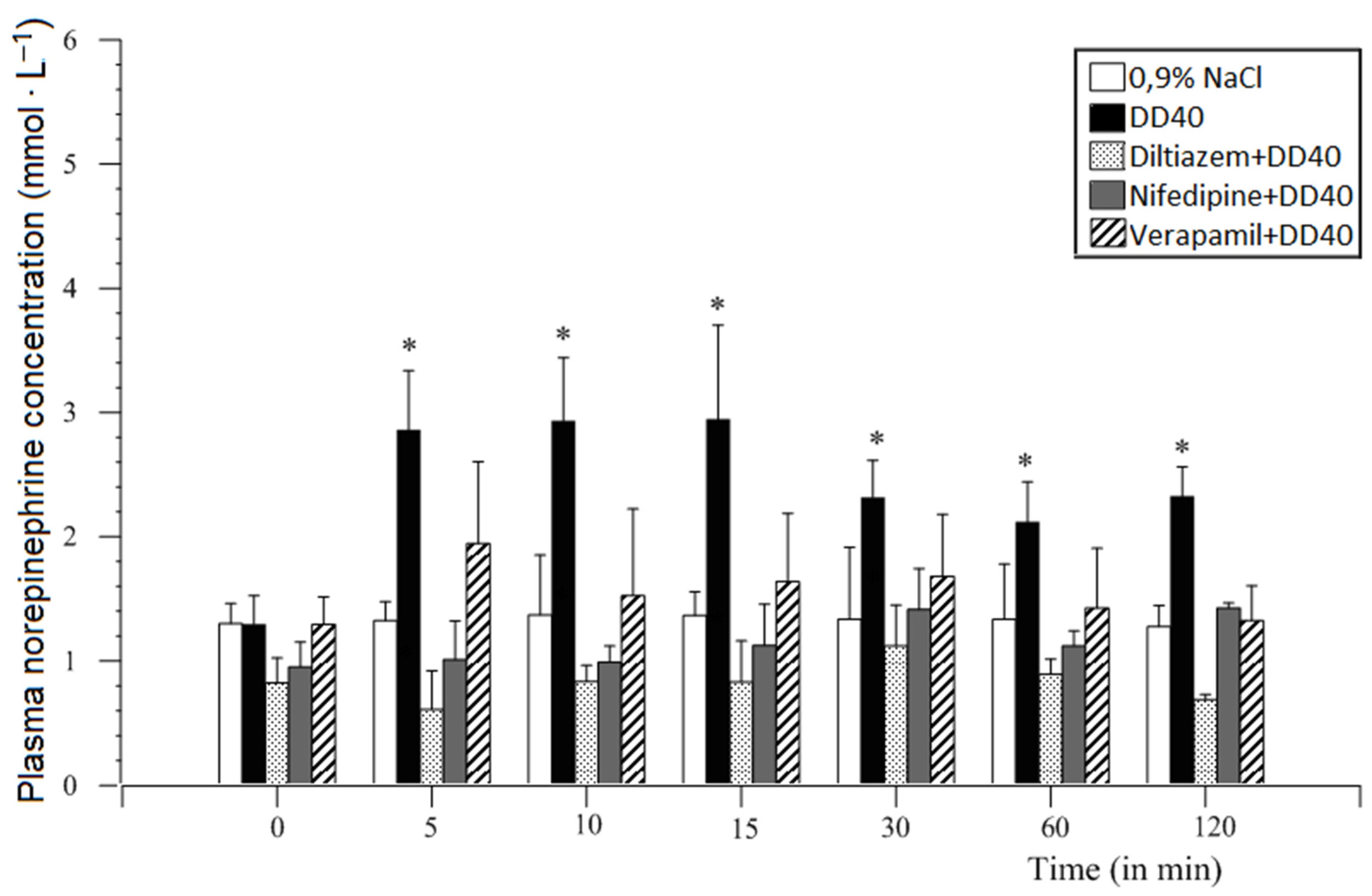
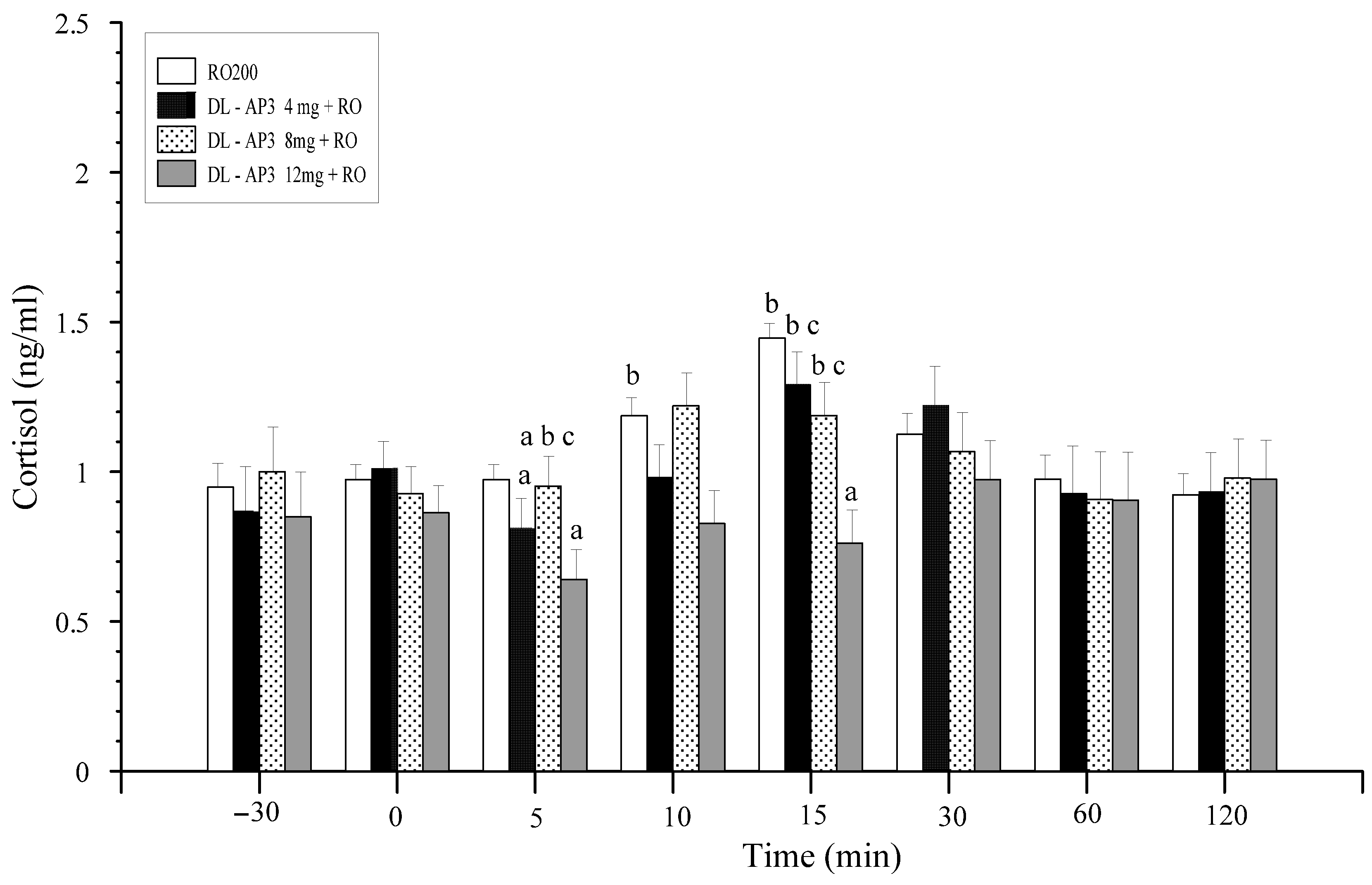
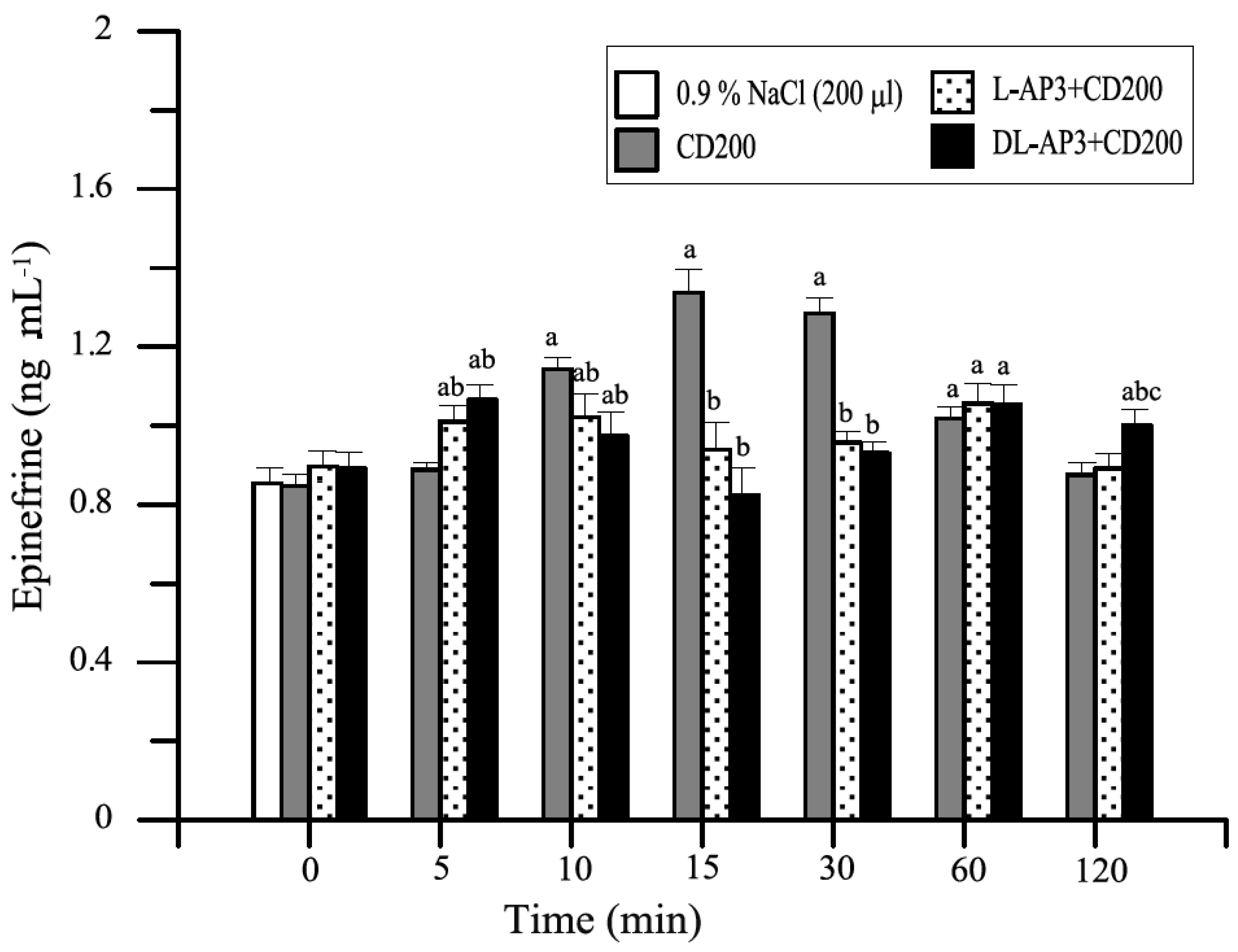
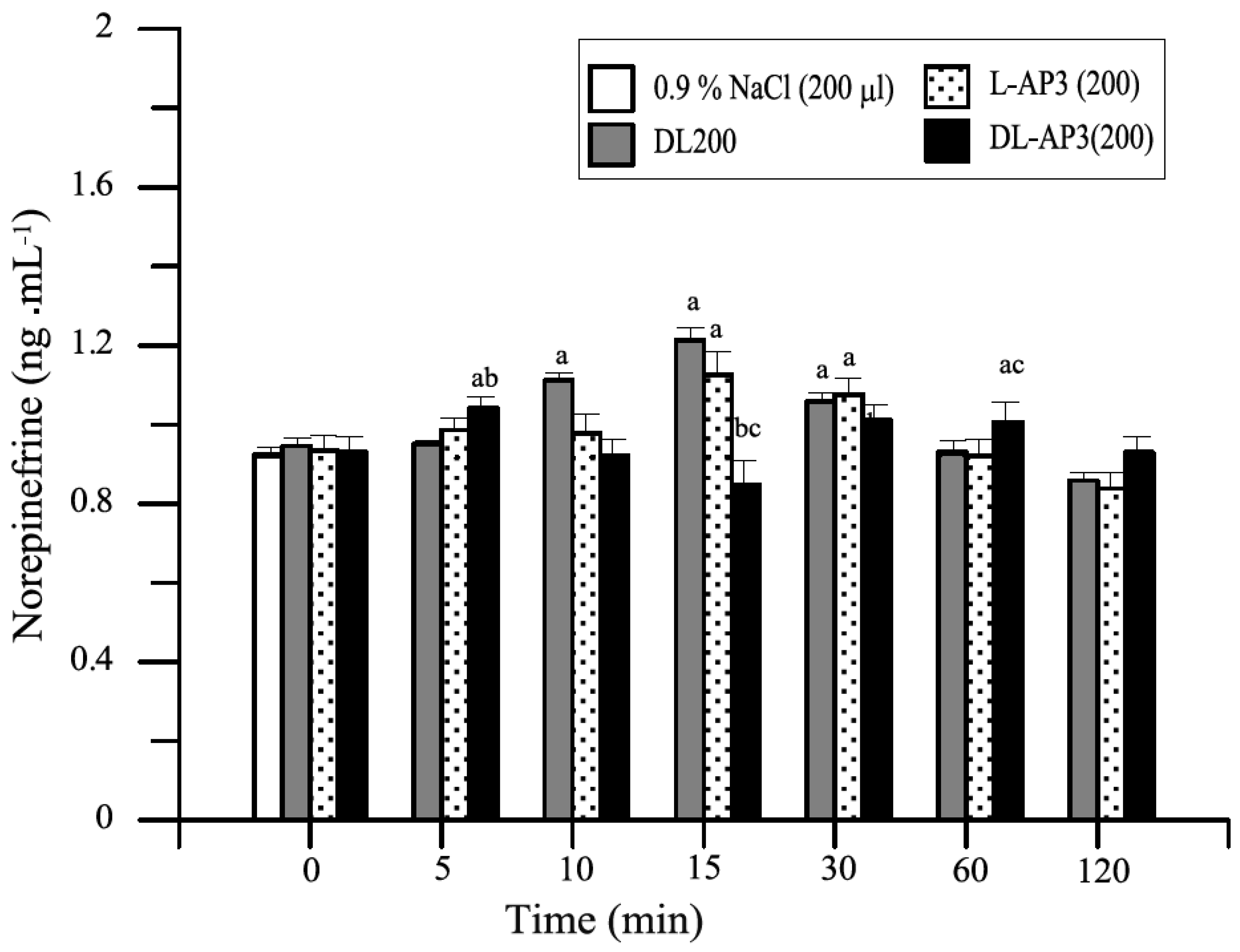
12.2. Methods and Results
12.3. Inhibitors of Cholecystokinin (CCK)
12.4. Voltage-Dependent Calcium Channel Inhibitors
12.5. Inhibitors of Metabotropic Glutaminergic Receptors (mGluR1)
13. Discussion
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kania, B.F.; Kania, K. Endogenous opioid peptides: Action and significance for therapy. Med. Weter. 2002, 58, 487–491. [Google Scholar]
- Parikh, D.; Hamid, A.; Friedman, T.C.; Nguyen, K.; Tseng, A.; Marquez, P.; Lutfy, K. Stress-induced Analgesia and Endogenous Opioid Peptides: The Importance of Stress Duration. Eur. J. Pharmacol. 2011, 650, 563–567. [Google Scholar] [CrossRef]
- Przewłocka, B. Basic mechanisms of analgesic effects of opioids (pol.). Med. Paliatywna Prakt. 2017, 11, 48–54. [Google Scholar]
- Przewłocki, R. Opioid peptids. In Neuroscience in 21 Century; Pfaff, D.W., Volkov, N.D., Eds.; Springer Science + Business Media: New York, NY, USA, 2015; pp. 1783–1810. [Google Scholar]
- Yamanoto, T.; Nozaki-kguchi, N.; Sakashjta, Y.; Kinlura, S. Noclceptin/orphanin FQ: Role in nociceptive infomation processing. Prog. Neurobiol. 1999, 57, 527–535. [Google Scholar]
- Henderson, G.; Mc Knight, A.Z. The orphan opioid receptor and its endogenous 1igand-nociceptin/orphanin FQ. TIPS 1997, 18, 293–303. [Google Scholar]
- Meunier, J.C.; Mollereau, C.; Tol1, L.; Suaudeau, C.; Moisand, C.; Alvinerie, P.; Blltour, J.L.; Guitlemot, L.C.; Ferrara, P.; Monsatat, B. Isolation and structure of the endogenous agonist of opioid receptor-Iike ORL1 receptor. Nature 1995, 37, 532–535. [Google Scholar] [CrossRef]
