Comparison of Three Feeding Regimens on Blood Fatty Acids Metabolites of Wujumqin Sheep in Inner Mongolia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Blood Sample Preparation
2.3. Metabolomics Analysis
2.4. Statistical Analysis
3. Results
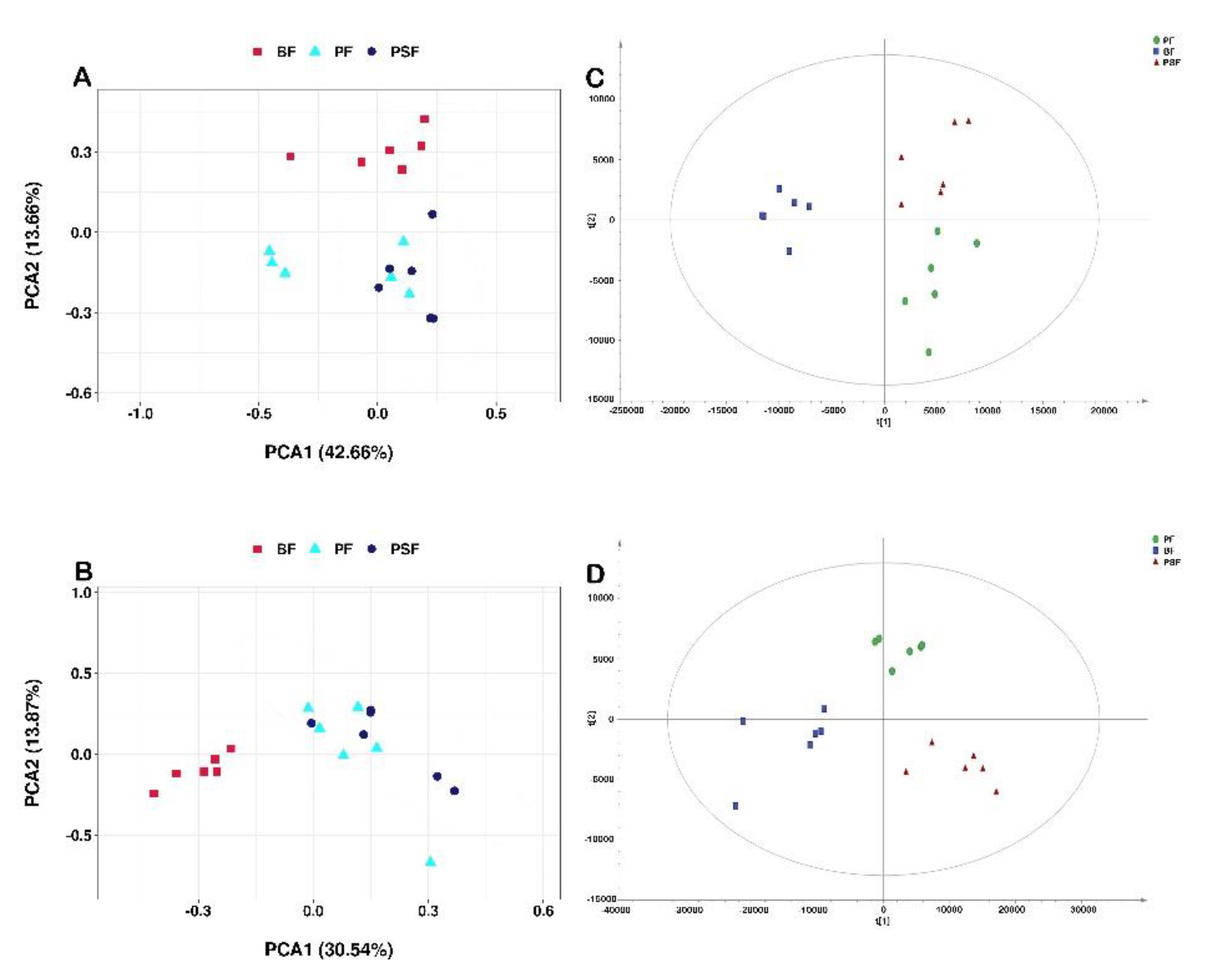
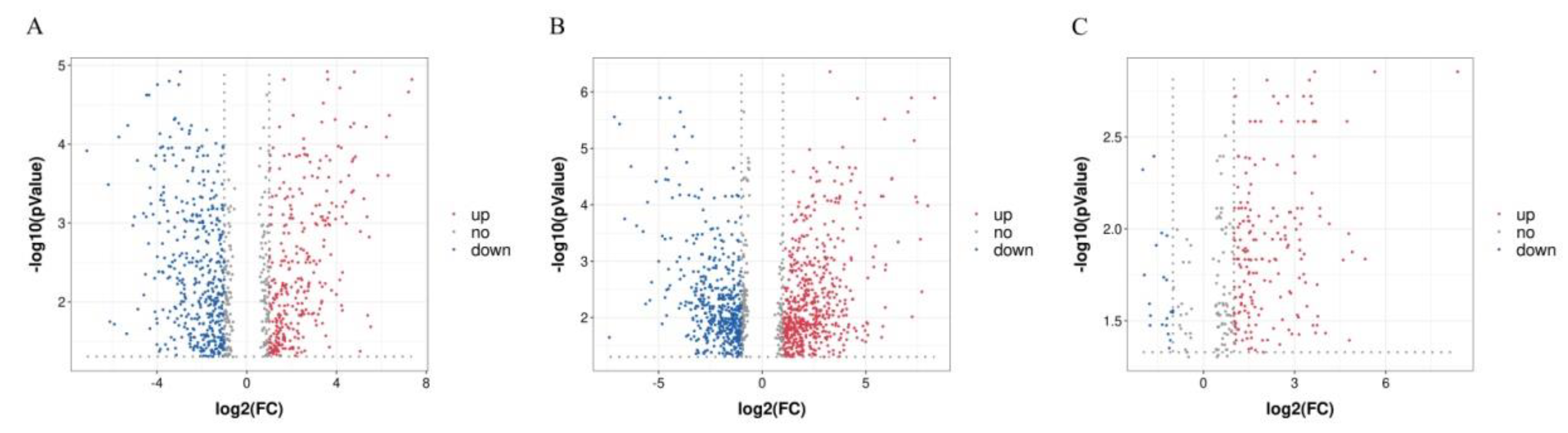
3.1. Metabolite Profiling of the PF, PSF and BF Groups
3.2. Identification of Potential Metabolites and the Metabolic Pathways
4. Discussion
4.1. Effect of Different Feeding Regimens on N-6 and N-3 PUFA Synthesis
4.2. Effect of Different Feeding Regimens on PUFA Metabolism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- SBXL (Statistics Bureau of Xilinguole League). Available online: http://tjj.xlgl.gov.cn/ywlm/tjsj/lnsj/sczz (accessed on 25 March 2020).
