Melatonin Restores the Developmental Competence of Heat Stressed Porcine Oocytes, and Alters the Expression of Genes Related to Oocyte Maturation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Oocytes Collection and In Vitro Maturation
2.3. Assessment of Oocyte Survival Rate and Maturation Rate
2.4. Immunofluorescence, α-Tubulin and F-Actin Staining
2.5. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.6. Statistical Analysis
3. Results
3.1. Effects of HS and Melatonin on Oocyte Survival Rate and Maturation Rate
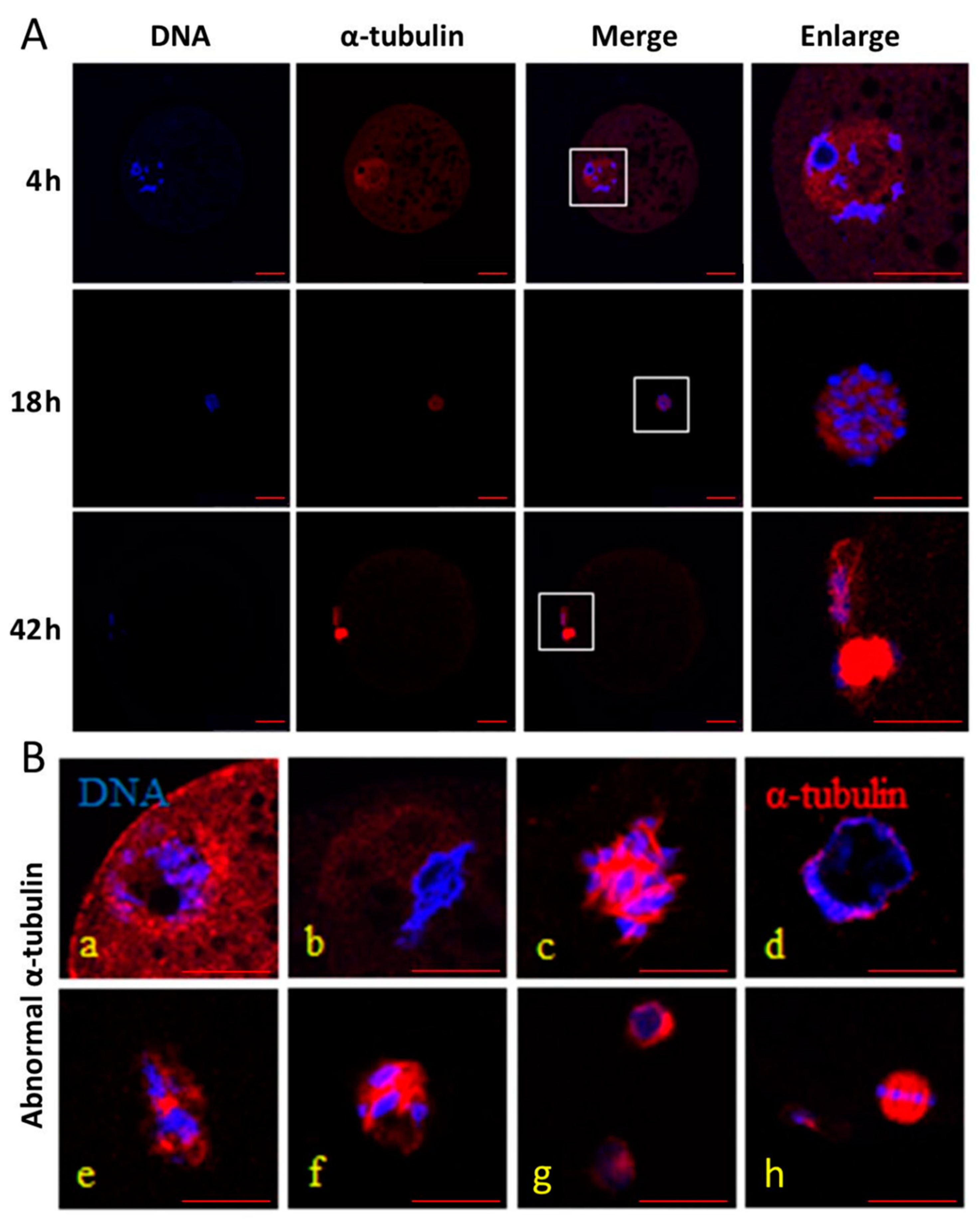
3.2. Effect of HS and Melatonin on the α-Tubulin Distribution in the Porcine Oocytes
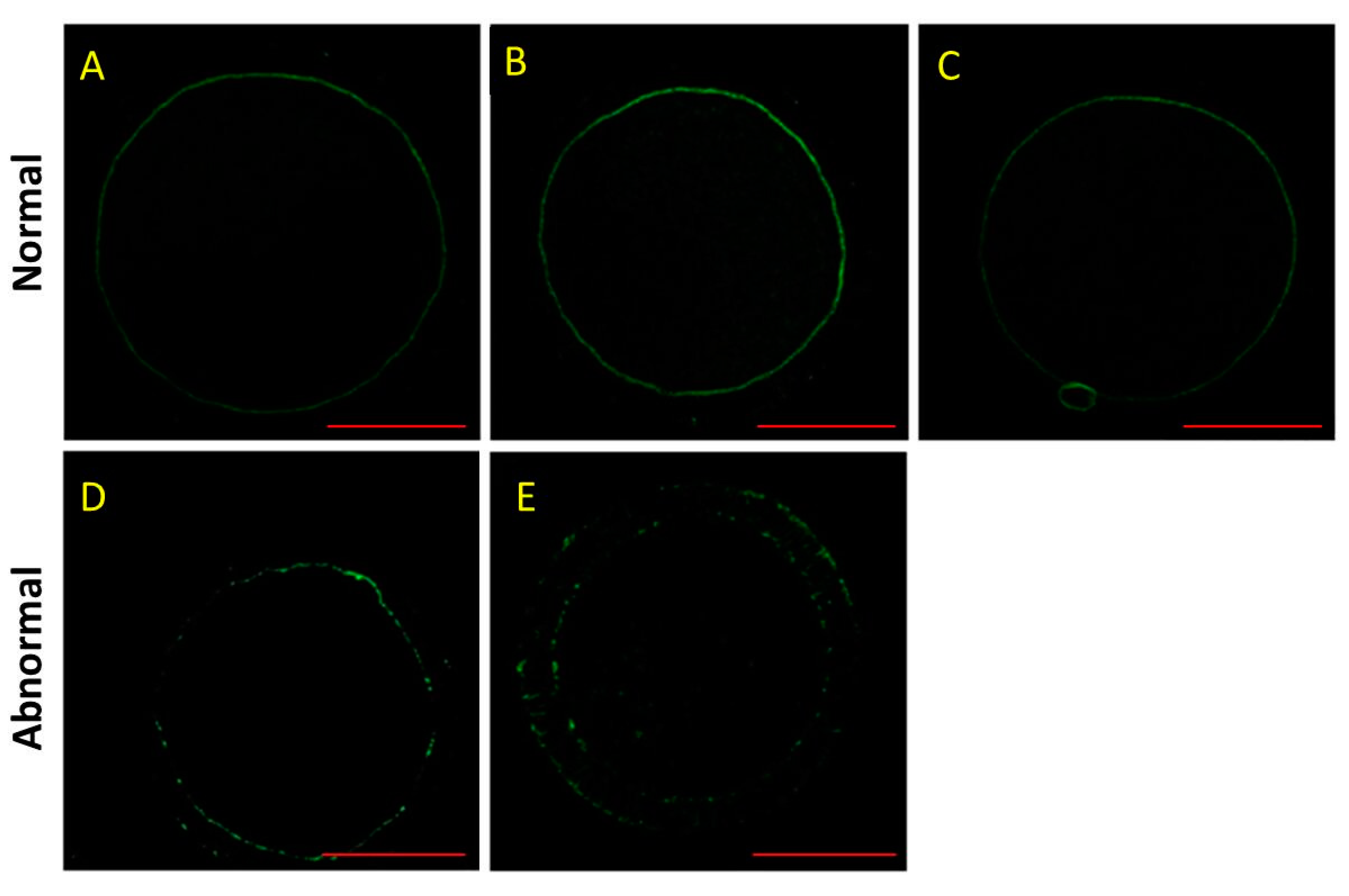
3.3. Effect of HS and Melatonin on F-Actin Distribution in the Porcine Oocytes
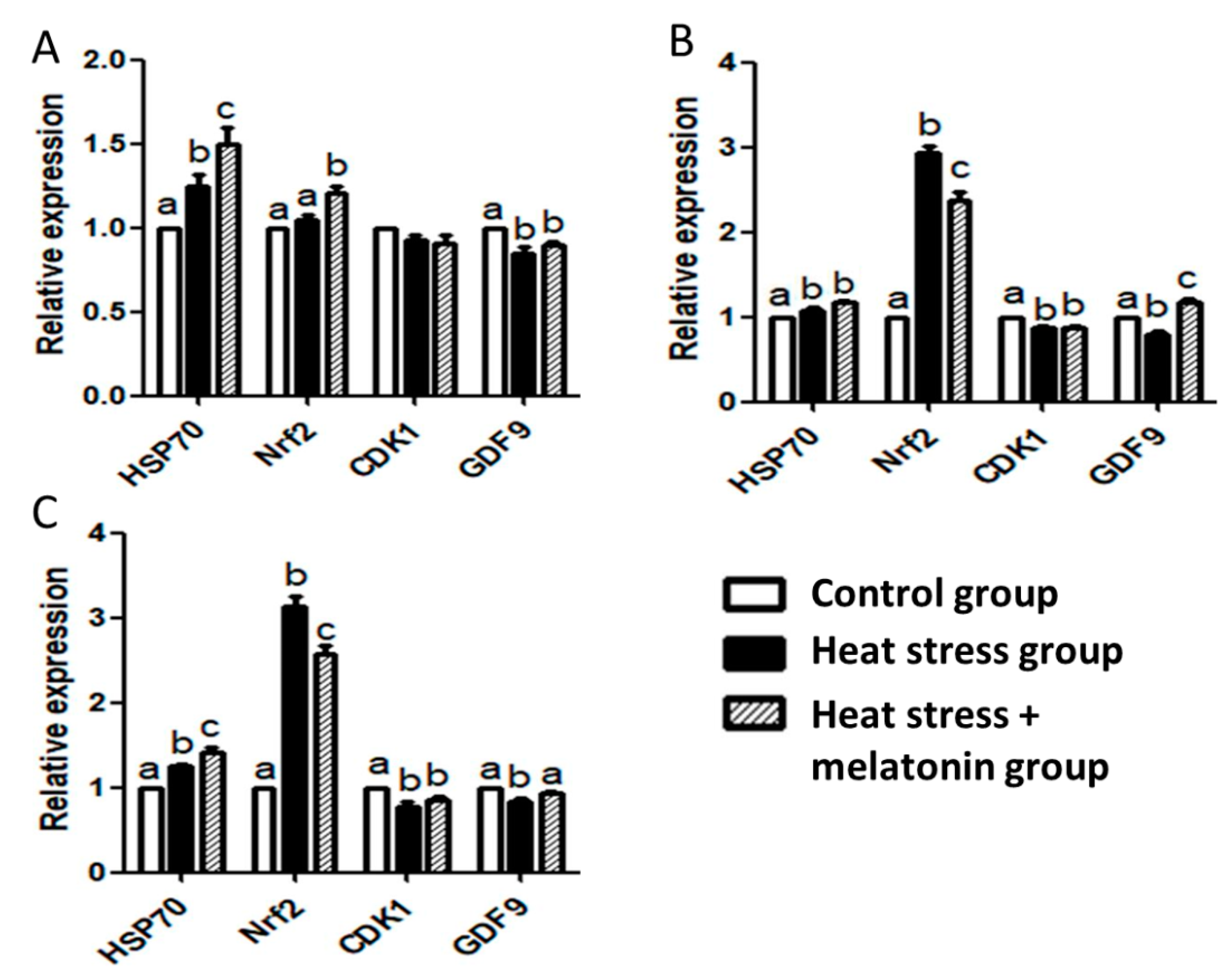
3.4. Effect of HS and Melatonin on Expression of HSP70, NRF2, CDK1 and GDF9 mRNA in the Porcine Oocytes during IVM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, Z.; Arav, A.; Bor, A.; Zeron, Y.; Braw-Tal, R.; Wolfenson, D. Improvement of quality of oocytes collected in the autumn by enhanced removal of impaired follicles from previously heat-stressed cows. Reproduction 2001, 122, 737–744. [Google Scholar] [CrossRef]
- Boma, M.H.; Bilkei, G. Seasonal infertility in Kenyan pig breeding units. Onderstepoort J. Vet. Res. 2006, 73, 229–232. [Google Scholar] [CrossRef]
- Naseer, Z.; Ahmad, E.; Epikmen, E.T.; Uçan, U.; Boyacioğlu, M.; İpek, E.; Akosy, M. Quercetin supplemented diet improves follicular development, oocyte quality, and reduces ovarian apoptosis in rabbits during summer heat stress. Theriogenology 2017, 96, 136–141. [Google Scholar] [CrossRef]
- Wang, J.Z.; Sui, H.S.; Miao, D.Q.; Liu, N.; Zhou, P.; Ge, L.; Tan, J.H. Effects of heat stress during in vitro maturation on cytoplasmic versus nuclear components of mouse oocytes. Reproduction 2009, 137, 181–189. [Google Scholar] [CrossRef]
- Nishio, K.; Yamazaki, M.; Taniguchi, M.; Besshi, K.; Morita, F.; Kunihara, T.; Tanihara, F.; Takemoto, T.; Otoi, T. Sensitivity of the meiotic stage to hyperthermia during in vitro maturation of porcine oocytes. Acta Vet. Hung. 2017, 65, 115–123. [Google Scholar] [CrossRef][Green Version]