- Gaynor, J.S.; Muir, W.W. Handbook of Veterinary Pain Management; Mosby Elsevier: St. Louis, MO, USA, 2009. [Google Scholar]
- Martin, W.R. A new receptor nomenclature. Life Sci. 1981, 28, 1555–1557. [Google Scholar] [CrossRef]
- Stillman, M.W.; Whittaker, A.L. Use and efficacy of analgesic agents in sheep used in biomedical research. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 755–766. [Google Scholar] [CrossRef]
- Colditz, I.G.; Paull, D.R.; Lloyd, J.B.; Johnston, L.; Small, A.H. Efficacy of meloxicam in a pain model in sheep. Aust. Vet. J. 2019, 97, 23–32. [Google Scholar] [CrossRef]
- Willis, W.D.; Kenshalo, D.R., Jr.; Leonard, R.B. The cells of origin of the primate spinothalamic tract. J. Comp. Neurol. 1979, 188, 543–573. [Google Scholar] [CrossRef]
- Buéno, L.; Fioramonti, J.; Delvaux, M.; Fexinos, J. Mediators and pharmacology of visceral sensitivity: From basic to clinical investigations. Gastroenterology 1997, 112, 1714–1743. [Google Scholar] [CrossRef]
- Battaglia, A.A. An Introduction to Pain and Its Relation to Nervous System Disorders, 1st ed.; John Willey & Sons, Ltd.: New York, NY, USA, 2016. [Google Scholar]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Ninneru, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Jeffrey, S.; Mogil, J.S.; Ringkamp, M.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Gué, M.; de Rio, C.; Junien, J.L.; Buéno, L. Interaction between CCK and opioid in the modulation of the rectocolonic inhibitory reflex in rats. Am. J. Physiol. 1995, 269, G240–G245. [Google Scholar]
- Julia, V.; Buéno, L. Peripheral and central NK3 receptors are differentially involved in colonic and abdominal responses to rectal distension in rats (abstr.). Neurogastroenterol. Motil. 1995, 7, 264. [Google Scholar]
