Sequential Modulation of the Equine Fecal Microbiota and Fibrolytic Capacity Following Two Consecutive Abrupt Dietary Changes and Bacterial Supplementation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Experimental Design, Diets and Supplementation
2.3. Sample Collection
2.4. DNA Extraction and Sequencing of the V3–V4 Region of the 16S rRNA Gene
2.5. Sequence Processing and Data Analysis
2.6. Clostridioides difficile Quantification
2.7. Protozoa Concentration Analysis
2.8. Analysis of Functional Bacterial Groups
2.9. Determination of Fermentation End-Products
2.10. Statistical Analysis
3. Results
3.1. Clinical Follow-Up of Horses
3.2. Analysis of Bacterial 16S rRNA Gene Sequencing
3.2.1. Sequencing Metrics
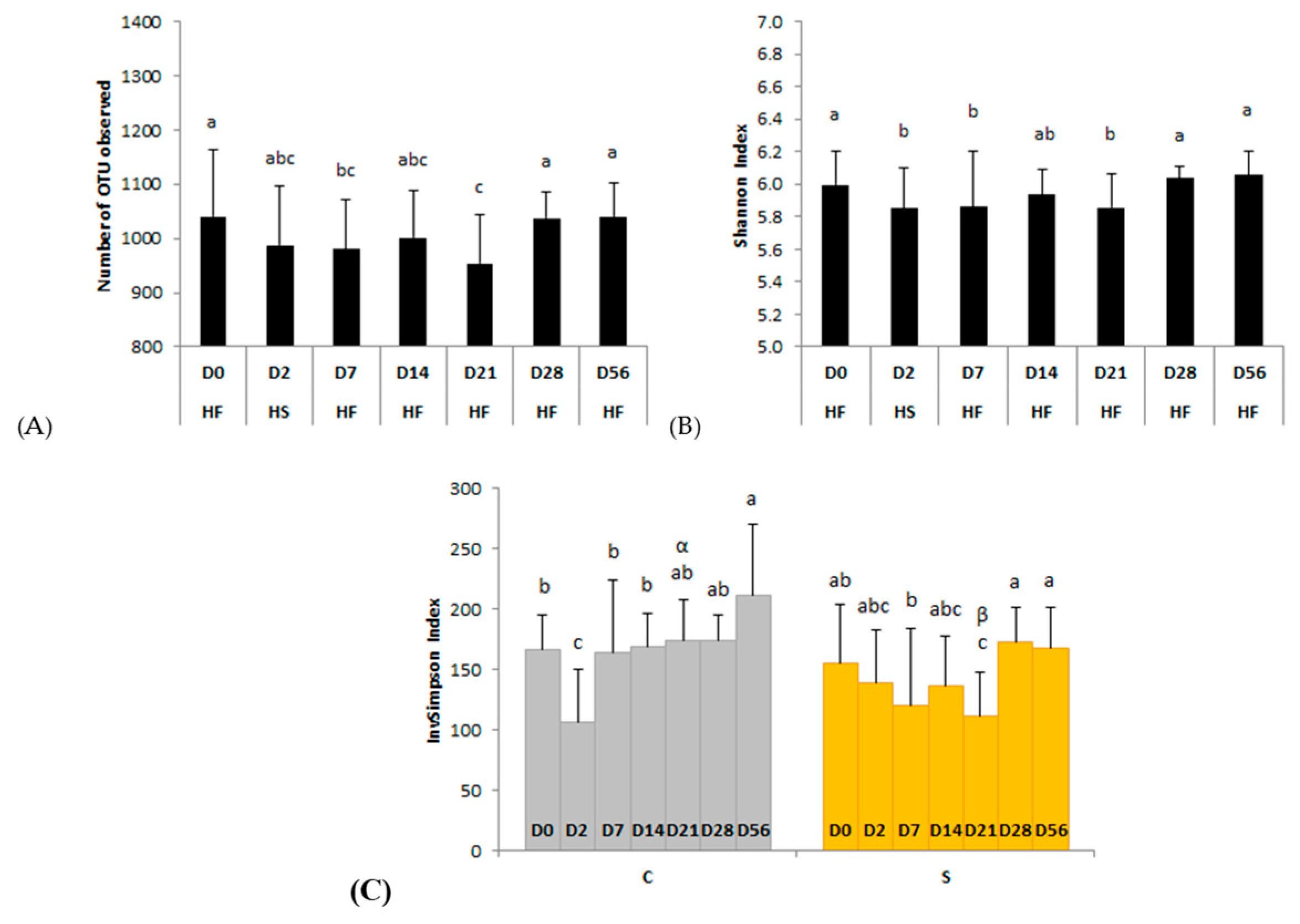
3.2.2. Richness and Diversity
3.2.3. Community Structure
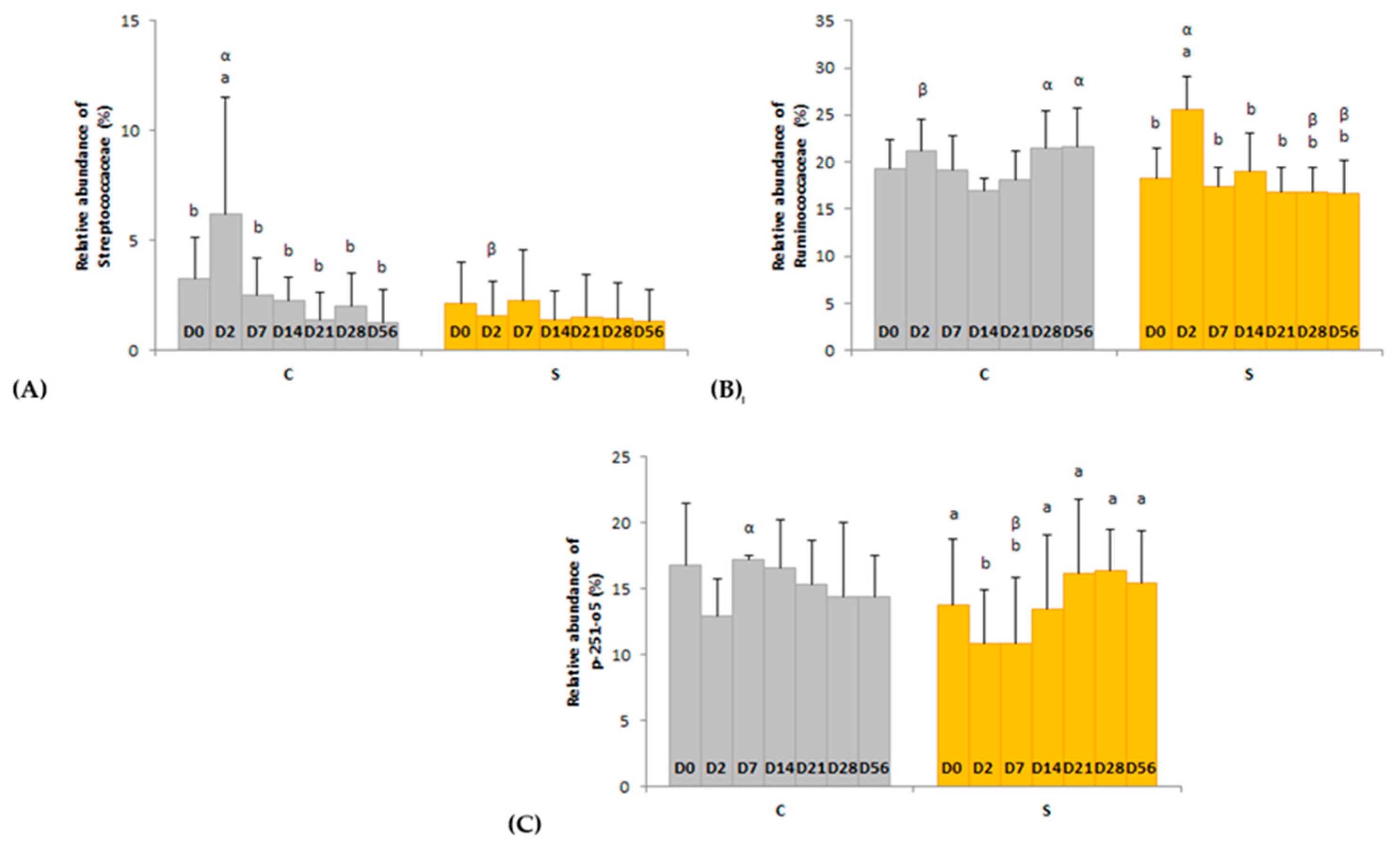
3.2.4. Bacterial Population Composition
3.3. Clostridioides difficile Quantification
3.4. Protozoa Concentration Analysis
3.5. Analysis of Bacterial Functional Groups
3.6. Microbial Activity
4. Discussion
4.1. A Sudden Change from HF to HS Alters the Diversity, Structure and Activity of the Ecosystem (D0–D2)
4.2. A Reverse Abrupt Change from HS to HF Induces Short and Long Term Modifications of the Fecal Ecosystem (D7–D56)
4.3. Effects of Dietary Supplementation with B. lactis, L. acidophilus and L. salivarius
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, N.A. Colic Prevalence, Risk Factors and Prevention. Aust. Equine Vet. 2009, 28, 42–49. [Google Scholar]
- Curtis, L.; Burford, J.H.; England, G.C.W.; Freeman, S.L.; Loor, J.J. Risk factors for acute abdominal pain (colic) in the adult horse: A scoping review of risk factors, and a systematic review of the effect of management-related changes. PLoS ONE 2019, 14, e0219307. [Google Scholar] [CrossRef] [PubMed]
- Julliand, V.; Grimm, P. The Impact of Diet on the Hindgut Microbiome. J. Equine Sci. 2017, 52, 23–28. [Google Scholar] [CrossRef]
- Garner, H.E.; Moore, J.N.; Johnson, J.H.; Clark, L.; Amend, J.F.; Tritschler, L.G.; Coffmann, J.R. Changes in the caecal flora associated with the onset of laminitis. Equine Vet. J. 1978, 10, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Goodson, J.; Tyznik, W.J.; Cline, J.H.; Dehority, B.A. Effect of an abrupt diet change from hay to concentrate on microbial numbers and physical environment in the cecum of the pony. Appl. Environ. Microbiol. 1988, 54, 1946–1950. [Google Scholar] [CrossRef] [Green Version]