- Lonergan, P.; Fair, T. Maturation of oocytes in vitro. Annu. Rev. Anim. Biosci. 2016, 4, 255–268. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.G.; Jeong, P.S.; Kim, M.J.; Park, S.H.; Song, B.S.; Sim, B.W.; Kim, S.U. Heat stress impairs oocyte maturation through ceramide-mediated apoptosis in pigs. Sci. Total Environ. 2021, 755, 144144. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z.; Hansen, P.J. Disruption of nuclear maturation and rearrangement of cytoskeletal elements in bovine oocytes exposed to heat shock during maturation. Reproduction 2005, 129, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Dang-Nguyen, T.Q.; Nguyen, H.T.; Somfai, T.; Wells, D.; Men, N.T.; Viet-Linh, N.; Noguchi, J.; Kaneko, H.; Kikuchi, K.; Nagai, T. Sucrose assists selection of high-quality oocytes in pigs. Anim. Sci. J. 2018, 89, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.Y.; Lai, L.; Park, K.W.; Kühholzer, B.; Prather, R.S.; Schatten, H. Dynamic events are differently mediated by microfilaments, microtubules, and mitogen-activated protein kinase during porcine oocyte maturation and fertilization in vitro. Biol. Reprod. 2001, 64, 879–889. [Google Scholar] [CrossRef]
- Yin, C.; Liu, J.; Chang, Z.; He, B.; Yang, Y.; Zhao, R. Heat exposure impairs porcine oocyte quality with suppressed actin expression in cumulus cells and disrupted F-actin formation in transzonal projections. J. Anim. Sci. Biotechnol. 2020, 11, 71. [Google Scholar] [CrossRef]
- Lánská, V.; Chmelíková, E.; Sedmíková, M.; Petr, J.; Rajmon, R.; Jeseta, M.; Rozinek, J. Expression of heat shock protein70 in pig oocytes: Heat shock response during oocyte growth. Anim. Reprod. Sci. 2006, 96, 154–164. [Google Scholar] [CrossRef]
- Ma, R.; Li, H.; Zhang, Y.; Lin, Y.; Qiu, X.; Xie, M.; Yao, B. The toxic effects and possible mechanisms of Brusatol on mouse oocytes. PLoS ONE 2017, 12, e0177844. [Google Scholar] [CrossRef]
- Oqani, R.K.; Lin, T.; Lee, J.E.; Kim, S.Y.; Kang, J.W.; Jin, D.I. Effects of CDK inhibitors on the maturation, transcription, and MPF activity of porcine oocytes. Reprod. Biol. 2017, 17, 320–326. [Google Scholar] [CrossRef]