- Kania, B.F. Glutamate as an Neural Factor for Stressoric Disorders; Warsaw University of Life Sciences SGGW: Warszawa, Poland, 2020; p. 127. [Google Scholar]
- Załucki, G. Animal Physiology (pol.); PWRiL: Warszawa, Poland, 1995. [Google Scholar]
- Priest, T.D.; Hoggard, B. Chronic pain: Mechanisms and treatment. Curr. Opin. Pharmacol. 2002, 3, 310–315. [Google Scholar] [CrossRef]
- Świderski, T. Participation of Opioid and Non-Opioid Components in Post-Stress Analgesia in Selected Mice. Ph.D. Thesis, PAN Institute of Animal Genetics and Breeding, Jastrzębiec, Poland, 1998. [Google Scholar]
- Werka, Z. Stress and pain (pol.). In Brain and Behavior; Górska, T., Grabowska, A., Zagrodzka, J., Eds.; PWN: Warszawa, Poland, 2011; pp. 252–268. [Google Scholar]
- Helleyer, P.W. Control of postoperative pain in dogs and cats. Weter. Dyplomie 1999, 1, 13–19. [Google Scholar]
- Khursheed, M. New possibilities to combat chronic pain in animals. Med. Dyplomie 1999, 393, 70–73. [Google Scholar]
- Holton, L.L.; Scott, E.M.; Nolan, A.M.; Reid, L.; Welsh, E.; Fłahelty, D. Comparison of three methods used for assessment of pain in dogs. JAVMA 1998, 212, 61–66. [Google Scholar]
- Tranquilli, W.L.; Ralfe, A.L.R. Understanding pain and analgesic therapy in pets. Yet. Med. 1989, 84, 680–686. [Google Scholar]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Cox, B.H.; Goldstejn, A.; Li, C.H. Opioid activity of a peptide, β-lipotropin (6 I-91) derived from β-lipotropin. Proc. Natl. Acad. Sci. USA 1976, 73, 1821–1823. [Google Scholar] [CrossRef]
- Ritter, J.M.; Flower, R.J.; Henderson, G.; Loke, J.K.; MacEven, D.; Rang, H.P. Rang @ Dale’s Pharmacology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sadowski, B. Biological Mechanisms of Behavior (pol.); PWN: Warszawa, Poland, 2016; pp. 163–164. [Google Scholar]