- De Fombelle, A.; Julliand, V.; Drogoul, C.; Jacotot, E. Feeding and Microbial Disorders in Horses: Effects of an Abrupt incorporation of Two Levels of Barley in a Hay Diet on Microbial Profile and Activities. J. Equine Vet. Sci. 2001, 21, 439–445. [Google Scholar] [CrossRef]
- Respondek, F.; Goachet, A.G.; Julliand, V. Effects of dietary short-chain fructooligosaccharides on the intestinal microflora of horses subjected to a sudden change in diet. J. Anim. Sci. 2008, 86, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ransom-Jones, E.; Jones, D.L.; Mcdonald, J.E. The Fibrobacteres: An Important Phylum of Cellulose-Degrading Bacteria. Microb. Ecol. 2012, 63, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, C.M.; Coverdale, J.A.; Janecka, J.E.; Leatherwood, J.L.; Pinchak, W.E.; Wickersham, T.A.; McCann, J.C. In-fluence of short-term dietary starch inclusion on the equine cecal microbiome. J. Anim. Sci. 2017, 95, 5077–5090. [Google Scholar] [CrossRef] [Green Version]
- Faubladier, C.; Jacotot, E.; Berger, C.; Julliand, V. Effects of a live or heat-treated lactic-acid bacteria versus a placebo on faecal microbial communities and activities in horses. In Proceedings of the 5th European Workshop on Equine Nutrition, Cirencester, UK, 19–22 September 2010; pp. 288–292. [Google Scholar]
- Goachet, A.G.; Berger, C.; Julliand, V. Effect of a new alive lactic bacteria as a probiotic on organic matter and cell-walls digestibility in competing horses. In Proceedings of the 5th European Workshop on Equine Nutrition, Cirencester, UK, 19–22 September 2010; pp. 319–322. [Google Scholar]
- Goachet, A.G.; Berger, C.; Julliand, V. Effect of Vivaflor 03 on the colonic ecosystem of horses fed a high forage diet. In Proceedings of the 6th European Workshop on Equine Nutrition, Lisbon, Portugal, 20–22 September 2012; pp. 263–266. [Google Scholar]
- Swyers, K.L.; Burk, A.O.; Hartsock, T.G.; Ungerfeld, E.M.; Shelton, J.L. Effects of direct-fed microbial supplementation on digestibility and fermentation end-products in horses fed low- and high-starch concentrates. J. Anim. Sci. 2018, 86, 2596–2608. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Horses: Sixth Revised Edition; The National Academies Press: Washington, DC, USA, 2007.
- Julliand, V.; De Fombelle, A.; Varloud, M. Starch digestion in horses: The impact of feed processing. Livest. Sci. 2006, 100, 44–52. [Google Scholar] [CrossRef]