- Wang, Z.; Deng, X.; Song, W.; Li, Z.; Chen, J. What is the main cause of grassland degradation? A case study of grassland ecosystem service in the middle-south Inner Mongolia. Catena 2017, 150, 100–107. [Google Scholar] [CrossRef]
- Robinson, B.E.; Li, P.; Hou, X. Institutional change in social-ecological systems: The evolution of grassland management in Inner Mongolia. Glob. Environ. Chang. 2017, 47, 64–75. [Google Scholar] [CrossRef]
- Silva, W.L.; Costa, J.P.R.; Caputti, G.P.; Lage Filho, N.M.; Ruggieri, A.C.; Reis, R.A. Effects of grazing intensity and supplementation strategies on Tifton 85 production and on sheep performance. Small Rum. Res. 2019, 174, 118–124. [Google Scholar] [CrossRef]
- Van Elswyka, M.E.; McNeill, S.H. Impact of grass/forage feeding versus grain finishing on beef nutrients and sensory quality: The U.S. experience. Meat Sci. 2014, 96, 535–540. [Google Scholar]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef]
- Scerra, M.; Caparra, P.; Foti, F.; Galofaro, V.; Sinatra, M.C.; Scerra, V. Influence of ewe feeding systems on fatty acid composition of suckling lambs. Meat Sci. 2007, 76, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Gonzales-Barron, U.; Cadavez, V. A meta-analysis of the effect of pasture access on the lipid content and fatty acid composition of lamb meat. Food Res. Int. 2015, 77, 476–483. [Google Scholar] [CrossRef]
- Nuernberg, K.; Fischer, A.; Nuernberg, G.; Ender, K.; Dannenberger, D. Meat quality and fatty acid composition of lipids in muscle and fatty tissue of Skudde lambs fed grass versus concentrate. Small Rum. Res. 2008, 74, 279–283. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar]
- Sinclair, L.A.; Cooper, S.L.; Chikunya, S.; Wilkinson, R.G.; Hallett, K.G.; Enser, M.; Wood, J.D. Biohydrogenation of n-3 polyunsaturated fatty acids in the rumen and their effects on microbial metabolism and plasma fatty acid concentrations in sheep. Anim. Sci. 2005, 81, 239–248. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Luo, H.L.; Hou, X.Y.; Badgery, W.B.; Zhang, Y.J.; Jiang, C. Effect of restricted access to pasture and indoor supplementation on ingestive behaviour, dry matter intake and weight gain of growing lambs. Livest. Sci. 2014, 167, 137–143. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.L.; Zeng, C.W.; Liu, S.Q. MetaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Doreau, M.; Ferlay, A. Digestion and utilisation of fatty acids by ruminants. Anim. Feed Sci. Technol. 1994, 45, 379–396. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed. Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Willems, H.; Kreuzer, M.; Leiber, F. Alpha-linolenic and linoleic acid in meat and adipose tissue of grazing lambs differ among alpine pasture types with contrasting plant species and phenolic compound composition. Small Rum. Res. 2014, 116, 153–164. [Google Scholar] [CrossRef]
