Genetic Diversity and Population Genetic Structure of a Guzerá (Bos indicus) Meta-Population
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction and Genotyping
2.3. Polymorphic Information Content and Average Exclusion Probabilities
2.4. Global Hardy–Weinberg Tests
2.5. F-Statistics and Genetic Distances
2.6. Quantification of the Contribution to Diversity
3. Results and Discussion
3.1. Performance of the Microsatellite Panel
3.2. Global Hardy–Weinberg Equilibrium Tests
3.3. F-Statistics and Genetic Distances
3.4. Quantifying Contributions to Diversity
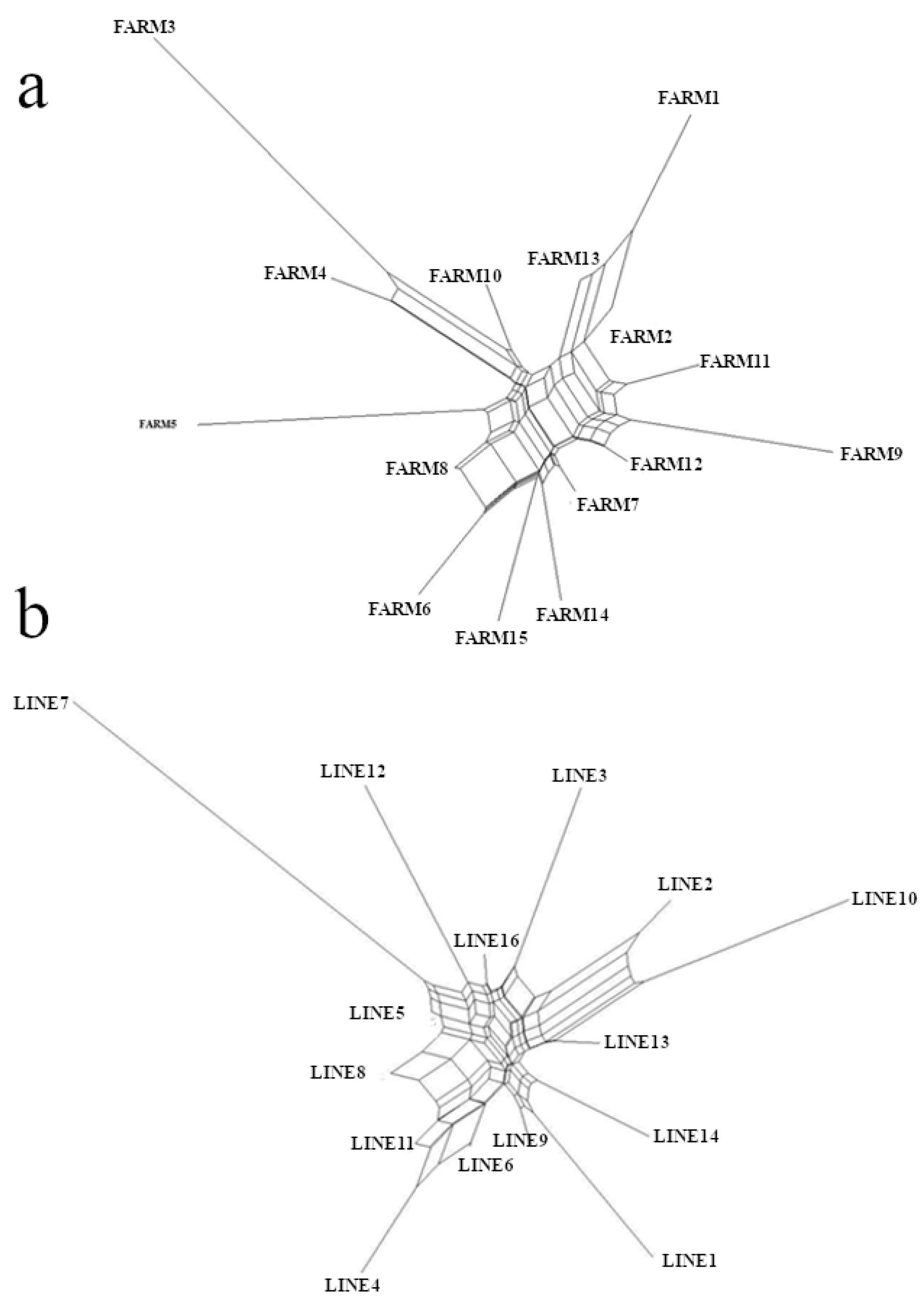
3.5. Numbers of Clusters and Lineages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- de Rochambeau, H.; Fournet-Hanocq, F.; Vu Tien Khang, J. Measuring and managing genetic variability in small populations. Ann. Zootech. 2000, 49, 77–93. [Google Scholar] [CrossRef]
- Taberlet, P.; Valentini, A.; Rezaei, H.R.; Naderi, S.; Pompanon, F.; Negrini, R.; Ajmone-Marsan, P. Are cattle, sheep, and goats endangered species? Mol. Ecol. 2008, 17, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.N.; Hoffmann, A.A.; Pertoldi, C.; Stronen, A.V. What can livestock breeders learn from conservation genetics and vice versa? Front. Genet. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boichard, D.; Ducrocq, V.; Fritz, S. Sustainable dairy cattle selection in the genomic era. J. Anim. Breed. Genet. 2015, 132, 135–143. [Google Scholar] [CrossRef]
- Toro, M.A.; Fernández, J.; Caballero, A. Molecular characterization of breeds and its use in conservation. Livest. Sci. 2009, 120, 174–195. [Google Scholar] [CrossRef]
- In Vivo Conservation of Animal Genetic Resources; FAO: Rome, Italy, 2013; ISBN 0306-5251.
- Peixoto, M.G.C.D.; Poggian, C.F.; Verneque, R.S.; Egito, A.A.; Carvalho, M.R.S.; Penna, V.M.; Bergmann, J.A.G.; Viccini, L.F.; Machado, M.A. Genetic basis and inbreeding in the Brazilian Guzerat (Bos indicus) subpopulation selected for milk production. Livest. Sci. 2010, 131, 168–174. [Google Scholar] [CrossRef]
- Santana, M.L.; Pereira, R.J.; Bignardi, A.B.; El Faro, L.; Pires, M.F.Á.; Andrade, R.G.; Perez, B.C.; Bruneli, F.A.T.; Peixoto, M.G.C.D. Dual-purpose Guzerá cattle exhibit high dairy performance under heat stress. J. Anim. Breed. Genet. 2020, 137, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Faria, F.J.C.; Filho, A.E.V.; Madalena, F.E.; Josahkian, L.A. Pedigree analysis in the Brazilian Zebu breeds. J. Anim. Breed. Genet. 2009, 126, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, M.G.C.D.; Verneque, R.S.; Teodoro, R.L.; Penna, V.M.; Martinez, M.L. Genetic trend for milk yield in Guzerat herds participating in progeny testing and MOET nucleus schemes. Genet. Mol. Res. 2006, 5, 454–465. [Google Scholar] [PubMed]