- Harris, P.A.; Ellis, A.D.; Fradinho, M.J.; Jansson, A.; Julliand, V.; Luthersson, N.; Santos, A.S.; Vervuert, I. Review: Feeding conserved forage to horses: Recent advances and recommendations. Animal 2016, 11, 958–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004, 36, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Grimm, P.; Combes, S.; Pascal, G.; Cauquil, L.; Julliand, V. Dietary composition and yeast/microalgae combination sup-plementation modulate the microbial ecosystem in the caecum, colon and faeces of horses. Br. J. Nutr. 2020, 123, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “all-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, 643–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavoine, S.; Dufour, A.B.; Chessel, D. From dissimilarities among species to dissimilarities among communities: A double principal coordinate analysis. J. Theor. Biol. 2004, 228, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, P.A.; Citron, D.M.; Tyrrell, K.L.; Finegold, S.M. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe 2016, 40, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Bandelj, P.; Logar, K.; Usenik, A.M.; Ocepek, M. An improved qPCR protocol for rapid detection and quantification of Clostridium difficile in cattle feces. FEMS Microbiol. Lett. 2013, 341, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Penders, J.; Vink, C.; Driessen, C.; London, N.; Thijs, C.; Stobberingh, E.E. Quantification of Bifidobacterium spp., Esch-erichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol. Lett. 2005, 243, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehority, B.A. Evaluation of subsampling and fixation procedures used for counting rumen protozoa. Appl. Environ. Microbiol. 1984, 48, 182–185. [Google Scholar] [CrossRef] [Green Version]
- D’Agosto, M.; Carneiro, M.E. Evaluation of lugol solution used for counting rumen ciliates. Rev. Bras. Zool. 1999, 16, 725–729. [Google Scholar] [CrossRef]
- Hungate, R.E.; Macy, J. The Roll-Tube Method for Cultivation of Strict Anaerobes. Bull. Ecol. Res. Commum. 1973, 17, 123–126. [Google Scholar]
- Bryant, M.P.; Burkey, L.A. Cultural Methods and Some Characteristics of Some of the More Numerous Groups of Bacteria in the Bovine Rumen. J. Dairy Sci. 1953, 36, 205–217. [Google Scholar] [CrossRef]