- Girard, M.; Dohme-Meier, F.; Silacci, P.; Kragten, S.A.; Kreuzer, M.; Bee, G. Forage legumes rich in condensed tannins may increase n-3 fatty acid levels and sensory quality of lamb meat. J. Sci. Food Agric. 2016, 96, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. PLEFA 2009, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Yang, L.; Luo, Y.L.; Su, R.; Su, L.; Zhao, L.H.; Jin, Y. Effects of feeding regimens on meat quality, fatty acid composition and metabolism as related to gene expression in Chinese Sunit sheep. Small Rum. Res. 2018, 169, 127–133. [Google Scholar] [CrossRef]
- Urrutia, O.; Mendizabal, J.A.; Insausti, K.; Soret, B.; Purroy, A.; Arana, A. Effect of linseed dietary supplementation on adipose tissue development, fatty acid composition, and lipogenic gene expression in lambs. Livest. Sci. 2015, 178, 345–356. [Google Scholar] [CrossRef]
- Tu, W.C.; Cook-Johnson, R.J.; James, M.J.; Mühlhäusler, B.S.; Gibson, R.A. Omega-3 long chain fatty acid synthesisis regulated more by substrate levels than gene expression. PLEFA 2010, 83, 61–68. [Google Scholar]
- Ponnampalam, E.N.; Hopkins, D.L.; Jacobs, J.L. Increasing omega-3 levels in meat from ruminants under pasture-based systems. Rev. Sci. Tech. 2018, 37, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.A.; Neumann, M.A.; Lien, E.L.; Boyd, K.A.; Tu, W.C. Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids. PLEFA 2013, 88, 139–146. [Google Scholar] [CrossRef]
- Barquissau, V.; Ghandour, R.A.; Ailhaud, G.; Klingenspor, M.; Langin, D.F.; Amri, E.Z.; Pisani, D.F. Control of adipogenesis by oxylipins, GPCRs and PPARs. Biochimie 2017, 136, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Buczynski, M.W.; Dumlao, D.S.; Dennis, E.A. Thematic review series: Proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009, 50, 1015–1038. [Google Scholar] [CrossRef]
- Furne, M.; Holen, E.; Araujo, P.; Lie, K.K.; Moren, M. Cytokine gene expression and prostaglandin production in head kidney leukocytes isolated from Atlantic cod (Gadus morhua) added different levels of arachidonic acid and eicosapentaenoic acid. Fish Shellfish Immunol. 2013, 34, 770–777. [Google Scholar] [CrossRef]
- Garreta-Lara, E.; Checa, A.; Fuchs, D.; Tauler, R.; Lacorte, S.; Wheelock, C.E.; Barata, C. Effect of psychiatric drugs on Daphnia magna oxylipin profiles. Sci. Total Environ. 2018, 644, 1101–1109. [Google Scholar] [CrossRef]