- Belli, M.; Shimasaki, S. Molecular aspects and clinical relevance of GDF9 and BMP15 in ovarian function. Vitam. Horm. 2018, 107, 317–348. [Google Scholar] [CrossRef]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Setyawan, E.M.N.; Lee, B.C. Interaction of the EGFR signaling pathway with porcine cumulus oocyte complexes and oviduct cells in a coculture system. J. Cell. Physiol. 2019, 234, 4030–4043. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Q.; Cui, M.; Li, Q.; Mu, S.; Zhao, Z. Effect of Melatonin on the in vitro maturation of porcine oocytes, development of parthenogenetically activated embryos, and expression of genes related to the oocyte developmental capability. Animals 2020, 10, 209. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; He, C.; Zhu, K.; Xu, Z.; Ma, T.; Tao, J.; Liu, G. Melatonin protects porcine oocyte in vitro maturation from heat stress. J. Pineal Res. 2015, 59, 365–375. [Google Scholar] [CrossRef]
- Cavallari, F.C.; Leal, C.L.V.; Zvi, R.; Hansen, P.J. Effects of melatonin on production of reactive oxygen species and developmental competence of bovine oocytes exposed to heat shock and oxidative stress during in vitro maturation. Zygote 2019, 27, 180–186. [Google Scholar] [CrossRef]
- Shi, J.M.; Tian, X.Z.; Zhou, G.B.; Wang, L.; Gao, C.; Zhu, S.E.; Zeng, S.M.; Tian, J.H.; Liu, G.S. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J. Pineal Res. 2009, 47, 318–323. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, Z.; Yi, J.; He, C.; Wang, F.; Tian, X.; Yang, M.; Song, Y.; He, P.; et al. Resveratrol compares with melatonin in improving in vitro porcine oocyte maturation under heat stress. J. Anim. Sci. Biotechnol. 2016, 7, 33. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, D.; Tong, X.; Xu, T.; Zhang, D.; Wang, Y.; Liu, Y.; Li, Y.; Zhang, Y.; Pu, Y. Melatonin improves developmental competence of oocyte-granulosa cell complexes from porcine preantral follicles. Theriogenology 2019, 133, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hartig, S.M. Basic image analysis and manipulation in ImageJ. Curr. Protoc. Mol. Biol. 2013, 102, 14–15. [Google Scholar] [CrossRef]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef]
- Cruz, M.H.; Leal, C.L.; da Cruz, J.F.; Tan, D.X.; Reiter, R.J. Role of melatonin on production and preservation of gametes and embryos: A brief review. Anim. Reprod. Sci. 2014, 145, 150–160. [Google Scholar] [CrossRef]
