Weaning Induced Gut Dysfunction and Nutritional Interventions in Nursery Pigs: A Partial Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Weaning Stress Disturbs the Gut Microbiota and the Importance of a Healthy Gut
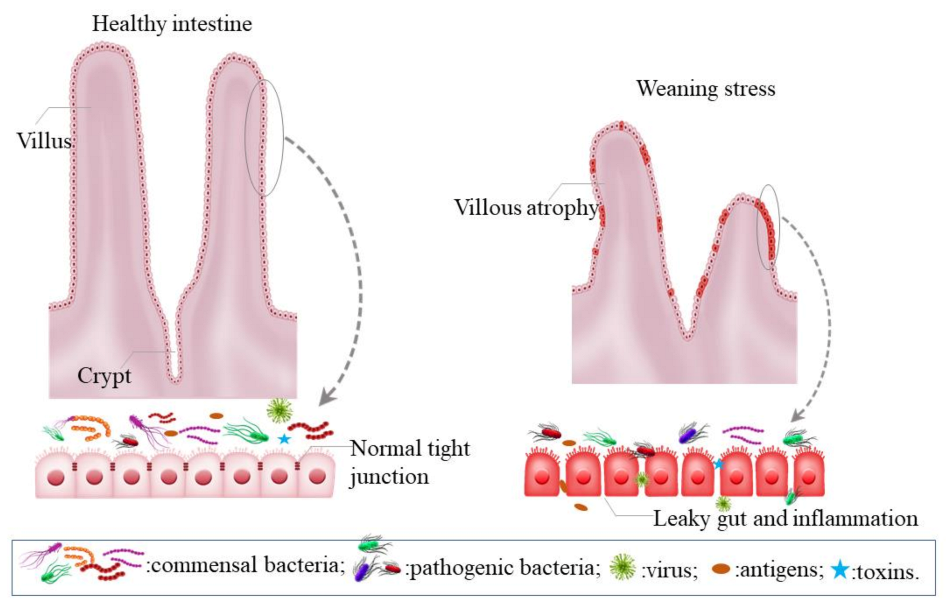
2.1. Intestinal Dysfunction Induced by Weaning Stress
2.2. The Importance of Maintaining a Healthy Gut
2.2.1. Nutrient Metabolism
2.2.2. Immunomodulatory and Anti-Inflammatory Effects
2.2.3. Impacts of Gut Microbiota on Intestinal Barrier Function and Gut Structure
3. The Effects of Nutritional Interventions on Swine Gut Health around Weaning
3.1. Zinc
3.1.1. Pharmacological Role of Zinc
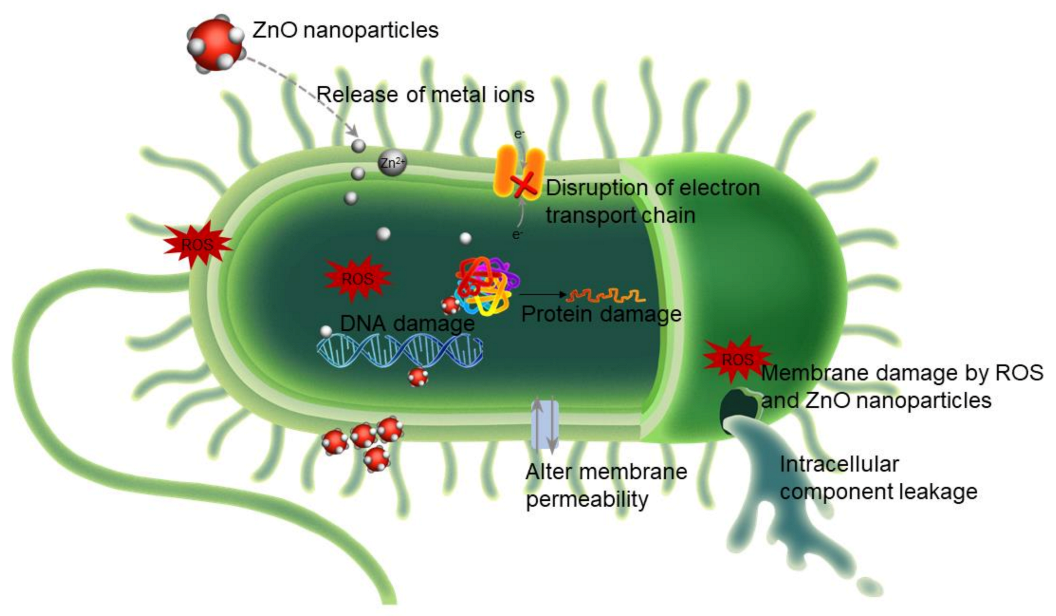
3.1.2. Antimicrobial Activity of Zinc
- (i)
- Reactive oxygen species (ROS) generation.
- (ii)
- Zinc ion (Zn2+) release.
- (iii)
- Changes in bacterial membrane permeability.