- Lenstra, J.A. Marker-assisted conservation of European cattle breeds: An evaluation. Anim. Genet. 2006, 37, 475–481. [Google Scholar]
- Shivaji, S.; Kholkute, S.D.; Verma, S.K.; Gaur, A.; Umapathy, G.; Singh, A.; Sontakke, S.; Shailaja, K.; Reddy, A.; Monika, S.; et al. Conservation of wild animals by assisted reproduction and molecular marker technology. Indian J. Exp. Biol. 2003, 41, 710–723. [Google Scholar] [PubMed]
- Allendorf, F.W.; Hohenlohe, P.A.; Luikart, G. Genomics and the future of conservation genetics. Nat. Rev. Genet. 2010, 11, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, P.G.; Martinez, A.; Cortes, O. Genomic tools for effective conservation of livestock breed diversity. Diversity 2020, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Egito, A.A.; Paiva, S.R.; Albuquerque, M.D.S.M.; Mariante, A.S.; Almeida, L.D.; Castro, S.R.; Grattapaglia, D. Microsatellite based genetic diversity and relationships among ten Creole and commercial cattle breeds raised in Brazil. BMC Genet. 2007, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadi, H.; Tibbo, M.; Takahashi, Y.; Nomura, K.; Hanada, H.; Amano, T. Microsatellite analysis reveals high genetic diversity but low genetic structure in Ethiopian indigenous cattle populations. Anim. Genet. 2008, 39, 425–431. [Google Scholar] [CrossRef]
- Dalvit, C.; De Marchi, M.; Dal Zotto, R.; Zanetti, E.; Meuwissen, T.; Cassandro, M. Genetic characterization of the Burlina cattle breed using microsatellites markers. J. Anim. Breed. Genet. 2008, 125, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, L.F.; Lenstra, J.A.; Eding, H.; Toro, M.A.; Scherf, B.; Pilling, D.; Negrini, R.; Finlay, E.K.; Jianlin, H.; Groeneveld, E.; et al. Genetic diversity in farm animals—A review. Anim. Genet. 2010, 41, 6–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, S.D.; Schnabel, R.D.; Murdoch, B.M.; Matukumalli, L.K.; Aerts, J.; Coppieters, W.; Crews, D.; Neto, E.D.; Gill, C.A.; Gao, C.; et al. An assessment of population structure in eight breeds of cattle using a whole genome SNP panel. BMC Genet. 2008, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flury, C.; Tapio, M.; Sonstegard, T.; Drögemüller, C.; Leeb, T.; Simianer, H.; Hanotte, O.; Rieder, S. Effective population size of an indigenous Swiss cattle breed estimated from linkage disequilibrium. J. Anim. Breed. Genet. 2010, 127, 339–347. [Google Scholar] [CrossRef]