- Wu, T.; Lane, S.I.R.; Morgan, S.L.; Jones, K.T. Spindle tubulin and MTOC asymmetries may explain meiotic drive in oocytes. Nat. Commun. 2018, 9, 2952. [Google Scholar] [CrossRef]
- Liu, H.; Yin, F.X.; Bai, C.L.; Shen, Q.Y.; Wei, Z.Y.; Li, X.X.; Liang, H.; Bou, S.; Li, G.P. TFIIB co-localizes and interacts with α-tubulin during oocyte meiosis in the mouse and depletion of TFIIB causes arrest of subsequent embryo development. PLoS ONE 2013, 8, e80039. [Google Scholar]
- Serra, E.; Succu, S.; Berlinguer, F.; Porcu, C.; Leoni, G.G.; Naitana, S.; Gadau, S.D. Tubulin posttranslational modifications in in vitro matured prepubertal and adult ovine oocytes. Theriogenology 2018, 114, 237–243. [Google Scholar] [CrossRef]
- Lee, J.; Miyano, T.; Moor, R.M. Spindle formation and dynamics of gamma-tubulin and nuclear mitotic apparatus protein distribution during meiosis in pig and mouse oocytes. Biol. Reprod. 2000, 62, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Wang, W.; Shen, W.; Sun, Q.Y.; Yin, S. Melatonin protects against Fenoxaprop-ethyl exposure-induced meiotic defects oocytes. Toxicology 2019, 425, 152241. [Google Scholar] [CrossRef]
- Ju, J.C.; Tseng, J.K. Nuclear and cytoskeletal alterations of in vitro matured porcine oocytes under hyperthermia. Mol. Reprod. Dev. 2004, 68, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, J.; Carlton, C.; McGinnis, L.K.; Kinsey, W.H. Sperm-oocyte contact induces outside-in signaling via PYK2 activation. Dev. Biol. 2017, 428, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Liu, J.; He, B.; Jia, L.; Gong, Y.; Guo, H.; Zhao, R. Heat stress induces distinct responses in porcine cumulus cells and oocytes associated with disrupted gap junction and trans-zonal projection colocalization. J. Cell. Physiol. 2019, 234, 4787–4798. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Lu, Y.; Zhang, M.; Miao, Y.; Zhou, C.; Cui, Z.; Xiong, B. Melatonin improves the fertilization ability of post-ovulatory aged mouse oocytes by stabilizing ovastacin and Juno to promote sperm binding and fusion. Hum. Reprod. 2017, 32, 598–606. [Google Scholar] [CrossRef]