- Panocka, I. Differentiation of Opioid Anti-Nociceptive Systems in Mice under the Influence of Selection for High and Low Post-stress Analgesia (pol.). Ph.D. Thesis, Institute of Experimental Biology of the Polish Academy of Sciences PAN, Warszawa, Poland, 1989. [Google Scholar]
- Dannemann, P.J. Monitoring of analgesia. Anaesth. Analg. 1997, 15, 83–103. [Google Scholar]
- Cesare, P.; Naughton, P. Peripheral pain mechanisms. Curr. Opin. Neurobiol. 1997, 7, 493–499. [Google Scholar] [CrossRef]
- Kania, B.F.; Wrońska, D. Voltage-gated calcium channel antagonists: Potential analgesic for jejunal pain. In Pain Relief–from Analgesics to Alternative Therapies; Maldonado, D., Ed.; IntechOpen: London, UK, 2017; pp. 226–243. [Google Scholar]
- Tracey, I. Imaging pain. BJA Br. J. Anaesth. 2008, 101, 32–39. [Google Scholar] [CrossRef]
- Raver, C.; Uddin, O.; Ji, Y.; Cramer, N.; Jenne, C.; Morales, M.; Masri, R.; Keller, A. An amgdalo-parabrachial pathway regulates pain perception and chronic pain. J. Neurosci. 2020, 40, 3424–3442. [Google Scholar] [CrossRef]
- Fields, H. Syte-dependent opioid control of pain. Nat. Rev. Neurosci. 2004, 5, 565–575. [Google Scholar] [CrossRef]
- Zagon, I.S.; Goodman, S.R.; MacLaughin, P.J. Characterization of zetal: A new opioid receptor involved in growth. Brain Res. 1989, 20, 297–305. [Google Scholar] [CrossRef]
- Hughes, J.; Smith, T.W.; Kosterlitz, H.W.; Fothergill, L.A.; Morgan, B.A.; Morris, H.R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 1975, 258, 577–579. [Google Scholar] [CrossRef]
- Simon, E.J.; Hiller, J.M.; Edelman, J. Stereospecific binding of the potent narcotic analgesic 3H-etorphine to rat brain homogenate. Proc. Natl. Acad. Sci. USA 1973, 190, 389–390. [Google Scholar]