- Sanz, A.; Martin-Burriel, I.; Cons, C.; Reta, M.; Poblador, A.; Rodellar, C.; Zaragoza, P. Genetic diversity, structure and individual assignment of Casta Navarra cattle: A well-differentiated fighting bull population. J. Anim. Breed. Genet. 2014, 131, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.M.; do Carmo, A.S.; do Egito, A.A.; da Mariante, A.S.; do Albuquerque, M.S.M.; de Gouveia, J.J.S.; Malhado, C.H.M.; Verardo, L.L.; da Silva, M.V.G.B.; Carneiro, P.L.S. Genetic diversity, population structure, and correlations between locally adapted zebu and taurine breeds in Brazil using SNP markers. Trop. Anim. Health Prod. 2017, 49, 1677–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosse, I.C.; Assis, J.G.; Oliveira, F.S.; Leite, L.R.; Araujo, F.; Zerlotini, A.; Volpini, A.; Dominitini, A.J.; Lopes, B.C.; Arbex, W.A.; et al. Whole genome sequencing of Guzerá cattle reveals genetic variants in candidate genes for production, disease resistance, and heat tolerance. Mamm. Genome 2017, 28, 66–80. [Google Scholar] [CrossRef]
- de Souza Fonseca, P.A.; dos Santos, F.C.; Rosse, I.C.; Ventura, R.V.; Brunelli, F.Â.T.; Penna, V.M.; da Silva Verneque, R.; Machado, M.A.; da Silva, M.V.G.B.; Carvalho, M.R.S.; et al. Retelling the recent evolution of genetic diversity for Guzerá: Inferences from LD decay, runs of homozygosity and Ne over the generations. Livest. Sci. 2016, 193, 110–117. [Google Scholar] [CrossRef]
- Thomson, K. World Agriculture: Towards 2015/2030: An FAO Perspective; FAO: Rome, Italy, 2003. [Google Scholar]
- Machado, M.A.; Schuster, I.; Martinez, M.L.; Campos, A.L. Genetic Diversity of Four Cattle Breeds Using Microsatellite Markers. Rev. Bras. Zootec. 2003, 32, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Ruttan, V.W.; Alexandratos, N. World Agriculture: Towards 2010: An FAO Study; FAO: Rome, Italy, 1996. [Google Scholar]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F. GENEPOP’007: A complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Guo, S.W.; Thompson, E.A. Performing the Exact Test of Hardy-Weinberg Proportion for Multiple Alleles. Biometrics 1992, 48, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. Variability within and among natural populations. In Evolution and the Genetics of Populations; University of Chicago Press: Chicago, IL, USA, 1978. [Google Scholar]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 36, 1358–1370. [Google Scholar]
- Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Gutiérrez, J.P.; Royo, L.J.; Álvarez, I.; Goyache, F. MolKin v2.0: A computer program for genetic analysis of populations using molecular coancestry information. J. Hered. 2005, 96, 718–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Huson, D.H.; Bryant, D. Estimating Phylogenetic Trees and Networks Using SplitsTree4. 2014. Available online: www.splitstree.org (accessed on 23 February 2021).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, N.A. DISTRUCT: A program for the graphical display of population structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Caballero, A.; Toro, M.A. Analysis of genetic diversity for the management of conserved subdivided populations. Conserv. Genet. 2002, 3, 289–299. [Google Scholar] [CrossRef]
- Chen, B.; Cole, J.W.; Grond-Ginsbach, C. Departure from Hardy Weinberg Equilibrium and genotyping error. Front. Genet. 2017. [Google Scholar] [CrossRef] [PubMed]
- Garnier-Géré, P.; Chikhi, L. Population Subdivision, Hardy-Weinberg Equilibrium and the Wahlund Effect. In eLS; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Panetto, J.C.C.; Gutiérrez, J.P.; Ferraz, J.B.S.; Cunha, D.G.; Golden, B.L. Assessment of inbreeding depression in a Guzerat dairy herd: Effects of individual increase in inbreeding coefficients on production and reproduction. J. Dairy Sci. 2010, 93, 4902–4912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, J.V.; Martínez, A.M.; Acosta, A.; Álvarez, L.A.; Armstrong, E.; Camacho, E.; Cañõn, J.; Cortés, O.; Dunner, S.; Landi, V.; et al. Genetic characterization of Latin-American Creole cattle using microsatellite markers. Anim. Genet. 2012, 43, 2–10. [Google Scholar] [CrossRef]
- Kim, K.S.; Yeo, J.S.; Choi, C.B. Genetic diversity of north-east Asian cattle based on microsatellite data. Anim. Genet. 2002, 33, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Mukesh, M.; Sodhi, M.; Bhatia, S.; Mishra, B.P. Genetic diversity of Indian native cattle breeds as analysed with 20 microsatellite loci. J. Anim. Breed. Genet. 2004, 121, 416–424. [Google Scholar] [CrossRef]
- Sasazaki, S.; Honda, T.; Fukushima, M.; Oyama, K.; Mannen, H.; Mukai, F.; Tsuji, S. Genealogical relationship between pedigree and microsatellite information and analysis of genetic structure of a highly inbred Japanese black cattle strain. Asian Australas. J. Anim. Sci. 2004, 17, 1355–1359. [Google Scholar] [CrossRef]
- Chikhi, L.; Goossens, B.; Treanor, A.; Bruford, M.W. Population genetic structure of and inbreeding in an insular cattle breed, the Jersey, and its implications for genetic resource management. Heredity 2004, 92, 396–401. [Google Scholar] [CrossRef]
- Maretto, F.; Cassandro, M. Temporal variation in genetic diversity and population structure of Burlina cattle breed. Ital. J. Anim. Sci. 2014. [Google Scholar] [CrossRef] [Green Version]