- Neuer, A.; Spandorfer, S.D.; Giraldo, P.; Dieterle, S.; Rosenwaks, Z.; Witkin, S.S. The role of heat shock proteins in reproduction. Hum. Reprod. Update 2000, 6. [Google Scholar] [CrossRef]
- Khan, I.; Lee, K.L.; Xu, L.; Mesalam, A.; Chowdhury, M.M.; Joo, M.D.; Ihsan-Ul-Haq; Mirza, B.; Kong, I.K. Improvement of in vitro-produced bovine embryo treated with coagulansin-A under heat-stressed condition. Reproduction 2017, 153, 421–431. [Google Scholar] [CrossRef]
- Souza-Cácares, M.B.; Fialho, A.L.L.; Silva, W.A.L.; Cardoso, C.J.T.; Pöhland, R.; Martins, M.I.M.; Melo-Sterza, F.A. Oocyte quality and heat shock proteins in oocytes from bovine breeds adapted to the tropics under different conditions of environmental thermal stress. Theriogenology 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Manejwala, F.M.; Logan, C.Y.; Schultz, R.M. Regulation of hsp70 mRNA levels during oocyte maturation and zygotic gene activation in the mouse. Dev. Biol. 1991, 144, 301–308. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, S.; Sun, Y.; Jiang, X.; Hao, H.; Du, W.; Zhu, H. Protective effects of melatonin on the in vitro developmental competence of bovine oocytes. Anim. Sci. J. 2018, 89, 648–660. [Google Scholar] [CrossRef]
- Tseng, J.K.; Tang, P.C.; Ju, J.C. In vitro thermal stress induces apoptosis and reduces development of porcine parthenotes. Theriogenology 2006, 66, 1073–1082. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell. Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liang, W.; Sun, Q.; Qiu, X.; Lin, Y.; Ge, X.; Jueraitetibaike, K.; Xie, M.; Zhou, J.; Huang, X.; et al. Sirt1/Nrf2 pathway is involved in oocyte aging by regulating Cyclin B1. Aging 2018, 10, 2991–3004. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, Y.L.; Zhu, Q.H.; Zhou, Z.K.; Gu, W.B.; Liu, Z.P.; Wang, L.Z.; Shu, M.A. Effects of heat stress on the liver of the Chinese giant salamander Andrias davidianus: Histopathological changes and expression characterization of Nrf2-mediated antioxidant pathway genes. J. Therm. Biol. 2018, 76, 115–125. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, G.A.; Taweechaipaisankul, A.; Lee, S.H.; Qasim, M.; Ahn, C.; Lee, B.C. Melatonin enhances porcine embryo development via the Nrf2/ARE signaling pathway. J. Mol. Endocrinol. 2019, 63, 175–185. [Google Scholar] [CrossRef]