- Grimm, P.; Philippeau, C.; Julliand, V. Faecal parameters as biomarkers of the equine hindgut microbial ecosystem under dietary change. Animal 2017, 11, 1136–1145. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, G.; Bryant, M.P. The Cellulolytic Activity of Pure Strains of Bacteria From the Rumen Cattle. J. Genet. Microbiol. 1963, 32, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouany, J.P. Volatile Fatty Acid and Alcohol Determination in Digestive Contents, Silage Juices, Bacterial Cultures and Anaerobic Fermentor Contents. Sci. Aliment. 1982, 2, 131–144. [Google Scholar]
- Sauvant, D.; Chapoutot, P.; Archimede, H. La digestion des amidons par les ruminants et ses conséquences. INRA Prod. Anim. 1994, 7, 115–124. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R.E. Deciphering di-versity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, J.B.; Muck, R.E.; Weimer, P.J. Quantitative analysis of cellulose degradation and growth of cellulolytic bacteria in the rumen. FEMS Microbiol. Ecol. 2009, 67, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by lachnospi-raceae and ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Wade, W.G. In Prokaryotes; Springer: New York, NY, USA, 2006; The Genus Eubacterium and Related Genera; pp. 823–835. [Google Scholar]
- Rosero, J.A.; Killer, J.; Sechovcová, H.; Mrázek, J.; Benada, O.; Fliegerová, K.; Havlík, J.; Kopečný, J. Reclassification of Eubacterium rectale (Hauduroy et al. 1937) prévot 1938 in a new genus agathobacter gen. nov. as Agathobacter rectalis comb. nov., and description of Agathobacter ruminis sp. nov., isolated from the rumen contents of sheep and cows. Int. J. Syst. Evol. Microbiol. 2016, 66, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Leth, L.M.; Ejby, M.; Madland, E.; Kitaoku, Y.; Slotboom, D.J.; Guskov, A.; Aachmann, F.L.; Abou Hachem, M. Molecular insight into a new low-affinity xylan binding module from the xylanolytic gut symbiont Roseburia intestinalis. Biochem. Eng. J. 2020, 287, 2105–2117. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Hold, G.L.; Barcenilla, A.; Stewart, C.S.; Flint, H.J. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 2002, 52, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Tanno, H.; Fujii, T.; Hirano, K.; Maeno, S.; Sakamoto, M.; Ohkuma, M.; Tochio, T. Characterization of fructooligosaccha-ride metabolism and fructooligosaccharide-degrading enzymes in human commensal butyrate producers. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, G.; Mikaelyan, A.; Fukui, C.; Matsuura, Y.; Watanabe, H.; Fujishima, M.; Brune, A. Fiber-associated spirochetes are major agents of hemicellulose degradation in the hindgut of wood-feeding higher termites. Proc. Natl. Acad. Sci. USA 2018, 115, E11996–E12004. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, M.M.; Harris, H.M.B.; Jeffery, I.B.; Claesson, M.J.; Younge, B.; O’Toole, P.W.; Ross, R.P. The core faecal bacterial microbiome of Irish Thoroughbred racehorses. Lett. Appl. Microbiol. 2013, 57, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Dougal, K.; De La Fuente, G.; Harris, P.A.; Girdwood, S.E.; Pinloche, E.; Geor, R.J.; Nielsen, B.D.; Schott, H.C.; Elzinga, S.; Jamie Newbold, C. Characterisation of the faecal bacterial community in adult and elderly horses fed a high fibre, high oil or high starch diet using 454 pyrosequencing. PLoS ONE 2014, 9, e87424. [Google Scholar] [CrossRef] [Green Version]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Oliver, O.E.; Stämpfli, H. Acute Diarrhea in the Adult Horse: Case Example and Review. Vet. Clin. N. Am. Equine Pract. 2006, 22, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Julliand, V.; Grimm, P. Horse species symposium: The microbiome of the horse hindgut: History and current knowledge. J. Anim. Sci. 2016, 94, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Goachet, A.-G. Effect of Physical Activity on Dietary Energetic Components Digestion in Horses. Ph.D. Thesis, University of Burgundy, Dijon, France, 2009; p. 207. [Google Scholar]
- Al Jassim, R.A.M.; Scott, P.T.; Trebbin, A.L.; Trott, D.; Pollitt, C.C. The genetic diversity of lactic acid producing bacteria in the equine gastrointestinal tract. FEMS Microbiol. Lett. 2005, 248, 75–81. [Google Scholar] [CrossRef]
- Liu, C.; Finegold, S.M.; Song, Y.; Lawson, P.A. Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydroge. Int. J. Syst. Evol. Microbiol. 2008, 58, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Collinet, A.; Grimm, P.; Julliand, S.; Julliand, V. Multidimensional Approach for Investigating the Effects of an Antibi-otic-Probiotic Combination on the Equine Hindgut Ecosystem and Microbial Fibrolysis. Front. Microbiol. 2021, 12, 646294. [Google Scholar] [CrossRef] [PubMed]
- Shirazi-Beechey, S.P. Molecular insights into dietary induced colic in the horse. Equine Vet. J. 2008, 40, 414–421. [Google Scholar] [CrossRef]
- Cai, S.; Li, J.; Hu, F.; Zhang, K.; Luo, Y.; Janto, B.; Boissy, R.; Ehrlich, G.; Dong, X. Cellulosilyticum ruminicola, a newly described rumen bacterium that possesses redundant Fibrolytic-protein-encoding genes and degrades lignocellulose with multiple carbohydrateborne fibrolytic enzymes. Appl. Environ. Microbiol. 2010, 76, 3818–3824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hespell, R.B. The Prokaryotes; Springer: New York, NY, USA, 1992; The Genera Succinivibrio and Succinimonas; pp. 3979–3982. [Google Scholar]