- Dey, A.; Williams, R.S.; Pollock, D.M.; Stepp, D.W.; Newman, J.W.; Hammock, B.D.; Imig, J.D. Altered kidney CYP2C and cyclooxygenase-2 levels are associated with obesity-related albuminuria. Obes. Res. 2004, 12, 1278–1289. [Google Scholar] [CrossRef]
- Williams, J.M.; Murphy, S.; Burke, M.; Roman, R.J. 20-Hydroxyeicosatetraeonic acid: A new target for the treatment of hypertension. J. Cardiovasc. Pharmacol. 2010, 56, 336–344. [Google Scholar] [CrossRef]
- Anthonymuthu, T.S.; Kenny, E.M.; Amoscato, A.A.; Lewis, J.; Kochanek, P.M.; Kagan, V.E.; Bayır, H. Global assessment of oxidized free fatty acids in brain reveals an enzymatic predominance to oxidative signaling after trauma. BBA-Mol. Basis. Dis. 2017, 1863, 2601–2613. [Google Scholar] [CrossRef]
- Thompson, D.A.; Hammock, B.D. Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. J. Biosci. 2007, 32, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prost. Leuk. Esent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D. Essential fatty acids and the immune response. FASEB J. 1989, 3, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: A review. J. Adv. Res. 2018, 11, 43–55. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Labelling reference intake values for n-3 and n-6 polyunsaturated fatty acids. EFSA J. 2009, 1176, 1–11. [Google Scholar]
- Bibus, D.; Lands, B. Balancing proportions of competing omega-3 and omega-6 highly unsaturated fatty acids (HUFA) in tissue lipids. PLEFA 2015, 99, 19–23. [Google Scholar] [CrossRef]
- Muturi, K.N.; Scaife, J.R.; Lomax, M.A.; Jackson, F.; Huntley, J.; Coop, R.L. The effect of dietary polyunsaturated fatty acids (PUFA) on infection with the nematodes Ostertagia ostertagi and Cooperia oncophora in calves. Vet. Parasitol. 2005, 129, 273–283. [Google Scholar] [CrossRef] [PubMed]
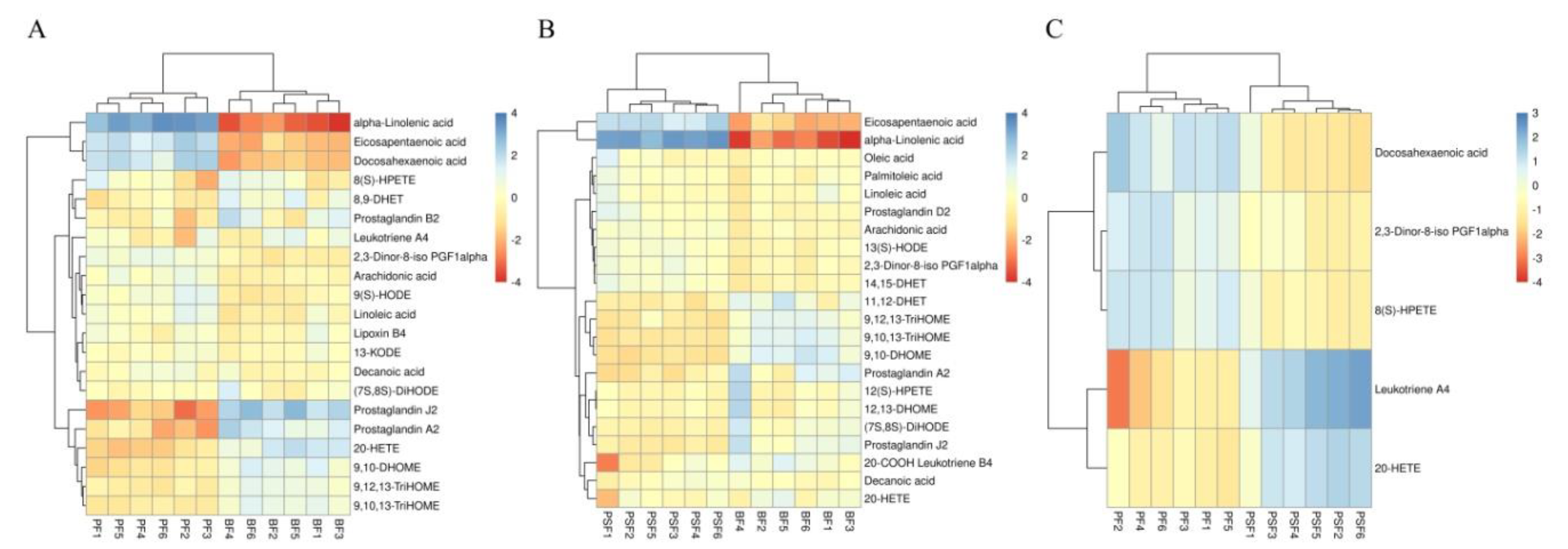
| Chemical Denomination | Formula | p-Value | VIP (Variable Importance of Projection) | m.z (Mass-to-Charge Ratio) | Retention Time (min) | Trend | Metabolic Pathway |
|---|---|---|---|---|---|---|---|
| 2,3-Dinor-8-iso PGF1alpha | C18H32O5 | 2.18 × 10−2 | 1.06 | 327.22 | 7.57 | up | Arachidonic acid metabolism |
| Leukotriene A4 | C20H30O3 | 1.48 × 10−2 | 1.86 | 317.21 | 8.04 | up | Arachidonic acid metabolism |
| Prostaglandin A2 | C20H30O4 | 4.09 × 10−4 | 2.96 | 333.21 | 7.11 | up | Arachidonic acid metabolism |
| Prostaglandin B2 | C20H30O4 | 5.84 × 10−3 | 2.37 | 333.21 | 7.99 | up | Arachidonic acid metabolism |
| Prostaglandin J2 | C20H30O4 | 4.32 × 10−5 | 3.68 | 333.21 | 7.67 | up | Arachidonic acid metabolism |
| 20-HETE | C20H32O3 | 1.21 × 10−5 | 3.25 | 319.23 | 8.31 | up | Arachidonic acid metabolism |
| 8(S)-HPETE | C20H32O4 | 3.75 × 10−2 | 1.54 | 335.22 | 6.84 | up | Arachidonic acid metabolism |
| Lipoxin B4 | C20H32O5 | 8.00 × 10−3 | 1.56 | 351.22 | 7.25 | up | Arachidonic acid metabolism |