- Kim, E.H.; Ridlo, M.R.; Lee, B.C.; Kim, G.A. Melatonin-Nrf2 signaling activates peroxisomal activities in porcine cumulus cell-oocyte complexes. Antioxidants 2020, 9, 1080. [Google Scholar] [CrossRef]
- Li, J.; Ouyang, Y.C.; Zhang, C.H.; Qian, W.P.; Sun, Q.Y. The cyclin B2/CDK1 complex inhibits separase activity in mouse oocyte meiosis I. Development 2019, 146, dev182519. [Google Scholar] [CrossRef]
- Xu, G.; Stevens, S.M., Jr.; Kobeissy, F.; Brown, H.; McClung, S.; Gold, M.S.; Borchelt, D.R. Identification of proteins sensitive to thermal stress in human neuroblastoma and glioma cell lines. PLoS ONE 2012, 7, e49021. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Reiter, R.J. Melatonin inhibits the proliferation of human osteosarcoma cell line MG-63. Bone 2013, 55, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Sanfins, A.; Rodrigues, P.; Albertini, D.F. GDF-9 and BMP-15 direct the follicle symphony. J. Assist. Reprod. Genet. 2018, 35, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Riepsamen, A.H.; Chan, K.; Lien, S.; Sweeten, P.; Donoghoe, M.W.; Walker, G.; Fraison, E.H.J.; Stocker, W.A.; Walton, K.L.; Harrison, C.A.; et al. Serum Concentrations of Oocyte-Secreted Factors BMP15 and GDF9 During IVF and in Women with Reproductive Pathologies. Endocrinology 2019, 160, 2298–2313. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Kim, G.A.; Choi, Y.B.; Jo, Y.K.; Setyawan, E.M.N.; Lee, B.C. Effect of co-culture canine cumulus and oviduct cells with porcine oocytes during maturation and subsequent embryo development of parthenotes in vitro. Theriogenology 2018, 106, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Gendelman, M.; Roth, Z. In vivo vs. in vitro models for studying the effects of elevated temperature on the GV-stage oocyte, subsequent developmental competence and gene expression. Anim. Reprod. Sci. 2012, 134, 125–134. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; He, C.; Zhang, L.; Tan, D.; Reiter, R.J.; Xu, J.; Ji, P.; Liu, G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–247. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′–3′) | Product Size (Bp) | GenBank Accession No. |
|---|---|---|---|