- Rendo, F.; Iriondo, M.; Jugo, B.M.; Aguirre, A.; Mazón, L.I.; Vicario, A.; Gómez, M.; Estonba, A. Analysis of the genetic structure of endangered bovine breeds from the Western Pyrenees using DNA microsatellite markers. Biochem. Genet. 2004, 42, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.D.; Do, D.N.; Binh, N.T.; Nam, L.Q.; Van Ba, N.; Thuy, T.T.T.; Hoan, T.X.; Cuong, V.C.; Kadarmideen, H.N. Assessment of genetic diversity and population structure of Vietnamese indigenous cattle populations by microsatellites. Livest. Sci. 2013, 155, 17–22. [Google Scholar] [CrossRef]


| Number of individuals: | 723 |
| Number of loci: | 21 |
| Mean number of alleles per locus: | 13.67 |
| Mean proportion of individuals typed: | 0.9187 |
| Mean expected heterozygosity: | 0.7746 |
| Mean polymorphic information content (PIC): | 0.7473 |
| Combined non-exclusion probability (first parent): | 1.17 × 10−5 |
| Combined non-exclusion probability (second parent): | 5.64 × 10−9 |
| Combined non-exclusion probability (parent pair): | 8.41 × 10−15 |
| Combined non-exclusion probability (identity): | 2.16 × 10−24 |
| Combined non-exclusion probability (sib identity): | 1.63 × 10−9 |
| F-Statistics | |||||||
|---|---|---|---|---|---|---|---|
| Farms as Subpopulations | Lineages as Subpopulations | ||||||
| Limits | Fis | Fst | Fit | Fis | Fst | Fit | |
| Value (SE) | - | 0.029 (0.022) | 0.027 (0.002) | 0.055 (0.021) | 0.022 (0.022) | 0.034 (0.002) | 0.055 (0.021) |
| 95% confidence interval | Min: | −0.007 | 0.023 | 0.021 | −0.014 | 0.029 | 0.021 |
| Max: | 0.076 | 0.031 | 0.100 | 0.070 | 0.038 | 0.100 | |
| Lineage | LINE1 | LINE2 | LINE3 | LINE4 | LINE5 | LINE6 | LINE7 | LINE8 | LINE9 | LINE10 | LINE11 | LINE12 | LINE13 | LINE14 | LINE15 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FARMS | |||||||||||||||||
| FARM1 | 0.018 0.003 | 0.019 | 0.031 | 0.017 | 0.010 | 0.013 | 0.058 | 0.010 | 0.008 | 0.031 | 0.010 | 0.035 | 0.013 | 0.024 | 0.008 | LlNE1 | |
| FARM2 | 0.013 | 0.008 0.033 | 0.019 | 0.036 | 0.020 | 0.017 | 0.058 | 0.024 | 0.022 | 0.027 | 0.025 | 0.024 | 0.019 | 0.022 | 0.019 | LINE2 | |
| FARM3 | 0.040 | 0.025 | 0.061 0.020 | 0.035 | 0.011 | 0.019 | 0.060 | 0.014 | 0.018 | 0.039 | 0.020 | 0.030 | 0.017 | 0.028 | 0.009 | LINE3 | |
| FARM4 | 0.032 | 0.015 | 0.025 | 0.003 0.022 | 0.024 | 0.014 | 0.066 | 0.018 | 0.022 | 0.039 | 0.009 | 0.032 | 0.030 | 0.023 | 0.027 | LINE4 | |
| FARM5 | 0.032 | 0.016 | 0.042 | 0.023 | 0.027 0.031 | 0.013 | 0.025 | 0.013 | 0.013 | 0.027 | 0.012 | 0.013 | 0.014 | 0.011 | 0.013 | LINE5 | |