| 8,9-DHET | C20H34O4 | 1.61× 10−4 | 2.68 | 337.24 | 6.66 | up | Arachidonic acid metabolism |
| 13-KODE | C18H30O3 | 8.65 × 10−4 | 1.95 | 293.21 | 8.74 | up | Linoleic acid metabolism |
| 9(S)-HODE | C18H32O3 | 2.52 × 10−2 | 1.09 | 295.23 | 8.15 | up | Linoleic acid metabolism |
| (7S,8S)-DiHODE | C18H32O4 | 1.05 × 10−2 | 1.93 | 311.22 | 8.23 | up | Linoleic acid metabolism |
| 9,10-DHOME | C18H34O4 | 4.88× 10−5 | 2.83 | 313.24 | 8.11 | up | Linoleic acid metabolism |
| 9,10,13-TriHOME | C18H34O5 | 1.51 × 10−5 | 2.70 | 329.23 | 7.25 | up | Linoleic acid metabolism |
| 9,12,13-TriHOME | C18H34O5 | 3.01 × 10−5 | 2.59 | 329.23 | 6.91 | up | Linoleic acid metabolism |
| alpha-Linolenic acid | C18H30O2 | 2.38 × 10−5 | 3.08 | 277.22 | 8.44 | down | Biosynthesis of unsaturated fatty acids |
| Linoleic acid | C18H32O2 | 4.08 × 10−2 | 1.13 | 279.23 | 9.33 | up | Biosynthesis of unsaturated fatty acids |
| Eicosapentaenoic acid | C20H30O2 | 8.05 × 10−4 | 1.65 | 301.22 | 9.01 | down | Biosynthesis of unsaturated fatty acids |
| Arachidonic acid | C20H32O2 | 5.3 9× 10−3 | 1.41 | 303.96 | 10.1 | up | Biosynthesis of unsaturated fatty acids |
| Docosahexaenoic acid | C22H32O2 | 5.69 × 10−3 | 1.81 | 327.23 | 9.23 | down | Biosynthesis of unsaturated fatty acids |
| Decanoic acid | C10H20O2 | 4.92 × 10−4 | 1.82 | 171.14 | 7.63 | up | Fatty acid biosynthesis |
| Chemical Denomination | Formula | p-Value | VIP | m.z | Retention Time (min) | Trend | Metabolic Pathway |
|---|---|---|---|---|---|---|---|
| 2,3-Dinor-8-iso PGF1alpha | C18H32O5 | 6.70 × 10−3 | 1.15 | 327.22 | 7.57 | up | Arachidonic acid metabolism |
| Thromboxane | C20H32O5 | 1.91 × 10−2 | 1.12 | 387.20 | 9.16 | up | Arachidonic acid metabolism |
| 20-COOH-Leukotriene B4 | C20H30O6 | 6.11 × 10−3 | 1.93 | 401.18 | 7.48 | up | Arachidonic acid metabolism |
| 14,15-DHET | C20H34O4 | 9.04 × 10−3 | 1.12 | 337.24 | 9.78 | up | Arachidonic acid metabolism |
| 11,12-DHET | C20H34O4 | 3.98 × 10−4 | 2.25 | 337.24 | 6.66 | up | Arachidonic acid metabolism |
| 12(S)-HPETE | C20H32O4 | 2.26 × 10−2 | 1.79 | 335.22 | 8.47 | up | Arachidonic acid metabolism |
| Prostaglandin A2 | C20H30O4 | 5.25 × 10−3 | 2.16 | 333.21 | 7.99 | up | Arachidonic acid metabolism |
| Prostaglandin J2 | C20H30O4 | 2.92 × 10−3 | 2.24 | 333.20 | 8.23 | up | Arachidonic acid metabolism |
| 20-HETE | C20H32O3 | 4.70 × 10−3 | 1.36 | 319.23 | 8.31 | up | Arachidonic acid metabolism |
| 13(S)-HODE | C18H32O3 | 7.38 × 10−3 | 1.24 | 295.23 | 8.15 | up | Linoleic acid metabolism |
| (7S,8S)-DiHODE | C18H32O4 | 5.82 × 10−3 | 2.03 | 311.22 | 8.23 | up | Linoleic acid metabolism |
| 9,10-DHOME | C18H34O4 | 4.42 × 10−5 | 2.55 | 313.24 | 8.11 | up | Linoleic acid metabolism |