3.2. Peptides
3.2.1. Functional Properties of Bioactive Peptides
3.2.2. Peptide Absorption and Utilization in Animal Nutrition
3.3. Organic Acids
3.3.1. Lowering Gastrointestinal pH
3.3.2. Inhibition of Pathogenic Bacteria
3.3.3. Energy Source
3.3.4. Beneficial Effects of Organic Acids on Swine
3.4. Probiotics
3.4.1. Probiotics in Swine Nutrition
3.4.2. Probiotics as Modulators of Swine Gut Microbiota
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudry, G.; Péron, V.; Le Huërou-Luron, I.; Lallès, J.P.; Sève, B. Weaning Induces Both Transient and Long-Lasting Modifications of Absorptive, Secretory, and Barrier Properties of Piglet Intestine. J. Nutr. 2004, 134, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Spreeuwenberg, M.A.M.; Verdonk, J.M.A.J.; Gaskins, H.R.; Verstegen, M.W.A. Small Intestine Epithelial Barrier Function Is Compromised in Pigs with Low Feed Intake at Weaning. J. Nutr. 2001, 131, 1520–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modesto, M.; D’Aimmo, M.R.; Stefanini, I.; Trevisi, P.; De Filippi, S.; Casini, L.; Biavati, B. A novel strategy to select Bifidobacterium strains and prebiotics as natural growth promoters in newly weaned pigs. Livest. Sci. 2009, 122, 248–258. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Awati, A.A.; Williams, B.A.; Miller, B.G.; Jones, P.; Stokes, C.R.; De Vos, W.M. Post-natal development of the porcine microbiota composition and activities. Environ. Microbiol. 2006, 8, 1191–1199. [Google Scholar] [CrossRef]
- Berkes, J.; Viswanathan, V.K.; Savkovic, S.D.; Hecht, G. Intestinal epithelial responses to enteric pathogens: Effects on the tight junction barrier, ion transport, and inflammation. Gut 2003, 52, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Blecha, F.; Pollman, D.S.; Nichols, D.A. Weaning Pigs at an Early Age Decreases Cellular Immunity. J. Anim. Sci. 1983, 56, 396–400. [Google Scholar] [CrossRef]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I.P. Weaning is Associated with an Upregulation of Expression of Inflammatory Cytokines in the Intestine of Piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs1. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Zhao, J. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Cranwell, P.D. The development of acid and pepsin (EC 3. 4. 23. 1) secretory capacity in the pig; the effects of age and weaning: 1. Studies in anaesthetized pigs. Br. J. Nutr. 1985, 54, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Manners, M.J. The development of digestive function in the pig. Proc. Nutr. Soc. 1976, 35, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82–93. [Google Scholar] [CrossRef]
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed. Sci. Technol. 2019, 250, 32–40. [Google Scholar] [CrossRef]
- Wei, X.; Bottoms, K.; Stein, H.; Blavi, L.; Bradley, C.; Bergstrom, J.; Knapp, J.; Story, R.; Maxwell, C.; Tsai, T.; et al. Dietary Organic Acids Modulate Gut Microbiota and Improve Growth Performance of Nursery Pigs. Microorganisms 2021, 9, 110. [Google Scholar] [CrossRef]
- Wei, X.; Tsai, T.; Knapp, J.; Bottoms, K.; Deng, F.; Story, R.; Zhao, J. ZnO modulates swine gut microbiota and improves growth performance of nursery pigs when combined with peptide cocktail. Microorganisms 2020, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.; Maxwell, C.; Erf, G.; Davis, M.; Singh, S.; Johnson, Z. The influence of different management systems and age on intestinal morphology, immune cell numbers and mucin production from goblet cells in post-weaning pigs. Veter. Immunol. Immunopathol. 2006, 111, 187–198. [Google Scholar] [CrossRef]
- Bomba, L.; Minuti, A.; Moisa, S.J.; Trevisi, E.; Eufemi, E.; Lizier, M.; Ajmone-Marsan, P. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct. Integr. Gen. 2014, 14, 657–671. [Google Scholar] [CrossRef]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; He, J. Alginate oligosaccharide-induced intestinal morphology, barrier function and epithelium apoptosis modifications have beneficial effects on the growth performance of weaned pigs. J. Anim. Sci. Biotechnol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional Intervention for the Intestinal Development and Health of Weaned Pigs. Front. Veter. Sci. 2019, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Bocci, V. The Neglected Organ: Bacterial Flora Has a Crucial Immunostimulatory Role. Perspect. Biol. Med. 1992, 35, 251–260. [Google Scholar] [CrossRef]
- Baquero, F.; Nombela, C. The microbiome as a human organ. Clin. Microbiol. Infect. 2012, 18, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Houghteling, P.D.; Walker, W.A. Why is initial bacterial colonization of the intestine important to the infant’s and child’s health? J. Pediatr. Gastroenterol. Nutr. 2015, 60, 294. [Google Scholar] [CrossRef] [Green Version]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999, 69, 1035s–1045s. [Google Scholar] [CrossRef]
- Martín, V.; Maldonado-Barragán, A.; Moles, L.; Rodriguez-Baños, M.; del Campo, R.; Fernández, L.; Jiménez, E. Sharing of bacterial strains between breast milk and infant feces. J. Hum. Lact. 2012, 28, 36–44. [Google Scholar] [CrossRef]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of Human Milk Oligosaccharides by Gut-Related Microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.E.; Niñonuevo, M.; Mills, D.A.; Lebrilla, C.B.; German, J.B. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res. 2007, 51, 1398–1405. [Google Scholar] [CrossRef]