| FARM6 | 0.036 | 0.022 | 0.048 | 0.027 | 0.023 | 0.037 0.048 | 0.052 | 0.011 | 0.012 | 0.025 | 0.009 | 0.020 | 0.013 | 0.018 | 0.014 | LINE6 | |
| FARM7 | 0.025 | 0.013 | 0.031 | 0.017 | 0.014 | 0.013 | 0.038 0.006 | 0.030 | 0.041 | 0.087 | 0.044 | 0.062 | 0.047 | 0.063 | 0.029 | LINE7 | |
| FARM8 | 0.025 | 0.013 | 0.028 | 0.019 | 0.014 | 0.009 | 0.011 | −0.030 0.033 | 0.014 | 0.034 | 0.007 | 0.016 | 0.018 | 0.013 | 0.014 | LINE8 | |
| FARM9 | 0.017 | 0.011 | 0.031 | 0.017 | 0.035 | 0.016 | 0.008 | 0.010 | −0.019 0.018 | 0.040 | 0.011 | 0.021 | 0.012 | 0.013 | 0.009 | LINE9 | |
| FARM10 | 0.029 | 0.014 | 0.030 | 0.017 | 0.019 | 0.021 | 0.008 | 0.014 | 0.012 | 0.020 0.046 | 0.034 | 0.042 | 0.029 | 0.036 | 0.034 | LINE10 | |
| FARM11 | 0.017 | 0.009 | 0.037 | 0.016 | 0.029 | 0.019 | 0.008 | 0.013 | 0.016 | 0.013 | 0.028 −0.068 | 0.019 | 0.019 | 0.014 | 0.016 | LINE11 | |
| FARM12 | 0.024 | 0.014 | 0.040 | 0.020 | 0.023 | 0.014 | 0.008 | 0.011 | 0.009 | 0.011 | 0.009 | 0.031 0.024 | 0.020 | 0.039 | 0.014 | LINE12 | |
| FARM13 | 0.010 | 0.006 | 0.024 | 0.016 | 0.010 | 0.012 | 0.011 | 0.009 | 0.005 | 0.013 | 0.006 | 0.009 | 0.032 0.017 | 0.016 | 0.011 | LINE13 | |
| FARM14 | 0.003 | 0.014 | 0.039 | 0.018 | 0.026 | 0.014 | 0.006 | 0.009 | 0.023 | 0.014 | 0.018 | 0.015 | 0.008 | −0.109 0.077 | 0.011 | LINE14 | |
| FARM15 | 0.0029 | 0.018 | 0.043 | 0.024 | 0.035 | 0.016 | 0.010 | 0.013 | 0.021 | 0.017 | 0.020 | 0.015 | 0.009 | 0.017 | 0.005 −0.043 | LINE15 | |
| FARM1 | FARM2 | FARM3 | FARM4 | FARM5 | FARM6 | FARM7 | FARM8 | FARM9 | FARM10 | FARM11 | FARM12 | FARM13 | FARM14 | FARM15 | |||
| Average Fst | 0.021 | 0.025 | 0.025 | 0.028 | 0.016 | 0.018 | 0.052 | 0.017 | 0.018 | 0.037 | 0.018 | 0.028 | 0.020 | 0.024 | 0.016 | Lineage | |
| 0.024 | 0.015 | 0.035 | 0.020 | 0.024 | 0.021 | 0.013 | 0.014 | 0.017 | 0.017 | 0.017 | 0.016 | 0.011 | 0.016 | 0.021 | Farm | ||
| Average D’s | 0.20 | 0.19 | 0.21 | 0.20 | 0.12 | 0.13 | 0.33 | 0.14 | 0.14 | 0.25 | 0.14 | 0.22 | 0.14 | 0.17 | 0.13 | Lineage | |
| 0.171 | 0.108 | 0.241 | 0.151 | 0.190 | 0.144 | 0.103 | 0.108 | 0.171 | 0.118 | 0.126 | 0.110 | 0.112 | 0.134 | 0.142 | Farm | ||
| Breed | Number of Individuals | Number of Markers | Mean Expected Heterozygosity (He) | Mean Observed Heterozygosity (Ho) | F-Statistics | Reference |
|---|---|---|---|---|---|---|
| Ethiopian Cattle | 351 | 30 | 0.726 | 0.674 | Fst = 0.013 | [16] |
| Creole Cattle | 857 | 19 | 0.738 | 0.718 | Fis = 0.028 | [45] |
| Korean Cattle Chinese Cattle Japanese Black Cattle European Holstein | 200 | 13 | 0.728 0.744 0.471 0.693 | 0.721 0.745 0.515 0.753 | Fst = 0.109 | [46] |