- Mendoza, G.D.; Britton, R.A.; Stock, R.A. Influence of ruminal protozoa on site and extent of starch digestion and ruminal fermentation. J. Anim. Sci. 1993, 71, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Gotić, J.; Grden, D.; Babić, N.P.; Mrljak, V. The use of probiotics in horses with gastrointestinal disease. Am. J. Anim. Vet. Sci. 2017, 12, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Krzyaściak, W.; Pluskwa, K.K.; Jurczak, A.; Koaścielniak, D. The pathogenicity of the Streptococcus genus. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1361–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a foodborne pathogen: A review. Front. Microbiol. 2011, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaka, S.; Matsuda, A.; Amagai, Y.; Oida, K.; Jang, H.; Ueda, Y.; Takai, M.; Tanaka, A.; Matsuda, H. Oral Administration of Fermented Probiotics Improves the Condition of Feces in Adult Horses. J. Eq. 2014, 25, 65–72. [Google Scholar] [CrossRef] [Green Version]





| Diet | High-Fiber (HF) | High-Starch (HS) |
|---|---|---|
| Hay (% DM) | 86 | 60 |
| Concentrate (% DM) | 14 | - |
| Barley (% DM) | - | 40 |
| Digestive energy (kcal/100 kg BW) | 4660 | 4660 |
| Dry matter (DM) (g/100 g) | 93.50 | 90.60 |
| Hay ingestion level (% BW DM/day) | 1.85 | 1.04 |
| Pellet ingestion level (% BW DM/day) | 0.30 | - |
| Rolled barley ingestion level (% BW DM/day) | - | 0.70 |
| Starch ingestion level (% BW DM/day) | 0.14 | 0.39 |
| Crude protein (CP, % DM) | 8.51 | 9.21 |
| Neutral detergent fiber (NDF, % DM) | 57.08 | 42.56 |
| Acid detergent fiber (ADF, % DM) | 34.31 | 24.77 |
| Acid detergent lignin (ADL, % DM) | 5.72 | 4.22 |
| Starch (% DM) | 6.59 | 22.19 |
| Phylum > Class > Order > Family > Genus > Species | Day | LDA | p-Value |
|---|---|---|---|
| Group C | |||
| Firmicutes > Clostridia > Clostridiales > Lachnospiraceae > Blautia | D7 | 4.275 | 0.030 |
| Proteobacteria > Deltaproteobacteria > Desulfovibrionales > Desulfovibrionaceae > Mailhella | D14 | 3.991 | 0.025 |
| Firmicutes > Clostridia > Clostridiales > Lachnospiraceae | D21 | 5.018 | 0.033 |
| Firmicutes > Clostridia > Clostridiales > Lachnospiraceae > Lachnoclostridium | D21 | 4.391 | 0.039 |