- Sela, D.A.; Mills, D.A. Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010, 18, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Petri, D.; Hill, J.; Van Kessel, A. Microbial succession in the gastrointestinal tract (GIT) of the preweaned pig. Livest. Sci. 2010, 133, 107–109. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Blaser, M.J. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.C.; Wedlund, L.; Cohen, D.A.; Stromberg, A.J.; Kaetzel, C.S. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc. Natl. Acad. Sci. USA 2014, 111, 3074–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planer, J.D.; Peng, Y.; Kau, A.L.; Blanton, L.V.; Ndao, I.M.; Tarr, P.I.; Gordon, J.I. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature 2016, 534, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.R.; Shin, J.; Lee, J.H.; Kim, H.B. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Ivarsson, E.; Roos, S.; Liu, H.Y.; Lindberg, J.E. Fermentable non-starch polysaccharides increases the abundance of Bacteroides–Prevotella–Porphyromonas in ileal microbial community of growing pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Bäckhed, F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular analysis of commensal host-microbial relationships in the intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Gordon, J.I. Honor thy symbionts. Proc. Natl. Acad. Sci. USA 2003, 100, 10452–10459. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Poradosu, R.; McLoughlin, R.M.; Lee, J.C.; Kasper, D.L. Bacteroides fragilis–Stimulated Interleukin-10 Contains Expanding Disease. J. Infect. Dis. 2011, 204, 363–371. [Google Scholar] [CrossRef]
- Meng, Q.; Luo, Z.; Cao, C.; Sun, S.; Ma, Q.; Li, Z.; Shan, A. Weaning alters intestinal gene expression involved in nutrient metabolism by shaping gut microbiota in pigs. Front. Microbiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Wen, Z.; Jiang, X.; Ma, X.; Han, X. Weaning Stress Perturbs Gut Microbiome and Its Metabolic Profile in Piglets. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Downes, J.; Dewhirst, F.E.; Tanner, A.C.R.; Wade, W.G. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 1214–1218. [Google Scholar] [CrossRef]
- Li, X.; Mao, M.; Zhang, Y.; Yu, K.; Zhu, W. Succinate Modulates Intestinal Barrier Function and Inflammation Response in Pigs. Biomolecules 2019, 9, 486. [Google Scholar] [CrossRef] [Green Version]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Lee, G.-H.; Rhee, M.-S.; Chang, D.-H.; Lee, J.; Kim, S.; Yoon, M.H.; Kim, B.-C. Oscillibacter ruminantium sp. nov., isolated from the rumen of Korean native cattle. Int. J. Syst. Evol. Microbiol. 2013, 63, 1942–1946. [Google Scholar] [CrossRef] [Green Version]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and related bacteria–From metagenomic species to metabolic features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Shetty, S.A.; Hugenholtz, F.; Lahti, L.; Smidt, H.; De Vos, W.M. Intestinal microbiome landscaping: Insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol. Rev. 2017, 41, 182–199. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Powrie, F. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 2019, 50, 432–445.e437. [Google Scholar] [CrossRef] [Green Version]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, R.; Krogfelt, K.A.; Cawthraw, S.A.; Van Pelt, W.; Wagenaar, J.A.; Owen, R.J. Host-Pathogen Interactions in Campylobacter Infections: The Host Perspective. Clin. Microbiol. Rev. 2008, 21, 505–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef] [Green Version]
- Jorup-Rönström, C.; Håkanson, A.; Sandell, S.; Edvinsson, O.; Midtvedt, T.; Persson, A.-K.; Norin, E. Fecal transplant against relapsingClostridium difficile-associated diarrhea in 32 patients. Scand. J. Gastroenterol. 2012, 47, 548–552. [Google Scholar] [CrossRef]
- Tsai, T.; Maxwell, C.V.; Zhao, J. 171 Fecal microbiota transplant at weaning improves growth performance and alters fecal microbiome in pigs. J. Anim. Sci. 2019, 97, 175. [Google Scholar] [CrossRef]
- Cheng, C.S.; Wei, H.K.; Wang, P.; Yu, H.C.; Zhang, X.M.; Jiang, S.W.; Peng, J. Early intervention with faecal microbiota transplantation: An effective means to improve growth performance and the intestinal development of suckling piglets. Animal 2019, 13, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Coates, M.E.; Fuller, R.; Harrison, G.F.; Lev, M.; Suffolk, S.F. A comparision of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplements of penicillin. Br. J. Nutr. 1963, 17, 141–150. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microb. 2012, 3, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef]
- Tungland, B. Chapter 2-Short-Chain Fatty Acid Production and Functional Aspects on Host Metabolism. In Human Microbiota in Health and Disease; Tungland, B., Ed.; Academic Press: Cambridge, UK, 2018; pp. 37–106. [Google Scholar]
- Salyers, A.; Vercellotti, J.R.; West, S.E.; Wilkins, T.D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl. Environ. Microbiol. 1977, 33, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Gherardini, F.C.; Salyers, A.A. Characterization of an outer membrane mannanase from Bacteroides ovatus. J. Bacteriol. 1987, 169, 2031–2037. [Google Scholar] [CrossRef] [Green Version]
- Weaver, J.; Whitehead, T.R.; Cotta, M.A.; Valentine, P.C.; Salyers, A.A. Genetic analysis of a locus on the Bacteroides ovatus chromosome which contains xylan utilization genes. Appl. Environ. Microbiol. 1992, 58, 2764–2770. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, G.T.; Englyst, H.N. Starch utilization by the human large intestinal microflora. J. Appl. Bacteriol. 1986, 60, 195–201. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Gordon, J.I. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Morowitz, M.J.; Carlisle, E.M.; Alverdy, J.C. Contributions of Intestinal Bacteria to Nutrition and Metabolism in the Critically Ill. Surg. Clin. N. Am. 2011, 91, 771–785. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Frenette, P.S. Cross talk between neutrophils and the microbiota. Blood 2019, 133, 2168–2177. [Google Scholar] [CrossRef]
- Owaga, E.; Hsieh, R.-H.; Mugendi, B.; Masuku, S.; Shih, C.-K.; Chang, J.-S. Th17 Cells as Potential Probiotic Therapeutic Targets in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2015, 16, 20841–20858. [Google Scholar] [CrossRef] [Green Version]
- Usami, M.; Kishimoto, K.; Ohata, A.; Miyoshi, M.; Aoyama, M.; Fueda, Y.; Kotani, J. Butyrate and trichostatin A attenuate nuclear factor κB activation and tumor necrosis factor α secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr. Res. 2008, 28, 321–328. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, S.; Wang, C.; Jiang, R.; Wan, J. Histone Deacetylase Inhibitors Attenuate Acute Lung Injury During Cecal Ligation and Puncture-Induced Polymicrobial Sepsis. World J. Surg. 2010, 34, 1676–1683. [Google Scholar] [CrossRef]