- Snyder, S.H. Opiate receptor in normal and drug altered brain function. Nature 1975, 257, l85–l189. [Google Scholar] [CrossRef]
- Terenius, I.; Walstrom, A. Search for an endogenous ligand for opiate receptor. Acta Physiol. Scand. 1975, 94, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A. Back pain and the mineralocorticoid receptor: Is there a connection? Anesthesiology 2012, 117, 951–952. [Google Scholar] [CrossRef]
- Ganong, W.F. Physiology (pol.); Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2007; pp. 143–147. [Google Scholar]
- Al.-Chaer, E.D.; Traub, R.J. 2002 Biological basis of visceral pain: Recent developments. Pain 2002, 96, 221–225. [Google Scholar] [CrossRef]
- Cervero, F.; Laird, J.M. Visceral pain. Lancet 1999, 353, 2145–2148. [Google Scholar] [CrossRef]
- Goldstein, A.; Tachibuna, S.; Lowney, I.I.; Hunkapiller, M.; Hood, L. Dynorphin–(1–13) an extraordinarily potent opioid peptide. Proc. Natl. Acad. Sci. USA 1979, 76, 6666–6670. [Google Scholar] [CrossRef] [PubMed]
- Kopaczewska, M.; Kopaczewski, B.; Cichy, W.; Nowak, S. Bol trzewny w zaburzeniach czynnościowych przewodu pokarmowego. Pediatr. Współczesna Gastroenterol. Hepatol. Zyw. Dziecka 2004, 6, 369–373. [Google Scholar]
- Krajnik, M.; Żylicz, Z. Antinociceptive effects of opioids (pol.). Med. Paliatywna 2003, 2, 111–118. [Google Scholar]
- Kania, B.F.; Wrońska, D. Glutamate and metabotropic glutamate receptors: Physiology, function, and roles in neurological disorders. In Metabotropic Glutamate Receptors, Classification, Structure and Roles in Disease; O’Keeffe, J., Ed.; IntechOpen: London, UK, 2018; pp. 1–80. [Google Scholar]
- Dolan, S.; Nolan, A.M. Behavioral evidence supporting a differential role for spinal group I and II metabotropic glutamate receptors in inflammatory hyperalgesia in sheep. Neuropharmacology 2002, 43, 126–319. [Google Scholar] [CrossRef]