| 12,13-DHOME | C18H34O4 | 2.81 × 10−2 | 1.74 | 313.24 | 8.48 | up | Linoleic acid metabolism |
| 9,10,13-TriHOME | C18H34O5 | 1.77 × 10−5 | 2.42 | 329.23 | 7.25 | up | Linoleic acid metabolism |
| 9,12,13-TriHOME | C18H34O5 | 3.37 × 10−4 | 2.16 | 329.23 | 6.91 | up | Linoleic acid metabolism |
| Linoleic acid | C18H32O2 | 1.04 × 10−2 | 1.40 | 279.23 | 9.33 | up | Linoleic acid metabolism Biosynthesis of unsaturated fatty acids |
| alpha-Linolenic acid | C18H30O2 | 6.04 × 10−5 | 2.99 | 277.22 | 8.44 | down | Biosynthesis of unsaturated fatty acids |
| Oleic acid | C18H34O2 | 1.84 × 10−2 | 1.29 | 281.25 | 9.66 | up | Biosynthesis of unsaturated fatty acids |
| Stearic acid | C18H36O2 | 3.31 × 10−2 | 1.41 | 285.28 | 9.68 | up | Biosynthesis of unsaturated fatty acids |
| Eicosapentaenoic acid | C20H30O2 | 3.65 × 10−4 | 1.82 | 301.22 | 9.01 | down | Biosynthesis of unsaturated fatty acids |
| Arachidonic acid | C20H32O2 | 8.40 × 10−4 | 1.19 | 303.96 | 9.58 | up | Biosynthesis of unsaturated fatty acids |
| Palmitoleic acid | C16H30O2 | 5.47 × 10−3 | 1.36 | 253.22 | 9.17 | up | Fatty acid biosynthesis |
| Decanoic acid | C10H20O2 | 8.47 × 10−4 | 1.57 | 171.14 | 7.63 | up | Fatty acid biosynthesis |
| Chemical Denomination | p-Value | VIP | Trend | Metabolic Pathway |
|---|---|---|---|---|
| 2,3-Dinor-8-iso PGF1alpha | 3.72 × 10−2 | 1.45 | down | Arachidonic acid metabolism |
| Leukotriene A4 | 8.51 × 10−3 | 4.82 | up | Arachidonic acid metabolism |
| 20-HETE | 1.09 × 10−2 | 2.93 | up | Arachidonic acid metabolism |
| 8(S)-HPETE | 3.35 × 10−2 | 1.19 | down | Arachidonic acid metabolism |
| Docosahexaenoic acid | 3.32 × 10−2 | 2.47 | down | Biosynthesis of unsaturated fatty acids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Zhang, X.; Zhang, J.; Zhang, Q.; Tana. Comparison of Three Feeding Regimens on Blood Fatty Acids Metabolites of Wujumqin Sheep in Inner Mongolia. Animals 2021, 11, 1080. https://doi.org/10.3390/ani11041080
Jin Y, Zhang X, Zhang J, Zhang Q, Tana. Comparison of Three Feeding Regimens on Blood Fatty Acids Metabolites of Wujumqin Sheep in Inner Mongolia. Animals. 2021; 11(4):1080. https://doi.org/10.3390/ani11041080
Chicago/Turabian StyleJin, Yanmei, Xiaoqing Zhang, Jize Zhang, Qian Zhang, and Tana. 2021. "Comparison of Three Feeding Regimens on Blood Fatty Acids Metabolites of Wujumqin Sheep in Inner Mongolia" Animals 11, no. 4: 1080. https://doi.org/10.3390/ani11041080
APA StyleJin, Y., Zhang, X., Zhang, J., Zhang, Q., & Tana. (2021). Comparison of Three Feeding Regimens on Blood Fatty Acids Metabolites of Wujumqin Sheep in Inner Mongolia. Animals, 11(4), 1080. https://doi.org/10.3390/ani11041080