| GAPDH | F: TCAAATGGGGTGATGCTGGT R: GCAGAAGGGGCAGAGATGAT | 124 | XM_021091114 |
| GDF-9 | F: CCTCTACAACACTGTCCGGC R: GTCCCCTGATGGAAGGGTTC | 91 | NM_001001909.1 |
| CDK1 | F: TAATAAGCTGGGATCTACCACATC R: TGGCTACCACTTGACCTGTA | 130 | NM_001159304 |
| HSP70 | F: TGAATCCGCAGAATACCGTG R: CTCCGCAGTCTCCTTCATC | 215 | NM_001123127.1 |
| NRF2 | F: AAGCCTTCAACCAAGACCA R: AGAATCACTGAAGCCAAGCA | 179 | MH101365.1 |
| Group | Number of Oocytes | Replicates | Survival Rate (%) | Maturation Rate (%) |
|---|---|---|---|---|
| Control | 276 | 3 | 96.86 b ± 2.14 | 72.4 b ± 2.13 |
| HS | 252 | 3 | 88.72 a ± 3.42 | 59.6 a ± 3.06 |
| HSMT | 282 | 3 | 90.23 a ± 2.85 | 68.3 b ± 1.95 |
| Group | 4 h | 18 h | 42 h |
|---|---|---|---|
| Control | 75.56 ± 4.84 | 76.67 a ± 3.85 | 77.78 a ± 4.45 |
| HS | 73.33 ± 1.93 | 41.11 b ± 2.94 | 43.33 b ± 3.85 |
| HSMT | 72.22 ± 3.68 | 62.22 c ± 2.94 | 61.11 c ± 4.01 |
| Group | 4 h | 18 h | 42 h |
|---|---|---|---|
| Control | 84.44 a ± 1.11 | 83.33 a ± 1.87 | 85.56 a ± 2.94 |
| HS | 53.33 b ± 1.92 | 55.56 b ± 1.11 | 58.89 b ± 4.01 |
| HSMT | 54.45 b ± 4.01 | 66.67 c ± 3.85 | 71.11 c ± 2.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhao, Z.; Cui, M.; Zhang, L.; Li, Q. Melatonin Restores the Developmental Competence of Heat Stressed Porcine Oocytes, and Alters the Expression of Genes Related to Oocyte Maturation. Animals 2021, 11, 1086. https://doi.org/10.3390/ani11041086
Yang L, Zhao Z, Cui M, Zhang L, Li Q. Melatonin Restores the Developmental Competence of Heat Stressed Porcine Oocytes, and Alters the Expression of Genes Related to Oocyte Maturation. Animals. 2021; 11(4):1086. https://doi.org/10.3390/ani11041086
Chicago/Turabian StyleYang, Ling, Zimo Zhao, Maosheng Cui, Leying Zhang, and Qianjun Li. 2021. "Melatonin Restores the Developmental Competence of Heat Stressed Porcine Oocytes, and Alters the Expression of Genes Related to Oocyte Maturation" Animals 11, no. 4: 1086. https://doi.org/10.3390/ani11041086
APA StyleYang, L., Zhao, Z., Cui, M., Zhang, L., & Li, Q. (2021). Melatonin Restores the Developmental Competence of Heat Stressed Porcine Oocytes, and Alters the Expression of Genes Related to Oocyte Maturation. Animals, 11(4), 1086. https://doi.org/10.3390/ani11041086