- Ye, J.; Qiu, J.; Bostick, J.W.; Ueda, A.; Schjerven, H.; Li, S.; Zhou, L. The aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep. 2017, 21, 2277–2290. [Google Scholar] [CrossRef] [Green Version]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Romani, L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Barcik, W.; Wawrzyniak, M.; Akdis, C.A.; O’Mahony, L. Immune regulation by histamine and histamine-secreting bacteria. Curr. Opin. Immunol. 2017, 48, 108–113. [Google Scholar] [CrossRef]
- Podolsky, D.K.V. Innate mechanisms of mucosal defense and repair: The best offense is a good defense. Am. J. Physiol. Liver Physiol. 1999, 277, G495–G499. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Washington, M.K.; Shi, Y.; Liu, L.; Polk, D.B. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Investig. 2011, 121, 2242–2253. [Google Scholar] [CrossRef] [Green Version]
- Lutgendorff, F.; Akkermans, L.M.A.; Soderholm, J.D. The Role of Microbiota and Probiotics in Stress-Induced Gastrointestinal Damage. Curr. Mol. Med. 2008, 8, 282–298. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Cani, P.D. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Stein, H.H. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Swine, 11th ed.; Natl. Acad. Press: Washington, DC, USA, 2012. [Google Scholar]
- Poulsen, H.D. Zinc and copper as feed additives, growth factors or unwanted environmental factors. J. Anim. Feed. Sci. 1998, 7 (Suppl. S1), 135–142. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.W.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Richert, B.T. Effects of the interrelationship between zinc oxide and copper sulfate on growth performance of early-weaned pigs. J. Anim. Sci. 1997, 75, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.M.; Cromwell, G.L.; Crenshaw, T.D.; Dove, C.R.; Ewan, R.C.; Knabe, D.A.; Veum, T.L. Growth promotion effects and plasma changes from feeding high dietary concentrations of zinc and copper to weanling pigs (regional study). J. Anim. Sci. 2000, 78, 1010–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Song, J.; You, Z.; Luan, Z.; Li, W. Zinc Oxide–Montmorillonite Hybrid Influences Diarrhea, Intestinal Mucosal Integrity, and Digestive Enzyme Activity in Weaned Pigs. Biol. Trace Element Res. 2012, 149, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.D.; Baker, D.H. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J. Anim. Sci. 1993, 71, 3020–3024. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.S.; Hill, G.M.; Link, J.E. Early- and traditionally weaned nursery pigs benefit from phase-feeding pharmacological concentrations of zinc oxide: Effect on metallothionein and mineral concentrations. J. Anim. Sci. 1999, 77, 1199–1207. [Google Scholar] [CrossRef]
- Rajendran, D. Application of nano minerals in animal production system. Res. J. Biotechnol. 2013, 8, 1–3. [Google Scholar]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population, and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef]
- Li, M.-Z.; Huang, J.-T.; Tsai, Y.-H.; Mao, S.-Y.; Fu, C.-M.; Lien, T.-F. Nanosize of zinc oxide and the effects on zinc digestibility, growth performances, immune response and serum parameters of weanling piglets. Anim. Sci. J. 2016, 87, 1379–1385. [Google Scholar] [CrossRef]
- Sturniolo, G.C.; Di Leo, V.; Ferronato, A.; D’Odorico, A.; D’Incà, R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm. Bowel Dis. 2001, 7, 94–98. [Google Scholar] [CrossRef]
- Hu, C.H.; Song, Z.H.; Xiao, K.; Song, J.; Jiao, L.F.; Ke, Y.L. Zinc oxide influences intestinal integrity, the expressions of genes associated with inflammation and TLR4-myeloid differentiation factor 88 signaling pathways in weanling pigs. Innate Immun. 2013, 20, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Zhong, W.; McClain, C.J.; Cave, M.; Kang, Y.J.; Zhou, Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am. J. Physiol. Liver Physiol. 2010, 298, G625–G633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finamore, A.; Massimi, M.; Devirgiliis, L.C.; Mengheri, E. Zinc Deficiency Induces Membrane Barrier Damage and Increases Neutrophil Transmigration in Caco-2 Cells. J. Nutr. 2008, 138, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Katouli, M.; Melin, L.; Jensen-Waern, M.; Wallgren, P.; Möllby, R. The effect of zinc oxide supplementation on the stability of the intestinal flora with special reference to composition of coliforms in weaned pigs. J. Appl. Microbiol. 1999, 87, 564–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Zhu, C.; Chen, S.; Gao, L.; Lv, H.; Feng, R.; Jiang, Z. Dietary high zinc oxide modulates the microbiome of ileum and colon in weaned piglets. Front. Microbiol. 2017, 8, 825. [Google Scholar] [CrossRef] [Green Version]
- Vahjen, W.; Pieper, R.; Zentek, J. Increased dietary zinc oxide changes the bacterial core and enterobacterial composition in the ileum of piglets1. J. Anim. Sci. 2011, 89, 2430–2439. [Google Scholar] [CrossRef] [Green Version]
- Højberg, O.; Canibe, N.; Poulsen, H.D.; Hedemann, M.S.; Jensen, B.B. Influence of Dietary Zinc Oxide and Copper Sulfate on the Gastrointestinal Ecosystem in Newly Weaned Piglets. Appl. Environ. Microbiol. 2005, 71, 2267–2277. [Google Scholar] [CrossRef] [Green Version]
- Starke, I.; Pieper, R.; Vahjen, W.; Zentek, J. The impact of dietary Zinc Oxide on the bacterial diversity of the small intestinal microbiota of weaned piglets. J. Vet. Sci. Technol. 2014, 5, 42424. [Google Scholar]
- Starke, I.C.; Pieper, R.; Neumann, K.; Zentek, J.; Vahjen, W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 2014, 87, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Agriculture of the People’s Republic of China. Regulations on the Safe. Use of Feed Additives Issued by Ministry of Agriculture of the People’s Republic of China (No. 2625); Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2018.