| Firmicutes > Clostridia > Clostridiales > Lachnospiraceae > Lachnoclostridium > Clostridium asparagiforme | D21 | 4.391 | 0.001 |
| Firmicutes > Clostridia > Clostridiales > Ruminococcaceae > Eubacterium coprostanoligenes group | D28 | 4.042 | 0.028 |
| Firmicutes > Clostridia | D56 | 5.161 | 0.034 |
| Firmicutes > Clostridia > Clostridiales | D56 | 5.161 | 0.034 |
| Firmicutes > Clostridia > Clostridiales > Ruminococcaceae > Pygmaiobacter | D56 | 4.099 | 0.014 |
| Firmicutes > Clostridia > Clostridiales > Lachnospiraceae > Moryella | D56 | 4.310 | 0.044 |
| Group S | |||
| Proteobacteria | D2 | 5.038 | 0.006 |
| Proteobacteria > Gammaproteobacteria | D2 | 5.015 | 0.002 |
| Proteobacteria > Gammaproteobacteria > Aeromonadales | D2 | 5.025 | 0.001 |
| Proteobacteria > Gammaproteobacteria > Aeromonadales > Succinivibrionaceae | D2 | 5.025 | 0.001 |
| Proteobacteria > Gammaproteobacteria > Aeromonadales > Succinivibrionaceae > Succinivibrio | D2 | 5.025 | 0.001 |
| Firmicutes > Clostridia > Clostridiales > Ruminococcaceae > Ruminococcaceae UCG-011 | D7 | 3.924 | 0.044 |
| Firmicutes > Clostridia > Clostridiales > Lachnospiraceae > Lachnospiraceae FCS020 group | D7 | 4.277 | 0.027 |
| Firmicutes > Clostridia > Clostridiales > Lachnospiraceae > FD2005 | D14 | 3.881 | 0.025 |
| Firmicutes > Clostridia > Clostridiales > Lachnospiraceae > Lachnospiraceae AC2044 group | D21 | 3.853 | 0.036 |
| Bacteroidetes > Bacteroidia > Bacteroidales > Bacteroidetes BD2 2 | D28 | 4.169 | 0.026 |
| Bacteroidetes > Bacteroidia > Bacteroidales > Bacteroidaceae | D28 | 4.006 | 0.017 |
| Bacteroidetes > Bacteroidia > Bacteroidales > Bacteroidaceae > Bacteroides | D28 | 3.988 | 0.017 |
| Spirochaetes > Spirochaetia > Spirochaetales > Spirochaetaceae > Treponema 2 | D28 | 4.077 | 0.050 |
| Spirochaetes > Spirochaetia > Spirochaetales > Spirochaetaceae > Treponema 2 > Treponema pectinovorum | D28 | 3.537 | 0.016 |
| Days | D0 | D2 | D7 | D14 | D21 | D28 | D56 | Mean ± Standard Deviation | p-Values | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diets | HF | HS | HF | HF | HF | HF | HF | D | S | D × S | |
| Dry matter and pH in feces | |||||||||||
| Dry matter (%) | 21.04 d | 21.16 cd | 22.16 bc | 22.65 ab | 23.47 a | 21.22 cd | 22.33 b | 22.00 ± 1.80 | <0.0001 | 0.7909 | 0.2532 |
| pH | 6.80 ab | 6.63 cd | 6.77 abcd | 6.74 abc | 6.60 d | 6.92 a | 6.77 abc | 6.74 ± 0.27 | 0.0081 | 0.3168 | 0.7490 |
| Volatile Fatty Acid (VFA) concentration and proportion in feces | |||||||||||