| Sahiwal Hariana Deoni | 136 | 20 | 0.61 0.66 0.70 | 0.42 0.53 0.59 | Fst = 0.067 | [47] |
| Japanese Black Cattle | 252 | 20 | 0.618 | 0.623 | Fst = 0.151 | [48] |
| Jersey | 223 | 12 | 0.643 | 0.6355 | Fst = 0.013 † Fst = 0.035 †† | [49] |
| Burlina Cattle | 279 | 24 | 0.69 | 0.63 | Fst = 0.036 | [50] |
| Pirenaica Betizu Terreña Monchina | 302 | 11 | 0.688 0.715 0.747 0.762 | 0.682 0.651 0.737 0.757 | Fst = 0.041 | [51] |
| Caracu; Criolo Lageano; Curraleiro; Mocho Nacional and Pantaneiro; Holstein and Jersey | 623 | 22 | 0.793 | 0.695 | Fst = 0.061 | [15] |
| Nellore; Gyr and Guzerá | 292 | 22 | 0.748 | 0. 645 | Fst = 0.040 | [15] |
| Vietnamese indigenous cattle | 410 | 27 | 0.760 | 0.680 | Fst = 0.04 | [52] |
| Lineage | GD | Internal Diversity | Mean Distance | Loss/Gain |
|---|---|---|---|---|
| LINE1 | 0.7749 | 0.0046 | −0.0358 | −0.0312 |
| LINE2 | 0.7731 | −0.1360 | −0.1267 | −0.2627 |
| LINE3 | 0.7750 | 0.0484 | −0.0671 | −0.0186 |
| LINE4 | 0.7759 | 0.2753 | −0.1740 | 0.1013 |
| LINE5 | 0.7764 | −0.0883 | 0.2467 | 0.1585 |
| LINE6 | 0.7745 | −0.2157 | 0.1370 | −0.0787 |
| LINE7 | 0.7759 | 0.5530* | −0.4474* | 0.1056 |
| LINE8 | 0.7751 | −0.1929 | 0.1903 | −0.0026 |
| LINE9 | 0.7747 | −0.1712 | 0.1201 | −0.0511 |
| LINE10 | 0.7739 | 0.2400 | −0.3954 | −0.1554 |
| LINE11 | 0.7746 | −0.2397 | 0.1690 | −0.0707 |
| LINE12 | 0.7746 | 0.0260 | −0.0925 | −0.0665 |
| LINE13 | 0.7759 | 0.0362 | 0.0609 | 0.0971 |
| LINE14 | 0.7759 | 0.1356 | −0.0359 | 0.0997 |
| LINE15 | 0.7739 | −0.3457 | 0.1868 | −0.1589 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, M.G.C.D.; Carvalho, M.R.S.; Egito, A.A.; Steinberg, R.S.; Bruneli, F.Â.T.; Machado, M.A.; Santos, F.C.; Rosse, I.C.; Fonseca, P.A.S. Genetic Diversity and Population Genetic Structure of a Guzerá (Bos indicus) Meta-Population. Animals 2021, 11, 1125. https://doi.org/10.3390/ani11041125
Peixoto MGCD, Carvalho MRS, Egito AA, Steinberg RS, Bruneli FÂT, Machado MA, Santos FC, Rosse IC, Fonseca PAS. Genetic Diversity and Population Genetic Structure of a Guzerá (Bos indicus) Meta-Population. Animals. 2021; 11(4):1125. https://doi.org/10.3390/ani11041125
Chicago/Turabian StylePeixoto, Maria Gabriela C. D., Maria Raquel S. Carvalho, Andrea A. Egito, Raphael S. Steinberg, Frank Ângelo T. Bruneli, Marco Antônio Machado, Fernanda C. Santos, Izinara C. Rosse, and Pablo Augusto S. Fonseca. 2021. "Genetic Diversity and Population Genetic Structure of a Guzerá (Bos indicus) Meta-Population" Animals 11, no. 4: 1125. https://doi.org/10.3390/ani11041125