- Nitrayova, S.; Windisch, W.; Von Heimendahl, E.; Müller, A.; Bartelt, J. Bioavailability of zinc from different sources in pigs. J. Anim. Sci. 2012, 90, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.L.; Asche, G.L.; Louis, G.F.; Pollmann, D.S. Zinc-methionine improves growth performance of starter pigs. J. Anim. Sci. 1996, 74, 182. [Google Scholar]
- Hollis, G.R.; Carter, S.D.; Cline, T.R.; Crenshaw, T.D.; Cromwell, G.L.; Hill, G.M.; Veum, T.L. Effects of replacing pharmacological levels of dietary zinc oxide with lower dietary levels of various organic zinc sources for weanling pigs. J. Anim. Sci. 2005, 83, 2123–2129. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D.W. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Yamamoto, O.; Hotta, M.; Sawai, J.; Sasamoto, T.; Kojima, H. Influence of Powder Characteristic of ZnO on Antibacterial Activity. J. Ceram. Soc. Jpn. 1998, 106, 1007–1011. [Google Scholar] [CrossRef] [Green Version]
- Sawai, J.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Effect of particle size and heating temperature of ceramic powders on antibacterial activity of their slurries. J. Chem. Eng. Jpn. 1996, 29, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 1998, 86, 521–522. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids–A potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, A.M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, S.-J.; Lee, Y.-S.; Song, M.-D.; Kim, I.-H.; Won, H.-S. De novo generation of short antimicrobial peptides with simple amino acid composition. Regul. Pept. 2011, 166, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nat. Cell Biol. 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Pouny, Y.; Rapaport, D.; Mor, A.; Nicolas, P.; Shai, Y. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogs with phospholipid membranes. Biochemistry 1992, 31, 12416–12423. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Murase, O.; Fujii, N.; Miyajima, K. An Antimicrobial Peptide, Magainin 2, Induced Rapid Flip-Flop of Phospholipids Coupled with Pore Formation and Peptide Translocation. Biochemistry 1996, 35, 11361–11368. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Genet. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of Action of the Antimicrobial Peptide Buforin II: Buforin II Kills Microorganisms by Penetrating the Cell Membrane and Inhibiting Cellular Functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Münch, D.; Sahl, H.-G. Structural variations of the cell wall precursor lipid II in Gram-positive bacteria—Impact on binding and efficacy of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 3062–3071. [Google Scholar] [CrossRef] [Green Version]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Afacan, N.J.; Yeung, A.T.; Pena, O.M.; Hancock, R.E. Therapeutic Potential of Host Defense Peptides in Antibiotic-resistant Infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef]
- Payoungkiattikun, W.; Joompang, A.; Thongchot, S.; Nowichai, B.; Jangpromma, N.; Klaynongsruang, S. Evidence of multi-functional peptide activity: Potential role of KT2 and RT2 for anti-inflammatory, anti-oxidative stress, and anti-apoptosis properties. Appl. Biol. Chem. 2020, 63, 1–13. [Google Scholar] [CrossRef]
- Gitler, C. Protein Digestion and Absorption in Nonruminants. Mamm. Protein Metab. 1964, 1, 35–69. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kim, Y.S.; Shadchehr, A.; Garrido, A.; Macgregor, I.L.; Sleisenger, M.H. Protein digestion and absorption in human small intestine. Gastroenterology 1979, 76, 1415–1421. [Google Scholar] [CrossRef]
- Silk, D.B.; Chung, Y.C.; Berger, K.L.; Conley, K.; Beigler, M.; Sleisenger, M.H.; Kim, Y.S. Comparison of oral feeding of peptide and amino acid meals to normal human subjects. Gut 1979, 20, 291–299. [Google Scholar] [CrossRef]
- Webb, K., Jr. Amino acid and peptide absorption from the gastrointestinal tract. Fed. Proc. 1986, 45, 2268–2271. [Google Scholar]
- Zimmerman, D. Interaction of Intestinal Hydrolysate and Spray-Dried Plasma Fed to Weanling Pigs; Experiment; Iowa State University: Ames, IA, USA, 1996; p. 9615. [Google Scholar]
- Zimmerman, D. The Duration of Carry-Over Growth Response to Intestinal Hydrolysate Fed to Weanling Pigs; Experiment; Iowa State University: Ames, IA, USA, 1996; p. 9612. [Google Scholar]
- Kim, J.H.; Chae, B.J.; Kim, Y.G. Effects of Replacing Spray Dried Plasma Protein with Spray Dried Porcine Intestine Hydrolysate on Ileal Digestibility of Amino Acids and Growth Performance in Early-Weaned Pigs. Asian-Australas. J. Anim. Sci. 2000, 13, 1738–1742. [Google Scholar] [CrossRef]