| Total VFAs (mmol/L) | 30.77 | 33.56 | 33.28 | 34.92 | 34.57 | 29.74 | 31.65 | 32.64 ± 10.04 | 0.7345 | 0.4071 | 0.1641 |
| C2 (%) | 67.56 a | 65.15 b | 69.01 a | 67.80 a | 68.06 a | 68.43 a | 68.62 a | 67.80 ± 3.40 | 0.0023 | 0.9080 | 0.7573 |
| C3 (%) | 20.60 | 21.20 | 19.29 | 20.71 | 20.48 | 19.67 | 20.53 | 20.36 ± 3.06 | 0.9513 | 0.5686 | 0.4974 |
| iC4 (%) | 1.94 | 2.45 | 2.01 | 1.84 | 1.88 | 2.00 | 1.76 | 1.98 ± 0.68 | 0.3142 | 0.6917 | 0.493 |
| C4 (%) | 6.75 | 7.50 | 6.53 | 6.85 | 6.68 | 6.83 | 6.45 | 6.80 ± 0.95 | 0.306 | 0.6203 | 0.4372 |
| iC5 (%) | 0.93 | 1.17 | 0.96 | 0.81 | 0.92 | 0.93 | 0.79 | 2.13 ± 0.72 | 0.1885 | 0.6171 | 0.4108 |
| C5 (%) | 2.22 bc | 2.53 a | 2.21 ab | 1.98 c | 1.97 bc | 2.14 bc | 1.85 c | 0.93 ± 0.28 | 0.0023 | 0.7236 | 0.096 |
| (C2 + C4)/C3 | 3.71 | 3.61 | 4.02 | 3.69 | 3.74 | 3.94 | 3.77 | 3.78 ± 0.81 | 0.9305 | 0.6743 | 0.4984 |
| Lactic acid (LA) concentration and proportion in feces | |||||||||||
| Total LA (mmol/L) | 0.98 | 1.08 | 1.08 | 0.92 | 1.08 | 0.92 | 1.17 | 1.03 ± 0.26 | 0.1342 | 0.4908 | 0.5077 |
| D-LA (%) | 51.28 a | 47.41 abc | 43.63 c | 49.38 ab | 46.66 abc | 45.35 bc | 45.69 bc | 51.28 ± 47.41 | 0.0449 | 0.4242 | 0.2612 |
| L-LA (%) | 48.72 c | 52.59 abc | 56.37 a | 50.62 bc | 53.34 abc | 54.65 ab | 54.31 ab | 48.72 ± 52.59 | 0.0449 | 0.4242 | 0.2612 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collinet, A.; Grimm, P.; Julliand, S.; Julliand, V. Sequential Modulation of the Equine Fecal Microbiota and Fibrolytic Capacity Following Two Consecutive Abrupt Dietary Changes and Bacterial Supplementation. Animals 2021, 11, 1278. https://doi.org/10.3390/ani11051278
Collinet A, Grimm P, Julliand S, Julliand V. Sequential Modulation of the Equine Fecal Microbiota and Fibrolytic Capacity Following Two Consecutive Abrupt Dietary Changes and Bacterial Supplementation. Animals. 2021; 11(5):1278. https://doi.org/10.3390/ani11051278
Chicago/Turabian StyleCollinet, Axelle, Pauline Grimm, Samy Julliand, and Véronique Julliand. 2021. "Sequential Modulation of the Equine Fecal Microbiota and Fibrolytic Capacity Following Two Consecutive Abrupt Dietary Changes and Bacterial Supplementation" Animals 11, no. 5: 1278. https://doi.org/10.3390/ani11051278
APA StyleCollinet, A., Grimm, P., Julliand, S., & Julliand, V. (2021). Sequential Modulation of the Equine Fecal Microbiota and Fibrolytic Capacity Following Two Consecutive Abrupt Dietary Changes and Bacterial Supplementation. Animals, 11(5), 1278. https://doi.org/10.3390/ani11051278