- Sotowska-Brochocka, J. Fizjologia Zwierząt, Zagadnienia Wybrane; Wyd, Uniwersytetu Warszawskiego: Warszawa, Poland, 2001; pp. 912–919. [Google Scholar]
- Osikowicz, M.; Mika, J.; Przewłocka, B. The glutamatergic system as a target for neuropathic pain relief. Exp. Physiol. 2013, 98, 372–384. [Google Scholar] [CrossRef]
- Vincent, K.; Corneal, V.M.; Jong, Y.-J.I.; Lafferiere, A.; Kumar, N.; Mickeviciute, A.; Fung, J.S.T.; Bandegi, P.; Ribeiro-da-Silva, A.; O’Malley, K.L.; et al. Intracellular mGluR5 plays a critical role in neuropathic pain. Nat. Commun. 2016, 7, 10604. [Google Scholar] [CrossRef] [PubMed]



| Receptor | Subtypes | Location | Function | G Protein Subunit |
|---|---|---|---|---|
| delta (δ) DOR OP1 (I) | δ1, δ2 |
|
| Gi |
| kappa (κ) KOR OP2 (I) | κ1, κ2, κ3 |
|
| Gi |
| mu (μ) MOR OP3 (I) | μ1, μ2, μ3 |
| μ1:
| Gi |
| Nociceptin receptor NOR OP4 (I) | ORL1 |
|
| |
| zeta (ζ) ZOR |
|
|
| Receptor Subtype | µ | δ | κ | |
|---|---|---|---|---|
| Effect | ||||
| Analgesia | ||||
| Supraspinal | +++ | − | − | |
| Spinal | ++ | ++ | + | |
| Peripheral | ++ | − | ++ | |
| Inhibition of respiration | +++ | ++ | − | |
| Miosis | ++ | − | + | |
| Inhibition of gastrointestinal motility | ++ | ++ | + | |
| Euphoria | +++ | − | − | |
| Dysphoria | − | − | +++ | |
| Currently Used Terminology According to the IUPHAR Committee on Receptor Nomenclature and Drug Classification | Previous Terminology | Main Endogenous Agonist | Genes |
|---|---|---|---|
| m-receptor (m receptor) (MOP, μ-opioid peptide) receptor | MOR-1, MOR (mu-opioid receptor) OP3 | [β-endorphin] [Met]enkefalin [Leu]enkefalin Endomorphin 1 and 2 | OPRM-1 (Hs), Oprm1 (Mm), Oprm1 (Rn) ? |