- Opheim, M.; Sterten, H.; Øverland, M.; Kjos, N. Atlantic salmon (Salmo salar) protein hydrolysate—Effect on growth performance and intestinal morphometry in broiler chickens. Livest. Sci. 2016, 187, 138–145. [Google Scholar] [CrossRef]
- Tang, X.-S.; Tang, Z.-R.; Wang, S.-P.; Feng, Z.-M.; Zhou, N.; Li, T.-J.; Yin, Y.-L. Expression, Purification, and Antibacterial Activity of Bovine Lactoferrampin–Lactoferricin in Pichia pastoris. Appl. Biochem. Biotechnol. 2011, 166, 640–651. [Google Scholar] [CrossRef]
- Yin, Y.; Tang, X.; Fatufe, A.A.; Tang, Z.; Wang, S.; Liu, Z.; Li, T.-J. Dietary Supplementation with Recombinant Lactoferrampin-Lactoferricin Improves Growth Performance and Affects Serum Parameters in Piglets. J. Anim. Veter-Adv. 2012, 11, 2548–2555. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Yang, H.S.; Li, L.; Wang, Y.F.; Huang, R.L.; Li, F.N.; Qiu, W. Effects of antimicrobial peptides in nursery diets on growth performance of pigs reared on five different farms. Livest. Sci. 2014, 167, 206–210. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lee, S.H.; Park, Y.K.; Chae, B.J. Effects of dietary supplementation of antimicrobial peptide-A3 on growth performance, nutrient digestibility, intestinal and fecal microflora and intestinal morphology in weanling pigs. Anim. Feed Sci. Technol. 2012, 177, 98–107. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lohakare, J.; Park, Y.K.; Chae, B.J. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs. J. Sci. Food Agric. 2013, 93, 587–592. [Google Scholar] [CrossRef]
- Shan, T.; Wang, Y.; Liu, J.; Xu, Z. Effect of dietary lactoferrin on the immune functions and serum iron level of weanling piglets1. J. Anim. Sci. 2007, 85, 2140–2146. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, M.M.; Tan, B.E.; Yin, Y.L.; Li, T.J.; Xiao, D.F.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity1. J. Anim. Sci. 2013, 91, 4772–4780. [Google Scholar] [CrossRef]
- Xiao, H.; Tan, B.E.; Wu, M.M.; Yin, Y.L.; Li, T.J.; Yuan, D.X.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: II. Intestinal morphology and function1. J. Anim. Sci. 2013, 91, 4750–4756. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Shan, T.-Z.; Xu, Z.-R.; Feng, J.; Wang, Z.-Q. Effects of the lactoferrin (LF) on the growth performance, intestinal microflora and morphology of weanling pigs. Anim. Feed. Sci. Technol. 2007, 135, 263–272. [Google Scholar] [CrossRef]
- Tang, Z.; Yin, Y.; Zhang, Y.; Huang, R.; Sun, Z.; Li, T.; Tu, Q. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin–lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br. J. Nutr. 2008, 101, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhang, F.; Huang, Z.; Liu, H.; Xie, C.; Zhang, J.; Qiao, S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 2012, 35, 225–230. [Google Scholar] [CrossRef]
- Poudel, P.; Levesque, C.L.; Samuel, R.; St-Pierre, B. Dietary inclusion of Peptiva, a peptide-based feed additive, can accelerate the maturation of the fecal bacterial microbiome in weaned pigs. BMC Veter-Res. 2020, 16, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Erickson, R.H.; Kim, Y.S. Digestion and absorption of dietary protein. Ann. Rev. Med. 1990, 41, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Piper, D.W.; Fenton, B.H. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 1965, 6, 506–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrow, P.A.; Fuller, R.; Newport, M.J. Changes in the Microflora and Physiology of the Anterior Intestinal Tract of Pigs Weaned at 2 Days, with Special Reference to the Pathogenesis of Diarrhea. Infect. Immun. 1977, 18, 586–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radcliffe, J.S.; Zhang, Z.; Kornegay, E.T. The effects of microbial phytase, citric acid, and their interaction in a corn-soybean meal-based diet for weanling pigs. J. Anim. Sci. 1998, 76, 1880–1886. [Google Scholar] [CrossRef] [Green Version]
- Scipioni, R.; Zaghini, G.; Biavati, B. Researches on the use of acidified diets for early weaning of piglets. Zootec. Nutr. Anim. 1978, 4, 201–218. [Google Scholar]
- Burnell, T.W.; Cromwell, G.L.; Stahly, T.S. Effects of Dried Whey and Copper Sulfate on the Growth Responses to Organic Acid in Diets for Weanling Pigs. J. Anim. Sci. 1988, 66, 1100–1108. [Google Scholar] [CrossRef]
- Heo, J.M.; Kim, J.C.; Yoo, J.; Pluske, J.R. A between-experiment analysis of relationships linking dietary protein intake and post-weaning diarrhea in weanling pigs under conditions of experimental infection with an enterotoxigenic strain of E scherichia coli. Anim. Sci. J. 2015, 86, 286–293. [Google Scholar] [CrossRef]