| d-receptor (d receptor), (DOP, μ-opioid peptide) receptor | DOR-1, DOR (delta opioid receptor) OP1 | [Met]enkephalin [Leu]enkefalin)d β-endorphin | OPRD1 (Hs), Oprd1 (Mm), Oprd1 (Rn) |
| k-receptor (k receptor) (KOP, k-opioid peptide) receptor | KOR-1, KOR (kappa-opioid receptor) OP2 | Big dynorphin Dynorphin A α-neoendorphin | OPRK1 (Hs), Oprk1 (Mm), Oprkd1 (Rn) |
| Accompanying Symptoms | 0–5 | 5–10 | 10–15 | 25–30 | 55–60 | 120 min | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DD | 1 | 2 | 3 | DD | 1 | 2 | 3 | DD | 1 | 2 | 3 | DD | 1 | 2 | 3 | DD | 1 | 2 | 3 | DD | 1 | 2 | 3 | |
| Inhibition of ruminal activity | 4+ | − | + | − | 4+ | − | ± | − | 3+ | 2 | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| Looking around | 3+ | ± | ± | ± | 2+ | ± | ± | ± | + | + | + | + | − | − | − | + | − | − | − | − | − | − | − | − |
| Defecation | 3+ | − | − | − | + | − | ± | − | − | ± | − | ± | − | − | ± | + | − | − | − | − | − | − | − | − |
| Head movements | 3+ | − | − | − | 2+ | ± | ± | ± | − | − | − | − | − | − | ± | + | − | − | − | − | − | − | ± | − |
| Stretching | 2+ | − | − | − | + | − | − | − | − | − | + | ± | − | − | − | − | − | − | − | − | − | − | − | − |
| Grinding | 2+ | ± | ± | ± | − | + | + | + | − | ± | ± | ± | − | − | − | − | − | − | − | − | − | − | − | − |
| Lying down | 2+ | − | − | − | + | − | − | − | − | ± | ± | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Bleating | + | − | − | − | + | − | − | − | − | − | − | − | − | + | + | + | + | − | − | − | − | − | − | − |
| Tachycardia | 4+ | ± | 3+ | ± | 4+ | − | 3+ | ± | 4+ | − | 3+ | ± | 3+ | − | ± | ± | 3+ | − | ± | ± | 3+ | − | − | − |
| Hyperventilation | 4+ | ± | − | ± | 3+ | − | − | ± | 4+ | − | − | ± | 3+ | − | − | − | 3+ | − | − | − | 3+ | − | − | − |
| Drugs | Control | 5 min | 10 min | 15 min | 20 min | 25 min | 30 min |
|---|---|---|---|---|---|---|---|
| 0.9% NaCl | 6.45 ± 0.75 | 6.12 ± 0.28 | 6.00 ± 0.25 | 6.80 ± 0.33 | 6.10 ± 0.60 | 6.35 ± 0.15 | 6.11 ± 0.43 |
| DD40 | 6.15 ± 0.54 | 1.09 ± 0.33 * | 1.78 ± 0.49 * | 1.35 ± 0.52 * | 2.20 ± 0.31 * | 0.61 ± 0.12 * | 4.91 ± 0.75 |
| Diltiazem + DD40 | 5.00 ± 0.61 * | 4.00 ± 0.38 * | 3.80 ± 0.60 * | 4.23 ± 0.36 * | 5.14 ± 0.42 | 4.82 ± 0.64 | 6.12 ± 0.40 |
| Nifedipine + DD40 | 5.82 ± 0.45 | 1.89 ± 0.81 * | 5.54 ± 0.23 | 4.88 ± 0.62 | 5.12 ± 0.74 | 6.11 ± 1.11 | 5.59 ± 1.22 |
| Verapamil + DD40 | 6.12 ± 0.89 | 5.33 ± 0.51 | 5.75 ± 0.11 | 5.05 ± 0.80 | 4.97 ± 0.65 | 5.55 ± 1.02 | 5.85 ± 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, B.F.; Wrońska, D.; Bracha, U. Pain, Pathophysiological Mechanisms, and New Therapeutic Options for Alternative Analgesic Agents in Sheep: A Review and Investigation. Animals 2021, 11, 909. https://doi.org/10.3390/ani11030909
Kania BF, Wrońska D, Bracha U. Pain, Pathophysiological Mechanisms, and New Therapeutic Options for Alternative Analgesic Agents in Sheep: A Review and Investigation. Animals. 2021; 11(3):909. https://doi.org/10.3390/ani11030909
Chicago/Turabian StyleKania, Bogdan Feliks, Danuta Wrońska, and Urszula Bracha. 2021. "Pain, Pathophysiological Mechanisms, and New Therapeutic Options for Alternative Analgesic Agents in Sheep: A Review and Investigation" Animals 11, no. 3: 909. https://doi.org/10.3390/ani11030909
APA StyleKania, B. F., Wrońska, D., & Bracha, U. (2021). Pain, Pathophysiological Mechanisms, and New Therapeutic Options for Alternative Analgesic Agents in Sheep: A Review and Investigation. Animals, 11(3), 909. https://doi.org/10.3390/ani11030909