- Thomlinson, J.R.; Lawrence, T.L. Dietary manipulation of gastric pH in the prophylaxis of enteric disease in weaned pigs: Some field observations. Veter-Rec. 1981, 109, 120–122. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Boyen, F.; Gantois, I.; Timbermont, L.; Bohez, L.; Pasmans, F.; Ducatelle, R. Supplementation of coated butyric acid in the feed reduces colonization and shedding of Salmonella in poultry. Poult. Sci. 2005, 84, 1851–1856. [Google Scholar] [CrossRef]
- Fernández-Rubio, C.; Ordóñez, C.; Abad-González, J.; Garcia-Gallego, A.; Honrubia, M.P.; Mallo, J.J.; Balaña-Fouce, R. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult. Sci. 2009, 88, 943–948. [Google Scholar] [CrossRef]
- Kirchgessner, M. Fumaric acid as a feed additive in pig nutrition. Pig News Inform. 1982, 3, 259. [Google Scholar]
- Blank, R.; Mosenthin, R.; Sauer, W.C.; Huang, S. Effect of fumaric acid and dietary buffering capacity on ileal and fecal amino acid digestibilities in early-weaned pigs. J. Anim. Sci. 1999, 77, 2974–2984. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, J.F.; Aherne, F.X. Fumaric and Citric Acid as Feed Additives in Starter Pig Nutrition. J. Anim. Sci. 1984, 58, 935–938. [Google Scholar] [CrossRef]
- Giesting, D.W.; Easter, R.A. Response of Starter Pigs to Supplementation of Corn-Soybean Meal Diets with Organic Acids. J. Anim. Sci. 1985, 60, 1288–1294. [Google Scholar] [CrossRef] [Green Version]
- Halas, D.; Hansen, C.; Hampson, D.; Mullan, B.; Kim, J.; Wilson, R.; Pluske, J. Dietary supplementation with benzoic acid improves apparent ileal digestibility of total nitrogen and increases villous height and caecal microbial diversity in weaner pigs. Anim. Feed. Sci. Technol. 2010, 160, 137–147. [Google Scholar] [CrossRef]
- Diao, H.; Zheng, P.; Yu, B.; He, J.; Mao, X.; Yu, J.; Chen, D. Effects of benzoic acid on growth performance, serum biochemical parameters, nutrient digestibility and digestive enzyme activities of jejunal digesta in weaner piglets. Chin. J. Anim. Nutr. 2013, 25, 768–777. [Google Scholar]
- Piva, A.; Morlacchini, M.; Casadei, G.; Gatta, P.P.; Biagi, G.; Prandini, A. Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Ital. J. Anim. Sci. 2002, 1, 35–41. [Google Scholar] [CrossRef]
- Fang, C.L.; Sun, H.; Wu, J.; Niu, H.H.; Feng, J. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J. Anim. Physiol. Anim. Nutr. 2013, 98, 680–685. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Hwang, J.A.; Hoon, J.; Mun, H.S.; Yang, C.J. Comparison of Single and Blend Acidifiers as Alternative to Antibiotics on Growth Performance, Fecal Microflora, and Humoral Immunity in Weaned Piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 93–100. [Google Scholar] [CrossRef]
- Gabert, V.; Sauer, W. The effect of fumaric acid and sodium fumarate supplementation to diets for weanling pigs on amino acid digestibility and volatile fatty acid concentrations in ileal digesta. Anim. Feed. Sci. Technol. 1995, 53, 243–254. [Google Scholar] [CrossRef]
- Gabert, V.M.; Sauer, W.C.; Schmitz, M.; Ahrens, F.; Mosenthin, R. The effect of formic acid and buffering capacity on the ileal digestibilities of amino acids and bacterial populations and metabolites in the small intestine of weanling pigs fed semipurified fish meal diets. Can. J. Anim. Sci. 1995, 75, 615–623. [Google Scholar] [CrossRef]
- Eckel, B.; Kirchgessner, M.; Roth, F.X. Influence of formic acid on daily weight gain, feed intake, feed conversion rate and digestibility, 1: Investigations about the nutritive efficacy of organic acids in the rearing of piglets. J. Anim. Physiol. Anim. Nutr. 1992, 67, 93–100. [Google Scholar] [CrossRef]
- Guggenbuhl, P.; Séon, A.; Quintana, A.P.; Nunes, C.S. Effects of dietary supplementation with benzoic acid (VevoVitall®) on the zootechnical performance, the gastrointestinal microflora and the ileal digestibility of the young pig. Livest. Sci. 2007, 108, 218–221. [Google Scholar] [CrossRef]
- Food and Agriculture Organization; World Health Organization. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic acid Bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kyriakis, S.; Tsiloyiannis, V.; Vlemmas, J.; Sarris, K.; Tsinas, A.; Alexopoulos, C.; Jansegers, L. The effect of probiotic LSP 122 on the control of post-weaning diarrhoea syndrome of piglets. Res. Veter-Sci. 1999, 67, 223–228. [Google Scholar] [CrossRef]
- Chang, Y.H.; Kim, J.K.; Kim, H.J.; Kim, W.Y.; Kim, Y.B.; Park, Y.H. Probiotic effects of Lactobacillus reuteri BSA-131 on piglets. Korean J. Appl. Microb. Biotechnol. 2000, 28, 8–13. [Google Scholar]
- Xuan, Z.N.; Kim, J.D.; Heo, K.N.; Jung, H.J.; Lee, J.H.; Han, Y.K.; Han, I.K. Study on the development of a probiotics complex for weaned pigs. Asian-Aust. J. Anim. Sci. 2001, 14, 1425–1428. [Google Scholar]
- Giang, H.H.; Viet, T.Q.; Ogle, B.; Lindberg, J.E. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livest. Sci. 2010, 129, 95–103. [Google Scholar] [CrossRef]
- Huang, C.; Qiao, S.; Li, D.; Piao, X.; Ren, J. Effects of Lactobacilli on the Performance, Diarrhea Incidence, VFA Concentration and Gastrointestinal Microbial Flora of Weaning Pigs. Asian-Australas. J. Anim. Sci. 2004, 17, 401–409. [Google Scholar] [CrossRef]
- Zhao, P.; Kim, I. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhea score in weanling pigs. Anim. Feed. Sci. Technol. 2015, 200, 86–92. [Google Scholar] [CrossRef]
- Frantz, N.Z.; Nelssen, J.L.; DeRouchey, J.M.; Goodband, R.D.; Tokach, M.D.; Dritz, S.S. Effects of a prebiotic, Inulin, and a direct fed microbial on growth performance of weanling pigs. Kans. Agric. Exp. Stn. Res. Rep. 2003, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Dun, Y.; Li, S.; Zhang, D.; Peng, N.; Zhao, S.; Liang, Y. Dietary Enterococcus faecalis LAB31 Improves Growth Performance, Reduces Diarrhea, and Increases Fecal Lactobacillus Number of Weaned Piglets. PLoS ONE 2015, 10, e0116635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, M.-L.; Chen, H.-C.; Chen, K.-N.; Lin, Y.-C.; Lin, Y.-T.; Chen, M.-J. Optimizing Production of Two Potential Probiotic Lactobacilli Strains Isolated from Piglet Feces as Feed Additives for Weaned Piglets. Asian-Australas. J. Anim. Sci. 2015, 28, 1163–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowarah, R.; Verma, A.; Agarwal, N.; Patel, B.; Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest. Sci. 2017, 195, 74–79. [Google Scholar] [CrossRef]
- Barszcz, M.; Taciak, M.; Skomiał, J. The effects of inulin, dried Jerusalem artichoke tuber and a multispecies probiotic preparation on microbiota ecology and immune status of the large intestine in young pigs. Arch. Anim. Nutr. 2016, 70, 278–292. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.-H.; Zhou, D.; Wu, Q.; Song, D.; Dicksved, J.; Wang, J.-F. Oral Administration of a Select Mixture of Bacillus Probiotics Affects the Gut Microbiota and Goblet Cell Function following Escherichia coli Challenge in Newly Weaned Pigs of Genotype MUC4 That Are Supposed To Be Enterotoxigenic E. coli F4ab/ac Receptor Negative. Appl. Environ. Microbiol. 2016, 83, e02747-16. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tsai, T.; Wei, X.; Zuo, B.; Davis, E.; Rehberger, T.; Zhao, J. Effect of Lactylate and Bacillus subtilis on growth performance, peripheral blood cell profile, and gut microbiota of nursery pigs. Microorganisms 2021, 9, 803. [Google Scholar] [CrossRef]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.Y.; Choi, Y.J.; Heo, J. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Q.; Zhu, Y.H.; Zhang, H.F.; Yue, Y.; Cai, Z.X.; Lu, Q.P.; Wang, J.F. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: Intestinal microbiota and immune imbalances. PLoS ONE 2012, 7, e40666. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Li, X.-Q.; Zhang, W.; Zhou, N.; Liu, H.-Y.; Wang, J.-F. Dose-dependent effects of Lactobacillus rhamnosus on serum interleukin-17 production and intestinal T-cell responses in pigs challenged with Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 1787–1798. [Google Scholar] [CrossRef] [Green Version]
- Wei, X. Effects of Commercial Feed Additives on Growth Performance and Gut Microbiota of Nursery Pigs. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Tsai, T.; Howe, S.; Zhao, J. Weaning Induced Gut Dysfunction and Nutritional Interventions in Nursery Pigs: A Partial Review. Animals 2021, 11, 1279. https://doi.org/10.3390/ani11051279
Wei X, Tsai T, Howe S, Zhao J. Weaning Induced Gut Dysfunction and Nutritional Interventions in Nursery Pigs: A Partial Review. Animals. 2021; 11(5):1279. https://doi.org/10.3390/ani11051279
Chicago/Turabian StyleWei, Xiaoyuan, Tsungcheng Tsai, Samantha Howe, and Jiangchao Zhao. 2021. "Weaning Induced Gut Dysfunction and Nutritional Interventions in Nursery Pigs: A Partial Review" Animals 11, no. 5: 1279. https://doi.org/10.3390/ani11051279
APA StyleWei, X., Tsai, T., Howe, S., & Zhao, J. (2021). Weaning Induced Gut Dysfunction and Nutritional Interventions in Nursery Pigs: A Partial Review. Animals, 11(5), 1279. https://doi.org/10.3390/ani11051279






